Abstract
Brazil has been leading the consumer market for pesticides, mainly due to greater flexibility in registration for agricultural use. The constant application of chemicals increases the likelihood of contaminating adjacent aquatic environments. Furthermore, fluctuations in abiotic variables, especially in subtropical countries, can enhance the effects of pesticides on the aquatic community. The aims of the current study are to investigate the incidence of pesticides in a river in southern Brazil and to evaluate the water quality and toxicity biomarkers on fish species Astyanax jacuhiensis (Cope, 1894), in different crop seasons. Water, sediment, and fish samples were collected in the summer, autumn, and winter of 2019. The herbicides atrazine and clomazone and insecticide imidacloprid were identified in water throughout the sampling period—only atrazine has the maximum concentration established for surface water by Brazilian legislation. Redundancy analysis (RDA) mainly showed the effects of seasonality on biochemical responses such as pH and water temperature. Also, most A. jacuhiensis biomarkers alterations were associated with winter and autumn periods. Furthermore, it was possible to verify the relationship between non-enzymatic antioxidants (non-protein thiols) and oxidative damage (lipid peroxidation and protein carbonylation). Therefore, the results of pesticides detected in water emphasize the need to update Brazilian legislation for compounds registered in the country. Changes in the physicochemical variables of water can influence the responses of biomarkers in addition to pesticides. Finally, biomonitoring studies are important and necessary mainly in subtropical regions where there is variation in climatic conditions between periods of the year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides are used for pest control in agricultural crops grown worldwide. Population growth and the consequent need of increasing food production are the factors influencing the increased use of pesticides (Elibariki & Maguta, 2017). It happens due to increase in the number of cultivated areas and to intensification of crop systems (Mottes et al., 2017). The intensive agricultural model adopted in South America, which is based on the technological package GMO (genetically modified organisms), has been applied since the 1990s. The use of this model is associated with favorable regional features—such as extensive territories, medium to excellent quality soils, few or moderate climatic extremes, and technological adoption capacity—mainly in Brazil, Argentina, Paraguay, and Uruguay (Pengue, 2016). However, it has intense use and adopts a wide variety of pesticides (López et al., 2012).
Brazil is the largest pesticide consumer worldwide (Albuquerque et al., 2016), and such a consumption significantly increases on a yearly basis. Soy crops lead the pesticide consumption ranking in Brazil, since they account for more than half of pesticide trading in the country. They are followed by sugarcane and corn (10%), cotton (7%), and wheat (4%) crops (Bombardi, 2017). Approximately 95% of the agricultural area in Rio Grande do Sul State, southern Brazil, is used for grain production such as soybean, rice, corn, and wheat which are the main agricultural crops grown in this state (Feix et al., 2016). In southern Brazil, the largest volume of pesticides applied per area in the region is featured by the cultivation of soybean, corn, and wheat crops (Barreto et al., 2012).
Although the use of these chemicals has contributed to agricultural yield, water contamination with pesticides is a recurrent issue observed in rivers and lakes, and it has costly (Choudhary et al., 2018; Vieira et al., 2017; Loro et al., 2015, Amaral et al., 2020; Cerezer et al., 2020) and large-scale implications at global level. Pesticides mainly get to aquatic ecosystems through diffuse sources such as runoff, leaching, drainage, drift, atmospheric deposition, and groundwater flow (Dabrowski et al., 2014; Holvoet et al., 2007), since Brazil lacks monitoring programs, diffuse contamination of water resources becomes even more concerning. In addition, the National Environment Council Resolution (CONAMA, 2005) does not set maximum values allowed in surface waters for all active ingredients of pesticides registered in the country—values established for certain pesticides are much higher than values allowed in other countries, such as the one established by the Ministry of the Environment of Portugal (1998).
Thus, aquatic organisms such as fish are often exposed to a wide variety of pesticides, and, given their ability to metabolize and accumulate chemical pollutants, they have become one of the most suitable models to estimate likely risks to aquatic environments (Botelho et al., 2015). Biomarker use as biomonitoring tool is efficient and increasingly used to provide early information about river pollution to help prevent severe damage to these ecosystems (Amaral et al., 2018, 2020). The evaluation of individual substances, rather than mixtures found in the environment, is a limiting factor observed in chemical toxicity tests (Beyer et al., 2014). In addition, studies conducted in situ are increasingly relevant and necessary, mainly because they combine ecological importance and toxicity tests under natural field conditions (Vieira et al., 2014, 2017). Therefore, the use of tools that encompass contaminants and biomarkers provides an integrated and more comprehensive cause-and-effect response (Amaral et al., 2020; Cerezer et al., 2020).
Exposure of fish to pesticides can alter their metabolism and trigger disturbances in cellular components and tissues. These changes in organisms, induced by xenobiotics, are called biomarkers and reflect the water quality conditions in which the fish inhabit (Barata et al., 2007). Furthermore, fluctuations in abiotic variables can also affect biomarker responses (Lushchak, 2011; Lushchak & Bagnyukova, 2006). In addition, abiotic variables determine the behavior of pesticides in the aquatic environment, such as solubility, toxicity, and persistence. Therefore, it is necessary to conduct ecotoxicological studies focused on evaluating the effects of seasonality and pesticides in situ by taking into consideration the intense agricultural activity in southern Brazil, in association with the wide use of pesticides in prominent crops in the Brazilian scenario.
These studies are important mainly due to the lack of maximum values allowed for aquatic environments in Brazilian legislation, for all active ingredients of pesticides that are registered in the country. Thus, this study is a preliminary assessment of the area to assess the interaction between pesticides, abiotic variables, and non-target aquatic organisms (Astyanax jacuhiensis) in order to predict the impacts that may be generated by them and that are likely to trigger a series of adverse effects on the entire ecosystem. The current study aimed to evaluate the physicochemical variables in water and to investigate the presence of pesticides in water and sediment and biomarkers’ toxicity responses in fish species A. jacuhiensis in a river in southern Brazil, in different agricultural seasons.
2 Materials and Methods
2.1 Study Site
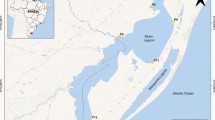
Potiribu River is the main river in the investigated basin (Potiribu River Basin), which is located in Rio Grande do Sul State (RS), southern Brazil; its total area covers approximately 600 km2 (Fig. 1). The main economic activities carried out in this region are associated with the primary sector. The main agricultural crops grown in the region comprise soybeans in summer and, to a lesser extent, corn (from September/October to April). On the other hand, wheat, oat, barley, triticale, and canola crops, among others, are grown at small scale in winter (from May to August/September).
According to Köppen’s classification, almost all RS territory has dubtropical temperate climate classified as humid mesothermal (Cfa). Thus, it presents great variation in temperature among seasons, such as hot summers and colder winters. Rainfall indices present balanced distribution throughout the year (Rio Grande do Sul, 2002). Soil in the investigated basin is classified as Oxisol (Soil Survey Staff, 2014).
Water, sediment, and fish sample collection place in Potiribu River is located upstream the urban region (Fig. 1). Consequently, it receives all the agricultural influence of the hydrographic basin. As the basin is inserted in essentially agricultural region, it was not possible determining a reference location without contamination, since places located far from anthropic activities, which have preserved riparian forest, can present contaminants in water (Cerezer et al., 2020). In addition, attempts were made to sample fish elsewhere in the river, upstream from the urban region, but no fish were found. Collections were carried out in summer (late January), autumn (early June), and winter (early September) of 2019.
2.2 Organisms
The genus Astyanax is one of the dominant fish genera in South America (Moreira-Filho & Bertollo, 1991) and the most diverse genus belonging to the family Characidae in Neotropical regions (Orsi et al., 2004). These fish are widely used as bioindicators in ecotoxicological studies conducted in situ (Bergmann et al., 2020; Lemos et al., 2008; Marins et al., 2020; Santana et al., 2015; Silva & Martinez, 2007) due to their abundance, sensitivity to variations in water quality, easy capture, small size, and lifestyle (Bueno-Krawczyk et al., 2015; Ghisi et al., 2017; Marcon et al., 2017; Rossi et al., 2011), as well as because they show preference for a specific site and do not present long-distance migratory behavior (Costa et al., 2013; Ghisi et al., 2014).
Female and male A. jacuhiensis individuals were collected with the aid of 2-mm-mesh fishing net (summer, n = 11; length, 8.32 ± 1.08 cm; mass, 9.09 ± 4.44 g; autumn, n = 10; length, 10.00 ± 1.50 cm; mass, 15.00 ± 8.00 g; and winter, n = 08, length, 7.00 ± 0.63 cm; mass, 5.67 ± 1.86 g). Fish of both sexes were collected to increase n, given the small size of these fish for biological analysis and to the small number of fish in the study site. Collected specimens were subjected to euthanasia through the spinal cord section. Brain, gill, liver, intestine, and muscle tissues were removed and stored in liquid nitrogen for further biochemical analysis. The current project was approved by Chico Mendes Biodiversity Conservation Institute (ICMBio), under license number 66994–1.
2.3 Water and Sediment Sample Collection and Analysis
Surface water samples were collected (around 10-cm deep) in plastic bottles for physical–chemical analysis. On the other hand, some samples were stored in amber borosilicate glass vials and kept under refrigeration for pesticide incidence analysis. An Ekman dredger was launched from the river bank to collect the sediments to a depth of around 2 m. Then, the sediments were packed in plastic containers and kept under refrigeration until analysis time (CETESB, 2011).
Water physicochemical variables such as temperature, pH, oxidation potential, electrical conductivity, turbidity, and total dissolved solids were determined in situ with the aid of Horiba® U-52 probe. Total ammonia was determined based on Verdouw et al. (1978), whereas total nitrogen, total carbon, total phosphorus, total hardness, and orthophosphate levels were determined based on the Standard Methods for the Examination of Water and Wastewater (APHA, 2012). Dissolved oxygen concentration along Potiribu River was ≥ 6 mg L−1 (SEMA, 2012).
Water and sediment samples were sent to the Laboratory for Analysis of Pesticide Residues—LARP—at UFSM (Universidade Federal de Santa Maria), for pesticide analysis, based on the methodology described by Sabin et al. (2009) and Martins et al. (2013). Water samples were analyzed based on the pesticide residue determination method by using solid phase extraction (SPE) and on liquid chromatography/tandem mass spectrometry analysis (LC–MS/MS). On the other hand, sediments were analyzed based on the modified QueChERS method and on LC–MS/MS. Pesticide incidence in fish muscle was also analyzed in the aforementioned laboratory, based on the modified QueChERS method and on LC–MS/MS, according to the methodology described by Munaretto et al. (2013).
Brazilian standards set for surface freshwater quality were defined by the National Environmental Council Resolution (CONAMA, 2005), which classifies water quality based on its use. Thus, values recorded for water physical–chemical variables, as well as for pesticide concentrations in it, were compared to those of the referred law (CONAMA, 2005).
2.4 Biochemical Analyses
Brain, gill, liver, intestine, and muscle tissues of the collected fish were homogenized with Tris–HCl buffer [50 mM] at pH 7.5 and centrifuged at 1400 × g, for 10 min. The supernatant was used for all analyses. Protein concentration in these tissues was determined based on Bradford (1976). Methodologies used for biochemical analyses were modified for microplate (except for catalase). Catalase (CAT) activity in the liver was determined according to Aebi (1984), based on the principle of decreasing hydrogen peroxide (H2O2) absorbance by CAT metabolism; results were expressed as µmol min−1 mg of protein−1. Glutathione S-transferase (GST) activity in the liver was measured based on Habig et al. (1974), by using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate; results were expressed as µmol GS-DNB min−1 mg of protein−1. Acetylcholinesterase (AChE) activity in brain and muscle was determined based on Ellman et al. (1961); results were expressed as µmol min−1 mg of protein−1. Non-enzymatic antioxidants were determined through non-protein thiol (NPSH) levels in all tissues, based on the reaction of 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) to thiol groups, as described by Ellman (1959); results were expressed as µmol SH g of tissue−1. Oxidative damage was measured through lipid peroxidation, based on Draper and Hadley (1990), by measuring substances reactive to thiobarbituric acid (TBARS) in all tissues; results were expressed as nmol MDA mg of tissue−1. Carbonyl protein (CP) assay in all tissues was carried out, as described by Yan et al. (1995); results were expressed as nmol carbonyl mg of protein−1.
2.5 Statistical Analysis
Shapiro–Wilk test and Bartlett test were used to test biochemical data normality and homogeneity, respectively. One-way analysis of variance (ANOVA), followed by Tukey post-test, was used to investigate the influence of time of the year on biochemical responses; results were expressed as mean ± standard deviation. Significant results presented p ≤ 0.05.
Two redundancy analyses were performed to assess biomarker responses: one analyzing the variables of water quality and pesticides and the other only pesticides. A matrix with 6 biomarkers (response variables), 12 environmental, and 3 pollutant parameters (predictor variables) was used. As it was not possible to collect the same number of fish in each investigated period in which resulted in a different number of samples collected for some tissues, we estimated the missing data using the “imputePCA” function from the missMDA package (Josse & Husson, 2016). Values missing in the data matrix were estimated through the “regularized iterative PCA algorithm,” whereby missing entries are imputed from a Gaussian distribution with mean and standard deviation calculated from the observed values (Josse & Husson, 2012). Posteriorly, redundancy analysis (RDA) was applied to simultaneously determine to what extent differences in the fish biomarkers’ response were related with differences in the water quality (pH, turbidity, among others) and pollutants (pesticides). RDA is an appropriate procedure to our data because the dominant gradient length revealed by the DCA (detrended correspondence analysis) was below 3 (ranging from 0.518 to 1.080; Leps & Smilauer, 2003). In addition, to assess the possible isolated relationship of pesticides on biomarkers, another RDA was carried out to 6 biomarkers (response variables) and pesticides (predictor variables). RDA analysis was carried out using the vegan package (Oksanen et al., 2020). Both missing data estimates and RDA procedures were performed in R software (R Core Team 2021).
3 Results
3.1 Water Quality Variables
Results recorded for physical–chemical variables of Potiribu River water are shown in Table 1. According to data analyzed in the current study, such as total dissolved solids, total phosphorus, total ammoniacal nitrogen, turbidity, and pH, the water quality in summer was classified as class 3 according to Brazilian legislation (CONAMA, 2005). Water qualities in the autumn and winter periods were classified into class 2 (CONAMA, 2005).
Pesticides such as atrazine (Atz), clomazone (Clo), and imidacloprid (Imi) were identified in water samples collected in summer; Clo and Imi were identified in samples collected in autumn; and Atz and Clo, in winter (Table 2). Pesticides were not found in sediment and fish muscle samples (the full list of pesticide residues analyzed in the current study can be found in supplementary material).
3.2 Biomarkers
Lipid peroxidation levels (TBARS) in fish’s gills and muscle tissues; NPSH levels in gills, brain, and intestine; brain AChE activity; and GST activity in liver were significantly higher in winter, compared to the summer and autumn periods. CP content in fish’s brain, in winter, was significantly lower. CP levels in fish’s brain, muscle, intestine, and liver tissue and CAT activity in the liver were significantly higher in autumn. On the other hand, AChE activity and NPSH levels in muscle were significantly lower compared to the other periods analyzed. In summer, it was possible to observe that TBARS levels in brain and NPSH levels in intestine were significantly lower than in the other periods. However, in this same period, NPSH levels in fish’s livers were significantly higher compared to autumn and winter (Figs. 2, 3, and 4).
Biochemical analyses applied to Astyanax jacuhiensis gills and intestine collected in Potiribu River, southern Brazil, in the summer, autumn and winter of 2019. A Carbonyl protein content, B TBA-reactive substance levels, C non-protein thiol levels. Different letters correspond to significant difference between the point and the analyzed seasons, at 95% probability level (p ≤ 0.05)
Biochemical analyses applied to Astyanax jacuhiensis brain and muscle collected in Rio Potiribu River, southern Brazil, in the summer, autumn and winter of 2019. A Acetylcholinesterase activity, B Carbonyl protein content, C TBA-reactive substance levels, D Non-protein thiol levels. Different letters correspond to significant difference between the point and the analyzed seasons, at 95% probability level (p ≤ 0.05)
Biochemical analyses applied to Astyanax jacuhiensis liver collected in Potiribu River, southern Brazil, in the summer, autumn and winter of 2019. A Catalase activity, B Glutathione-S-transferase activity, C Protein carbonyl content, D TBA-reactive substance levels, E Non-protein thiol levels. Different letters correspond to significant difference between the point and the analyzed seasons, at 95% probability level (p ≤ 0.05)
3.3 Redundancy analysis (RDA)
Redundancy analysis (RDA) was conducted to reveal more accurately the connection between biomarker response and environmental parameters (i.e., water quality and pollutants). Overall, the first two axes of the RDA accounted for 46% of the total variation and clearly separated samples by seasonality (Fig. 5A). The first canonical axis explained 26% of data variation and mainly distinguished samples collected in winter from those ones collected in autumn. In addition, pH and water temperature were the most important factors affecting biomarkers responses in the first axis. In fact, both variables were located in negative loadings, whereby temperature was clearly associated with summer samples. The second RDA axis explained 20% of data variation and separated samples collected in autumn–winter from those collected in summer. Similarly, pH and temperature were the predictor variables presenting the greatest positive and negative loadings in this axis, respectively. More specifically, pH was closely associated with the autumn–winter gradient, whereas temperature was closely associated with summer. In addition, the response of some biomarkers was associated with seasonality. For example, autumn has influenced A. jacuhiensis responses such as CAT and CP in the liver, and CP in the intestine, muscle, and brain of fish. NPSH and AChE in the brain, NPSH in the intestine and gills, TBARS in the muscle, and GST in the liver were influenced by winter season. Intestinal TBARS and gill CP were more associated with summer season.
Redundancy analysis (RDA) ordination plot depicting the relationship between Astyanax jacuhiensis biomarkers and environmental variables (i.e., water quality and pollutants) (A) and RDA ordination plot depicting the relationship between Astyanax jacuhiensis biomarkers and pollutants (pesticides) (B) over different seasons. Acronyms: CAT, catalase; CARB, carbonyl protein; NPSH, non-protein thiols; GST, glutathione S-transferase; AChE, acetylcholinesterase; li, liver; in, intestine; mu, muscle; br, brain; gi, gills; Clo, clomazone, Atz, atrazine
On the other hand, when we evaluated biomarkers and pesticides, without water quality variables, the first two axes of the RDA accounted for 46% of the total variation and also separated the samples by seasonality (Fig. 5B). The first canonical axis explained 26% of data variation and mainly distinguished samples collected in winter from those ones collected in autumn. Clo and Atz, located in negative and positive loadings, respectively, were the predictor variables with the greatest contribution in the first axis. The second RDA axis explained 20% of data variation and separated samples collected in autumn–winter from those collected in summer. Similarly, Clo and Atz were the predictor variables with the greatest positive and negative loadings in this axis, respectively. Specifically, Clo was more associated with autumn while Atz was more associated with the summer–winter gradient. Also, the response of some biomarkers was clearly influenced by Clo and Atz pesticides. Clo influenced the responses of A. jacuhiensis biomarkers such as liver CAT activity, and CP content in fish’s intestine, muscle, liver, and brain tissues. Muscle AChE activity, NPSH levels in muscle and liver, and TBARS levels in liver and gill were influenced by Atz.
4 Discussion
4.1 Water Physical–Chemical Variables
Overall, water physical–chemical variables did not show significantly different values between collection seasons (Table 1). Water temperature in summer was higher due to seasonality itself. According to INMET (2019), based on data provided by the Automatic Meteorological Station of Cruz Alta—RS: A853—which is the county where the head of the investigated basin is inserted in—air temperature in the 30 days preceding the summer collection date ranged from 17 to 33 °C; in autumn, it ranged from 7 to 25 °C, and in winter, from 0 to 30° C. Rainfall rate recorded in the aforementioned seasons was 263 mm, 304 mm, and 63 mm, respectively (INMET, 2019). The highest turbidity values recorded in summer and autumn were associated with high rainfall rates recorded in these seasons, which carried external particles into the river. Total phosphorus and orthophosphate concentrations were higher in summer, which is often featured by intense agricultural activity. Soils in agricultural regions often present higher phosphorus concentrations due to fertilizer using. Thus, water resources close to crops can be subjected to discharges deriving from runoff and erosion processes resulting from intense rainfall events (Bender et al., 2018; Tiecher et al., 2017, 2019).
In Brazilian legislation, surface fresh waters are classified according to the degree of pollution in classes 1, 2, 3, and 4, with class 1 being the lowest levels of pollution and class 4 the highest. In the current study, the Potiribu River water quality in summer was classified as class 3. The water of this class can be used for human consumption, after conventional or advanced treatment; as well as for tree, cereal, and forage crop irrigation; amateur fishing; secondary contact recreation; and animal desedentation. Winter and autumn received the water quality classification in class 2. Class 2 water can be used for more demanding purposes in comparison to that of the summer season, namely, supply for human consumption, after conventional treatment; aquatic community protection; primary contact recreation; vegetable, fruit plant and park, gardening, and sports and leisure field irrigation, whose water may have direct contact with humans; and aquaculture and fishing activity (Conama, 2005).
4.2 Pesticides
The most intense agricultural cultivation period in southern Brazil is in summer (IBGE, 2019) and as a consequence the greater use of pesticides. Several pesticides used in Brazil were banned in other countries. Among them, one finds Atz (Piancini et al., 2015), which was found in water analyzed in the current study. Atz is widely used to control certain broadleaf weeds, mainly in corn, sorghum, and sugarcane crops (Solomon et al., 1996), and Atz and its metabolites are well dispersed and persistent in the environment (Jablonowski & Schäffer, 2011). Atz identified in the water analyzed in the current study (in summer) likely derived from corn crops, which are often grown in the spring/summer season. Atz found in winter was likely associated with corn crops, since soil preparation for growing this crop has already started again at this time.
Clomazone (Clo) is widely used in agriculture to control pre- and post-emergent weeds (Pereira et al., 2013). It is mainly used in rice fields in southern Brazil (Jonsson et al., 1998; Menezes et al., 2014), as well as in soybean and cotton crops, among others (Liu et al., 1996). Residues of this herbicide can persist in agricultural water for up to 130 days (Zanella et al., 2002). According to Zanella et al. (2002), Clo was found in 90% of water samples collected from rivers in the central region of RS, which is characterized by irrigated rice culture. Clo was identified in all water samples collected in Potiribu River, in all seasons analyzed in the current study. This herbicide is recommended for some spring/summer crops grown in the region; however, it was not possible to accurately determine its origin in all collections. Although it can be used in soybean crops in RS, this active ingredient is mainly used to grow irrigated rice and it is not often found in the investigated region.
Imidacloprid (Imi) belongs to one of the most important chemical classes in the global market. In addition, it is the best-selling insecticide in the world (Nauen et al., 2008). Imi is used to control insects such as aphids, whiteflies, grasshoppers, and beetles, among other sucking and perforating insects (Jeschke et al., 2011). It is also used for seed, soil, and leaf applications (Tisler et al., 2009). There was incidence of Clo, Imi, and Atz in the investigated river in southern Brazil, which is a region featured by tobacco culture (Becker et al., 2009). Amaral et al. (2020) have found Atz in all collection seasons (summer, autumn, winter, and spring), whereas Imi was observed in autumn, winter, and spring in a reservoir in southern Brazil. Imi found in Potiribu River water (< LOQ) may be associated with agricultural corn and soybean cultivation in summer, as well as with wheat cultivation in autumn.
Among pesticides found in water during the sampling seasons, only Atz has the maximum limit value allowed for surface water (2 µg L−1) set by the Brazilian legislation (Conama, 2005). Although Atz concentrations found in the current study were in compliance with that established by law, it should be emphasized that these products are used intermittently throughout the year, as well as that they encompass a wide range of active ingredients depending on the crop grown in a given season. In addition, most of these products do not have maximum values established by law (Amaral et al., 2018), as observed in the current study.
4.3 Biomarkers and Redundancy Analysis (RDA)
The redundancy analysis (RDA) to variables of water quality, pesticides, and biomarkers showed that factors such as pH and water temperature were more related to biomarkers responses. However, all pesticides detected in this study can cause adverse effects on fish organism, such as changes in enzymatic activities (AChE, CAT, and GST), non-enzymatic antioxidant content (NPSH) and causing damage to lipids (TBARS) and proteins (CP) (Cattaneo et al., 2011; Murussi et al., 2015; Santos & Martinez, 2012; Schmidel et al., 2014; Topal et al., 2017; Vieira et al., 2018). And although pH and temperature had a greater relationship with biomarkers, we cannot rule out the pesticides effects, mainly because mixtures of these compounds occur in aquatic environment, and can be enhanced by abiotic fluctuations.
Both natural fluctuation of abiotic factors over seasons and pesticides’ toxic effect can cause changes in fish biomarkers. Fish are ectothermic animals whose metabolism is influenced by water temperature (Lushchak & Bagnyukova, 2006). The increase in temperature stimulates all metabolic processes in fish, such as increased oxygen consumption and therefore increased reactive oxygen species (ROS) production, which can result in oxidative stress (Lushchak, 2011).
Water contamination by pesticides is even more worrying in regions with a subtropical climate, such as in southern Brazil, due to the wide variation in temperature between periods of the year. The physicochemical characteristics of pesticide molecules depend, above all, on the temperature and pH values that are standardized under controlled conditions in the laboratory (Lewis et al., 2016). However, under field conditions, abiotic fluctuations influence the behavior of these chemical compounds in the environment, and consequently the exposed organisms. The direct influence of water temperature and pH on toxicity (Carvalho et al., 2015; Soares et al., 2020; Watson & Maly, 1987; Zebral et al., 2019) and on the persistence of xenobiotics in environment (Ragnarsdottir, 2000; Solomon et al., 1996, 2008) is well known. The presence of contaminants alters the ability of fish to adapt to seasonal changes in temperature, and with that, it can compromise population integrity, especially species that face global warming (Amaral et al., 2018; Zebral et al., 2019).
According to RDA, most biomarkers were associated with winter and autumn periods. In these seasons, the water temperature is colder, and as a consequence, the fish metabolism is slower, and it can enhance the pollution effect. The RDA associated the CP content of most tissues analyzed with autumn, as well as the liver CAT in fish. In addition, the RDA also linked Clo to this period. Murussi et al. (2015) demonstrated that Clo can increase CP levels and increase CAT activity in Cyprinus carpio. In fact, in the current study, the highest levels of CP and CAT activity were obtained in autumn, and this result may be associated with Clo. Therefore, the influence of pesticides on biomarkers’ responses at different times of the year is related to physicochemical characteristics of the pesticide molecule, time of exposure, pesticide concentrations, fluctuation of water abiotic variables, and fish species, in addition to indirect rainfall volume interference between periods.
While the RDA demonstrated the importance of an integrated approach to the assessed data, the isolated analysis of fish biomarkers mainly emphasized the relationships between antioxidants and oxidative damage from pollution. The highest NPSH levels recorded in winter were followed by the lowest TBARS levels in fish’s intestine, in the same collection season. This fact shows the important role played by non-enzymatic antioxidants in preventing lipid damage. On the other hand, NPSH depletion in A. jacuhiensis may also be linked to their exposure to agricultural herbicides and/or pesticides (Costa-Silva et al., 2015). Likewise, CP content decrease in fish gills was followed by increase in NPSH levels and vice versa, in the same period. This relationship suggests that increased content of non-enzymatic antioxidants in cells may have avoided damage to proteins. Furthermore, higher levels of CP in gills were obtained in summer and autumn rainy seasons. And as this organ is in permanent contact with the environment, it becomes vulnerable to variations in water quality and pollutants (Ghisi et al., 2014; Paulino et al., 2014), and this fact can be confirmed by the high levels of water turbidity in these periods.
The current study has found a significantly increase in liver GST activity of fish specimens collected in winter. This result corroborates to described by Amaral et al. (2018) in which the increased of GST activity may be associated with the transport of sex hormones in pre-reproductive period of fish (Zhou et al., 2009). In fact, Astyanax species have a reproductive period between the end of winter and spring (Dala-Corte and Azevedo, 2010; Hirt et al., 2011). Furthermore, the increase in GST activity may be associated with the pollutants concentration due to lower water dilution in rainless period. The lower dilution of chemical compounds found in water may also explain the high TBARS levels observed in fish’s muscles in winter. In addition, the highest brain AChE activity in fish was obtained in winter and may also be associated with Clo (Murussi et al., 2015).
5 Conclusion
Based on the classification established by the Brazilian legislation, the worst water quality recorded for Potiribu River was observed in summer, which also recorded a larger number of used pesticides (atrazine, clomazone, and imidacloprid). Atrazine is the only pesticide presenting maximum concentration value allowed for surface water resources established by Brazilian legislation. Thus, the current study emphasized the need of reassessing and including data in the legislation about all pesticides registered in the country in order to ensure the integrity and health of aquatic organisms.
Astyanax jacuhiensis biochemical biomarkers evaluated in this study responded differently to different collection periods and to different tissues analyzed. The integration between agricultural periods, biomarkers, and pesticides showed that many changes observed in biomarkers were mainly related to abiotic factors (pH and water temperature), but also to detected pesticides. Furthermore, pesticides’ behavior in aquatic environment is influenced by changes in seasonal abiotic variables. Thus, the importance of biomonitoring and complexity of evaluating all factors that may be contributing to biochemical changes in fish is proven, given the diversity of chemical composition that are present in river waters, along with fluctuations abiotic. Finally, we would like to emphasize the importance of evaluating changes in the physicochemical characteristics of pesticides, especially in subtropical regions whose temperatures fluctuate in a wide range between periods of the year.
Data Availability
Not applicable.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Albuquerque, A. F., Ribeiro, J. S., Kummrow, F., Nogueira, J. Á., Montagner, C. C., & Umbuzeiro, G. A. (2016). Pesticides in Brazilian freshwaters: A critical review. Environmental Science. Processes & Impacts, 18, 779–787. https://doi.org/10.1039/c6em00268d
Amaral, A. M. B., Gomes, J. L. C., Weimer, G. H., Marins, A. T., Loro, V. L., & Zanella, R. (2018). Seasonal implications on toxicity biomarkers of Loricariichthys anus (Valenciennes, 1835) from a subtropical reservoir. Chemosphere, 191, 876–885. https://doi.org/10.1016/j.chemosphere.2017.10.114
Amaral, A. M. B., Moura, L. K., Pellegrin, D., Guerra, L. J., Cerezer, F. O., Saibt, N., Prestes, O. D., Zanella, R., Loro, V. L., & Clasen, B. (2020). Seasonal factors driving biochemical biomarkers in two fish species from a subtropical reservoir in southern Brazil: An integrated approach. Environmental Pollution, 266, 115168. https://doi.org/10.1016/j.envpol.2020.115168
APHA. Standard Methods for the Examination of water and wastewater. 22 nd edition. American Public Health Association, American Water Works Association, Water Environmental Federation. Edited by: Eugene W. Rice, Rodger B. Baird, Andrew D. Eaton, Lenore S. Clesceri. 2012
Barata, C., Damasio, J., López, M. A., Kuster, M., López de Alda, M., Barceló, D., Riva, M. C., & Raldúa, D. (2007). Combined use of biomarkers and in situ bioassays in Daphnia magna to monitor environmental hazards of pesticides in the field. Environmental Toxicology and Chemistry, 26, 370. https://doi.org/10.1897/06-209r.1
Barreto, S., Herman, L., & Garibotti, V. (2012). Levantamento dos Agrotóxicos Usados no Estado do Rio Grande do Sul por Bacia Hidrográfica. Bol Epidemiol, 14, 3–6.
Becker, A. G., Moraes, B. S., Menezes, C. C., Loro, V. L., Santos, D. R., Reichert, J. M., & Baldisserotto, B. (2009). Pesticide contamination of water alters the metabolism of juvenile silver catfish, Rhamdia quelen. Ecotoxicology and Environmental Safety, 72, 1734–1739. https://doi.org/10.1016/j.ecoenv.2009.01.006
Bender, M. A., Santos, D. R., Tiecher, T., Minella, J. P. G., Barros, C. A. P., & Ramon, R. (2018). Phosphorus dynamics during storm events in a subtropical rural catchment in southern Brazil. Agriculture, Ecosystems & Environment, 261, 93–102. https://doi.org/10.1016/j.agee.2018.04.004
Bergmann, F. B., Amaral, A. M. B., Volcan, M. V., Leitemperger, J. W., Zanella, R., Prestes, O. D., Clasen, B., Guadagnin, D. L., & Loro, V. L. (2020). Organic and conventional agriculture: Conventional rice farming causes biochemical changes in Astyanax lacustris. Science of the Total Environment, 744, 140820. https://doi.org/10.1016/j.scitotenv.2020.140820
Beyer, J., Peterson, K., Song, Y., Ruus, A., Grung, M., Bakke, T., & Tollefsen, K. E. (2014). Environmental risk assessment of combined effects in aquatic ecotoxicology: A discussion paper. Marine Environment Research, 96, 81–91. https://doi.org/10.1016/j.marenvres.2013.10.008
Bombardi LM (2017) Geografia do Uso de Agrotóxicos no Brasil e Conexões com a União Europeia. - São Paulo: FFLCH - USP
Botelho, R. G., Monteiro, S. H., Christofoletti, C. A., Moura-Andrade, C. R., & Tornisielo, V. L. (2015). Environmentally Relevant Concentrations of Atrazine and Ametrine Induce Micronuclei Formation and Nuclear Abnormalities in Erythrocytes of Fish. Archives of Environmental Contamination and Toxicology, 69, 577–585. https://doi.org/10.1007/s00244-015-0171-6
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bueno-Krawczyk, A. C. D., Guiloski, I. C., Piancini, L. D. S., Azevedo, J. C., Ramsdorf, W. A., Ide, A. H., Guimarães, A. T. B., Cestari, M. M., & Silva de Assis, H. C. (2015). Multibiomarker in fish to evaluate a river used to water public supply. Chemosphere, 135, 257–264. https://doi.org/10.1016/j.chemosphere.2015.04.064
Carvalho, C. S., Bernusso, V. A., & Fernandes, M. N. (2015). Copper levels and changes in pH induce oxidative stress in the tissue of curimbata (Prochilodus lineatus). Aquatic Toxicology, 167, 220–227. https://doi.org/10.1016/j.aquatox.2015.08.003
Cattaneo, R., Moraes, B. S., Loro, V. L., Pretto, A., Menezes, C., Sartori, G. M. S., Clasen, B., Avila, L. A., Marchesan, E., & Zanella, R. (2011). Tissue Biochemical Alterations of Cyprinus carpio Exposed to Commercial Herbicide Containing Clomazone Under Rice-Field Conditions. Archives of Environmental Contamination and Toxicology, 62, 97–106. https://doi.org/10.1007/s00244-011-9669-8
Cerezer, C., Marins, A. T., Cerezer, F. O., Severo, E. S., Leitemperger, J. W., Grubel Bandeira, N. M., Zanella, R., Loro, V. L., & Santos, S. (2020). Influence of pesticides and abiotic conditions on biochemical biomarkers in Aegla aff longirostri (crustacea, anomura): Implications for conservation. Ecotoxicol Environ Saf, 203, 110982. https://doi.org/10.1016/j.ecoenv.2020.110982
Companhia Ambiental do Estado de São Paulo – CETESB (2011) Guia nacional de coleta e preservação de amostras: água, sedimento, comunidades aquáticas e efluentes líquidos/Companhia Ambiental do Estado de São Paulo; Organizadores: Carlos Jesus Brandão ... [et al.]. -- São Paulo: CETESB; Brasília: ANA. Accessed 10 June 2020
Choudhary, S., Yamini, R. N., Yadav, S. K., Kamboj, M. L., & Sharma, A. (2008). A review: Pesticide residue: Cause of many animal health problems. J Entomol Zool Stud, 6, 330–333.
CONAMA - Conselho Nacional do Meio Ambiente. Resolução nº 357, de 17 de março de 2005 (2005) Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. <http://www.mma.gov.br/port/conama/res/res05/res35705.pdf>. Accessed 15 June 2020
Costa-Silva, D., Nunes, M. E. M., Wallau, G. L., Martins, I. K., Zemolin, A. P. P., Cruz, L. C., Rodrigues, N. R., Lopes, A. R., Posser, T., & Franco, J. L. (2015). Oxidative stress markers in fish (Astyanax sp. and Danio rerio) exposed to urban and agricultural effluents in the Brazilian Pampa biome. Environmental Science and Pollution Research, 22, 15526–15535. https://doi.org/10.1007/s11356-015-4737-7
Dabrowski, J. M., Shadung, J. M., & Wepener, V. (2014). Prioritizing agricultural pesticides used in South Africa based on their environmental mobility and potential human health effects. Environment International, 62, 31–40.
Dala-Cort, R. B., & Azevedo, M. A. (2010). Biologia reprodutiva de Astyanax henseli (Teleostei, Characidae) do curso superior do rio dos Sinos, RS, Brasil. Iheringia Ser Zool, 100, 259–266. https://doi.org/10.1590/S0073-47212010000300012
Draper, H. H., & Hadley, M. (1990). Malondialdehyde determination as index of lipid peroxidation. Methods in Enzymology, 186, 421–431.
Elibariki, R., & Maguta, M. M. (2017). Status of pesticides pollution in Tanzania – A review. Chemosphere, 178, 154–164. https://doi.org/10.1016/j.chemosphere.2017.03.036
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70–77.
Ellman, G. L., Courtney, K. D., Andres, V., Jr., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88–95.
Feix RD, Leusin Júnior S, Agranonik C (2016) Painel do agronegócio no Rio Grande do Sul — 2016. In: Painel do Agronegócio no Rio Grande do Sul - 2016
Freire, C. A., Souza-Bastos, L. R., Chiesse, J., Tincani, F. H., Piancini, L. D. S., Randi, M. A. F., Prodocimo, V., Cestari, M. M., Silva-de-Assis, H. C., Abilhoa, V., Vitule, J. R. S., Bastos, L. P., & Oliveira-Ribeiro, C. A. (2015). A multibiomarker evaluation of urban, industrial, and agricultural exposure of small characins in a large freshwater basin in southern Brazil. Environmental Science and Pollution Research, 22, 13263–13277. https://doi.org/10.1007/s11356-015-4585-5
Ghisi, N. C., Oliveira, E. C., Fávaro, L. F., Assis, H. C. S., & Prioli, A. J. (2014). In situ assessment of a Neotropical fish to evaluate pollution in a river receiving agricultural and urban wastewater. Bulletin of Environment Contamination and Toxicology, 93, 699–709. https://doi.org/10.1007/s00128-014-1403-6
Ghisi, N. C., Oliveira, E. C., Guiloski, I. C., Lima, S. B., Assis, H. C. S., & Longhi, S. J. (2017). Prioli A (2017) Multivariate and integrative approach to analyze multiple biomarkers in ecotoxicology: A field study in Neotropical region. Science of the Total Environment, 609, 1208–1218. https://doi.org/10.1016/j.scitotenv.2017.07.266
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130–7139.
Hirt, L. M., Araya, P. R., & Flores, A. S. (2011). Population structure, reproductive biology and feeding of Astyanax fasciatus (Cuvier, 1819) in an Upper Paraná River tributary, Misiones, Argentina. Acta Limnologica Brasiliensia, 23, 1–12. https://doi.org/10.4322/actalb.2011.013
Holvoet, K. M. A., Seuntjens, P., & Vanrolleghem, P. A. (2007). Monitoring and modeling pesticide fate in surface waters at the catchment scale. Ecological Modelling, 209, 53–64. https://doi.org/10.1016/j.ecolmodel.2007.07.030
Instituto Brasileiro de Geografia e Estatística – IBGE (2019) Produção agrícola – Lavoura temporária. <https://cidades.ibge.gov.br/brasil/rs/pesquisa/14/10193>. Accessed 01 June 2020
Instituto Nacional de Meteorologia – INMET (2019) Estações automáticas - gráficos. <http://www.inmet.gov.br/portal/index.php?r=home/page&page=rede_estacoes_auto_graf> Accessed 01 June 2020
Jablonowski, N. D., & Schäffer, A. (2011). Still present after all these years: Persistence plus potential toxicity raise questions about the use of atrazine. Environmental Science and Pollution Research, 18, 328–331. https://doi.org/10.1007/s11356-010-0431-y
Jeschke, P., Nauen, R., Schindler, M., & Elbert, A. (2011). Overview of the status and global strategy for neonicotinoids. Journal of Agriculture and Food Chemistry, 59, 2897–2908. https://doi.org/10.1021/jf101303g
Jonsson, C. M., Maia, A. H. N., Ferreira, C. J. A., & Ribeiro, E. O. (1998). Risk assessment of the herbicide Clomazone to aquatic life. Verhandlungen - Internationale Vereinigung Fuer Theoretische Und Angewandte Limnologie, 26, 1724–1726. https://doi.org/10.1080/03680770.1995.11901028
Josse, J., & Husson, F. (2012). Handling missing values in exploratory multivariate data analysis methods. J Soc Fr Stat, 153, 79–99.
Josse, J., & Husson, F. (2016). missMDA: a package for handling missing values in multivariate data analysis. J Stat Softw, 70, 1–31. https://doi.org/10.18637/jss.v070.i01
Lemos, C. T., Iranço, F. A., Oliveira, N. C. D., Souza, G. D., & Fachel, J. M. G. (2008). Biomonitoring of genotoxicity using micronuclei assay in native population of Astyanax jacuhiensis (Characiformes: Characidae) at sites under petrochemical influence. Science of the Total Environment, 406, 337–343. https://doi.org/10.1016/j.scitotenv.2008.07.006
Leps, J., & Smilauer, P. (2003). Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press. https://doi.org/10.1017/CBO9780511615146
Lewis, K. A., Tzilivakis, J., Warner, D., & Green, A. (2016). An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment, 22, 1050–1064. https://doi.org/10.1080/10807039.2015.1133242
Liu, S. Y., Shocken, M., & Rosazza, J. P. N. (1996). Microbial transformations of Clomazone. Journal of Agriculture and Food Chemistry, 44, 313–319. https://doi.org/10.1021/jf9502663
López, S. L., Aiassa, D., BenÍtez-Leite, S., Lajmanovich, R., Mañas, F., Poletta, G., Sánchez, N., Simoniello, M. F., & Carrasco, A. E. (2012). Pesticides Used in South American GMO-Based Agriculture: A Review of Their Effects on Humans and Animal Models. Adv Mol Toxicol, 6, 41–75. https://doi.org/10.1016/B978-0-444-59389-4.00002-1
Loro, V. L., Murussi, C., Menezes, C., Leitemperger, J., Severo, E., Guerra, L., Costa, M., Perazzo, G. X., & Zanella, R. (2015). Spatial and temporal biomarkers responses of Astyanax jacuhiensis (Cope, 1894) (Characiformes: Characidae) from the middle Rio Uruguai, Brazil. Neotrop Ichthyol, 13, 569–578. https://doi.org/10.1590/1982-0224-20140146
Lushchak, V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicology, 101, 13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Lushchak, V. I., & Bagnyukova, T. V. (2006). Effects of different environmental oxygen levels on free radical processes in fish. Comparative Biochemistry and Physiology, 144, 283–289. https://doi.org/10.1016/j.cbpb.2006.02.014
Marcon, L., Thomé, R. G., Mounteer, A. H., Bazzoli, N., Rizzo, E., & Benjamin, L. A. (2017). Immunohistochemical, morphological and histometrical analyses of follicular development in Astyanax bimaculatus (Teleostei: Characidae) exposed to an organochlorine insecticide. Ecotoxicology and Environmental Safety, 143, 249–258. https://doi.org/10.1016/j.ecoenv.2017.05.029
Marins, A. T., Severo, E. S., Leitemperger, J. W., Cerezer, C., Muller, T. E., Costa, M. D., Weimer, G. H., Bandeira, M. N. G., Prestes, O. D., Zanella, R., & Loro, V. L. (2020). Assessment of river water quality in an agricultural region of Brazil using biomarkers in a native neotropical fish, Astyanax spp. (Characidae). Bulletin of Environment Contamination and Toxicology, 104, 575–581.
Martins, M. L., Donato, F. F., Prestes, O. D., Adaime, M. B., & Zanella, R. (2013). Determination of pesticide residues and related compounds in water and industrial effluent by solid-phase extraction and gas chromatography coupled to triple quadruple mass spectrometry. Analytical and Bioanalytical Chemistry, 405, 7697–7709. https://doi.org/10.1007/s00216-013-7235-0
Menezes, C., Leitemperger, J., Murussi, C., Toni, C., Araújo, M. C. S., Farias, I. L., Perazzo, G. X., Barbosa, N. V., & Loro, V. L. (2014). Herbicide Clomazone effects on δ-Aminolevulinic acid activity and metabolic parameters in Cyprinus carpio. Bulletin of Environment Contamination and Toxicology, 92, 393–398. https://doi.org/10.1007/s00128-014-1229-2
Ministério do Ambiente. Decreto-Lei n.º 236/1998 de 1 de agosto (1998) Estabelece normas, critérios e objectivos de qualidade com a finalidade de proteger o meio aquático e melhorar a qualidade das águas em. Diário da República, 1ª Série, n.º 176 de 1/8/1998, 3676–3722.
Moreira-Filho, O., & Bertollo, L. A. C. (1991). Astyanax scabripinnis (Pisces, Characidae): A species complex. Brazilian Journal of Genetics, 14, 331–357.
Mottes, C., Jannoyer, M. L., Le Bail, M., Guéné, M., Carles, C., & Malézieux, E. (2017). Relationships between past and present pesticide applications and pollution at a watershed outlet: The case of a horticultural catchment in Martinique, French West Indies. Chemosphere, 184, 762–773. https://doi.org/10.1016/j.chemosphere.2017.06.061
Munaretto, J. S., Ferronato, G., Ribeiro, L. C., Martins, M. L., Adaime, M. B., & Zanella, R. (2013). Development of a multiresidue method for the determination of endocrine disrupters in fish fillet using gas chromatography-triple quadrupole tandem mass spectrometry. Talanta, 116, 827–834. https://doi.org/10.1016/j.talanta.2013.07.047
Murussi, C. R., Costa, M., Menezes, C., Leitemperger, J., Guerra, L., Lópes, T., Severo, E., Zanella, R., & Loro, V. L. (2015). Integrated assessment of biomarker response in Carp (Cyprinus carpio) and Silver Catfish (Rhamdia quelen) exposed to Clomazone. Archives of Environmental Contamination and Toxicology, 68, 646–654. https://doi.org/10.1007/s00244-015-0145-8
Nauen, D. R., Jeschke, P., & Copping, L. (2008). In Focus: Neonicotinoid insecticides. Pest Management Science, 64, 1081. https://doi.org/10.1002/ps.1659
Oksanen J, Blanchet FG, Friendly M., Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara MRB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) Vegan: Community Ecology Package (Version 2.5–5)
Orsi, M. L., Carvalho, E. D., & Foresti, F. (2004). Biologia populacional de Astyanax altiparanae Garutti & Britski (Teleostei, Characidae) do médio Rio Paranapanema, Paraná, Brasil. Revista Brasileria De Zoologia, 21, 207–218.
Paulino, M. G., Benze, T. P., Sadauskas-Henrique, H., Sakuragui, M. M., Fernandes, J. B., & Fernandes, M. N. (2014). The impact of organochlorines and metals on wild fish living in a tropical hydroelectric reservoir: Bioaccumulation and histopathological biomarkers. Science of the Total Environment, 497–498, 293–306. https://doi.org/10.1016/j.scitotenv.2014.07.122
Pengue WA (2016) Cultivos transgénicos, ¿Hacia dónde fuimos? Veinte años después: La soja en Argentina 1996–2016. Fundación Heinrich Böll Stiftung, Buenos Aires/Santiago.
Pereira, L., Fernandes, M. N., & Martinez, C. B. R. (2013). Hematological and biochemical alterations in the fish Prochilodus lineatus caused by the herbicide clomazone. Environmental Toxicology and Pharmacology, 36, 1–8. https://doi.org/10.1016/j.etap.2013.02.019
Piancini, L. D. S., Guiloski, I. C., Assis, H. C. S., & Cestari, M. M. (2015). Mesotrione herbicide promotes biochemical changes and DNA damage in two fish species. Toxicology Reports, 2, 1157–1163. https://doi.org/10.1016/j.toxrep.2015.08.007
R Code Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ragnarsdottir, K. V. (2000). Environmental fate and toxicology of organophosphate pesticides. Journal of the Geological Society, 157, 859–876. https://doi.org/10.1144/jgs.157.4.859
Rio Grande do Sul. Secretaria da Coordenação e Planejamento (2002) Atlas Socioeconômico: Estado do Rio Grande do Sul/ Rio Grande do Sul. Porto Alegre: SCP, 2. ed.
Rossi, S. C., Silva, M. D., Piancini, L. D. S., Ribeiro, C. A. O., Cestari, M. M., & Assis, H. C. S. (2011). Sublethal effects of waterborne herbicides in tropical freshwater fish. Bulletin of Environment Contamination and Toxicology, 87, 603–607. https://doi.org/10.1007/s00128-011-0397-6
Sabin, G. P., Prestes, O. D., Adaime, M. B., & Zanella, R. (2009). Multiresidue determination of pesticides in drinking water by gas chromatography-mass spectrometry after solid-phase extraction. Journal of the Brazilian Chemical Society, 20, 918–925. https://doi.org/10.1590/S0103-50532009000500017
Santana, C. A., Andrade, L. H. C., Súarez, Y. R., Yukimitu, K., Moraes, J. C. S., & Lima, S. M. (2015). Fourier transform-infrared photoacoustic spectroscopy applied in fish scales to access environmental integrity: A case study of Astyanax altiparanae species. Infrared Physics & Technology, 72, 84–89. https://doi.org/10.1016/j.infrared.2015.07.005
Santos, T. G., & Martinez, C. B. R. (2012). Atrazine promotes biochemical changes and DNA damage in a Neotropical fish species. Chemosphere, 89, 1118–1125. https://doi.org/10.1016/j.chemosphere.2012.05.096
Schmidel, A. J., Assmann, K. L., Werlang, C. C., Bertoncello, K. T., Francescon, F., Rambo, C. L., Beltrame, G. M., Calegari, D., Batista, C. B., Blaser, R. E., Júnior, W. A. R., Conterato, G. M. M., Piato, A. L., Zanatta, L., Magro, J., & Rosemberg, D. (2014). Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebra fish. Neurotoxicology and Teratology, 44, 62–69. https://doi.org/10.1016/j.ntt.2014.05.006
Secretaria Estadual do Meio Ambiente – SEMA/RS (2012) Processo de Planejamento dos Usos da Água na Bacia Hidrográfica do Rio Ijuí: Enquadramento. Relatório final
Silva, A. G., & Martinez, C. B. R. (2007). Morphological changes in the kidney of a fish living in an urban stream. Environmental Toxicology and Pharmacology, 23, 185–192. https://doi.org/10.1016/j.etap.2006.08.009
Soares, M. P., Jesus, F., Almeida, A. R., Domingues, I., Hayd, L., & Soares, A. M. V. M. (2020). Effects of pH and nitrites on the toxicity of a cypermetrin-based pesticide to shrimps. Chemosphere, 241, 125089. https://doi.org/10.1016/j.chemosphere.2019.125089
Soil Survey Staff. (2014). Keys to Soil Taxonomy; Government Printing Office: Washington. DC.
Solomon, K. R., Baker, D. B., Richards, R. P., Dixon, K. R., Klaine, T. J., Point, T. W., Kendall, R. J., Weisskopf, C. P., Giddings, J. M., Giesy, J. P., Hall, L. W., & Williams, W. M. (1996). Ecological risk assessment of atrazine in North American surface waters. Environmental Toxicology and Chemistry, 15, 31–76. https://doi.org/10.1002/etc.5620150105
Solomon, K. R., Carr, J. A., Du Preez, L. H., Giesy, J. P., Kendall, R. J., Smith, E. E., & Van Der Kraak, G. J. (2008). Effects of Atrazine on Fish, Amphibians, and Aquatic Reptiles: A Critical Review. Critical Reviews in Toxicology, 38, 721–772. https://doi.org/10.1080/10408440802116496
Tiecher, T., Schenato, R. B., Santanna, M. A., Caner, L., & Santos, D. R. (2017). Phosphorus forms in sediments as indicators of anthropic pressures in an agricultural catchment in Southern Brazil. Rev Bras Cienc Solo, 41, 1–17. https://doi.org/10.1590/18069657rbcs20160569
Tiecher, T., Ramon, R., Laceby, J. P., Evrard, O., & Minella, J. P. G. (2019). Potential of phosphorus fractions to trace sediment sources in a rural catchment of Southern Brazil: Comparison with the conventional approach based on elemental geochemistry. Geoderma, 337, 1067–1076. https://doi.org/10.1016/j.geoderma.2018.11.011
Tišler, T., Jemec, A., Mozetic, B., & Trebse, P. (2009). Hazard identification of imidacloprid to aquatic environment. Chemosphere, 76, 907–914. https://doi.org/10.1016/j.chemosphere.2009.05.002
Topal, A., Alak, G., Ozkaraca, M., Yeltekin, A. C., Comakli, S., Acil, G., Kokturk, M., & Atamanalp, M. (2017). Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: Assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere, 175, 186–191. https://doi.org/10.1016/j.chemosphere.2017.02.047
Verdouw, H., Van Echteld, C. J. A., & Dekkers, E. M. J. (1978). Ammonia determinations based on indophenol formation with sodium salicylate. Water Research, 12, 399–402.
Vieira, C. E. D., Almeida, M. S., Galindo, B. A., Pereira, L., & Martinez, C. B. R. (2014). Integrated biomarker response index using a Neotropical fish to assess the water quality in agricultural areas. Neotrop Ichthyol, 12, 153–164. https://doi.org/10.1590/S1679-62252014000100017
Vieira, C. E. D., Costa, P. G., Cabrera, L. C., Primel, E. G., Fillmann, G., Bianchini, A., & Martinez, C. B. R. (2017). A comparative approach using biomarkers in feral and caged Neotropical fish: Implications for biomonitoring freshwater ecosystems in agricultural areas. Science of the Total Environment, 586, 598–609. https://doi.org/10.1016/j.scitotenv.2017.02.026
Vieira, C. E. D., Pérez, M. R., Acayaba, R. D., Raimundo, C. C. M., & Martinez, C. B. R. (2018). DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the Neotropical fish Prochilodus lineatus. Chemosphere, 195, 125–134. https://doi.org/10.1016/j.chemosphere.2017.12.077
Watson, S. J., & Maly, E. J. (1987). Thiocyanate toxicity to Daphnia magna: Modified by pH and temperature. Aquatic Toxicology, 10, 1–8. https://doi.org/10.1016/0166-445x(87)90023-3
Yan, L. J., Traber, M. G., & Packer, L. (1995). Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Analytical Biochemistry, 228, 349–351.
Zanella, R., Primel, E. G., Machado, S. L. O., Gonçalves, F. F., & Marchezan, E. (2002). Monitoring of the herbicide clomazone in environmental water samples by solid-phase extraction and high-performance liquid chromatographywith ultraviolet detection. Chromatographia, 55, 573–577. https://doi.org/10.1007/BF02492903
Zebral, Y. D., Roza, M., Fonseca, J. S., Costa, P. G., de Oliveira, C. S., Zocke, T. G., Dal Pizzol, J. L., Robaldo, R. B., & Bianchini, A. (2019). Waterborne copper is more toxic to the killifish Poecilia vivipara in elevated temperatures: Linking oxidative stress in the liver with reduced organismal thermal performance. Aquatic Toxicology, 209, 142–149. https://doi.org/10.1016/j.aquatox.2019.02.005
Zhou, J., Wang, W. N., Wang, A. L., He, W. Y., Zhou, Q. T., Liu, Y., & Xu, J. (2009). Glutathione S-transferase in the white shrimp Litopenaeus vannamei: Characterization and regulation under pH stress. Comp Biochem Physiol - Part c: Toxicol Pharmacol, 150, 224–230. https://doi.org/10.1016/j.cbpc.2009.04.012
Acknowledgements
The authors would like to thank Mr. Vilmar Souza for his assistance in collecting the fish, the Kroth Thomé da Cruz family for their support, and the Leal family for their collaboration.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) [Finance Code 001].
Author information
Authors and Affiliations
Contributions
Tamiris Rosso Storck: Study design, experimental execution (fish, water and sediment collection), water quality and biochemical analyses, data analyses and interpretation, and manuscript writing. Aline Monique Blank do Amaral: experimental execution, biochemical analyses, revision and writing of the manuscript. Taisson Kroth Thomé da Cruz: experimental execution and biochemical analyses. Dionatan de Pellegrin: Water quality and biochemical analyses. Jaíne Ames: Biochemical analyses. Felipe Osmari Cerezer: Statistical analysis and interpretation, and manuscript writing. Renato Zanella and Osmar Damian Prestes: Pesticides determination in fish muscle, water and sediments samples. Vania Lucia Loro: Financial support and manuscript revision. Barbara Clasen: Study conceptualization, financial support, data analyses and interpretation, and manuscript revision.
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by Chico Mendes Biodiversity Conservation Institute (ICMBio), under license number 66994–1.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Storck, T.R., do Amaral, A.M.B., da Cruz, T.K.T. et al. Biomarkers’ Responses in Neotropical Freshwater Fish Living in Southern Brazil: Agricultural Activity or Seasonal Interference?. Water Air Soil Pollut 233, 476 (2022). https://doi.org/10.1007/s11270-022-05956-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05956-4