Abstract
Throughout continents, reservoirs tend to have elevated methylmercury (MeHg) concentration transformed from mercury (Hg/total Hg). This impact may be pronounced in the reservoir with less velocity of water during the charging period resulted in the deposition of sediments. In sediments on favorable conditions, methylation may be enhanced by the decomposition of flood organic material, which can release Hg and enhance microbial activity. However, much less is known about the transfer ratio of Hg and its form MeHg from sediment to biota in the hydrological reservoir during the dam charging phase. The objective of our study was to understand the interrelationship between total Hg and MeHg in two key components sediment and fish in the reservoir ecosystem. This study was performed at the Three Gorges Reservoir (TGR) located on upstream of the Yangtze River in China. At the TGR charging phase, during winter time, the water level was high due to blockade of water by Three Gorges Dam (TGD). Sediment and fish samples were collected in winter season for total Hg, MeHg, and several ancillary parameters. The results showed that total Hg in sediment samples of the winter season were ranged from 6.2 ± 0.001 to 193.3 ± 0.001 × 10−3 mg/kg, with an average value of 53.76 ± 51.80 × 10−3 mg/kg, and for MeHg was ranged from 12.1 ± 0.04 to 348.7 ± 0.16 × 10−2 ng/g, with an average value of 98.96 ± 93.07 × 10−2 ng/g. Total Hg and MeHg in fish samples of the winter season were from 42.48 ± 6.71 to 166 ± 52.56 ng/g, with an average value of 76.22 ± 31.23 ng/g, and from 21.09 ± 2.31 to 61.60 ± 13.30 ng/g, with an average value of 37.89 ± 11.96 ng/g. The relationship of total Hg and MeHg concentrations in fish to those of sediments from corresponding sites showed a negative relationship. This might include a strong association of total Hg with an inorganic component of sediment (e.g., bound to sulfides or coprecipitated with other metal oxides such as manganese and iron). The average concentration of fish MeHg found in this study, at rates greater than 1.72 g/day, was estimated hazardous to human health. This study concludes sediment was acting as sequestrate for total Hg and MeHg in TGR. The bioaccumulation of total Hg and MeHg in fish was not controlled by sediment further investigation about pathological routes and dietary habits of fish needed to be identified for total Hg and MeHg study in TGR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a global pollutant released by natural agencies and anthropogenic activities (Zhou, Hopke, Zhou, & Holsen, 2019). Natural agencies include emission of Hg from vegetation, geo tectonics, forest fires, soil, and water (Ferrara, Mazzolai, Lanzillotta, Nucaro, & Pirrone, 2000). Anthropogenic sources include geological Hg mobilization, mining, extraction, and burning of fossil fuels which contain Hg as a trace contaminant to chlor-alkali industries (Pacyna et al., 2010). Hg accumulates in sediment through wet deposition/dry deposition (Durnford & Dastoor, 2011; R. P. Mason, Fitzgerald, & Morel, 1994; Schroeder and Munthe, 1998). Wet deposition of Hg from the atmosphere to sediment occurred through scavenging aerosols and clouds (Ferrara et al., 2000). Dry deposition includes the direct intact of Hg from the atmosphere to terrestrial and water bodies. The maximum deposition of atmospheric elemental Hg to the terrestrial and water bodies is carried by wet deposition as compared to dry deposition (Lindberg et al., 2007; Pacyna, Pacyna, Steenhuisen, & Wilson, 2006). Both inorganic and organic form of Hg can adversely affect human health (Ratcliffe, Swanson, & Fischer, 1996). However, MeHg is a bioavailable form of Hg and is toxic to all forms of Hg (Kotnik et al., 2015). MeHg gained a lot of attention since it was known as that it can bioaccumulate in the food chain (Elinder, Curhan, & Sheridan, 2011).
The sediment acts as a substrate for biological communities (Kamman et al., 2005). A number of studies have shown that 90 to 99% of total Hg in the environment accumulates in sediment and < 1% accumulates in biota, whereas, for MeHg, it has been reported that 90 to 99% of MeHg gets accumulated in biota and 1 to 10% gets accumulated in sediment (King, Kostka, Frischer, & Saunders, 2000). In sediment, inorganic form of Hg gets transformed into MeHg on favorable physicochemical conditions like redox potential, temperature, pH, availability of nutrients, and sulfide concentrations (Celo, Lean, & Scott, 2006; Ullrich, Tanton, & Abdrashitova, 2001). A number of scientific studies have also reported total organic carbon can enhance methylation, by stimulating the activity of heterotrophic microorganism/through direct abiotic methylation (Gorski, Armstrong, Hurley, & Krabbenhoft, 2008; Meili, 1995). Thus, sediment has the potential to release Hg especially MeHg in the aquatic environment which get consumed by biota through sorption/ingestion (J. Benoit, Gilmour, Mason, Riedel, & Riedel, 1998; J. M. Benoit, Gilmour, Mason, & Heyes, 1999; Schroeder and Munthe, 1998). The ecological risk assessment based on the sediment contamination was developed to infer potential risk in fish from reservoirs (Martinez et al., 2018).
Reservoirs are considered hotspots for mercury pollution (Mailman, Stepnuk, Cicek, & Bodaly, 2006). Reservoirs are also considered resourceful bodies, which provide a source of protein to human beings especially those inhabitants who live near its vicinity (Malik, Hashmi, & Huma, 2014). In the reservoir environment, maximum methylation occurs at the redox boundary which may vary seasonally and frequently at water-sediment interface (Korthals & Winfrey, 1987; Rudd, Turner, Furutani, Swick, & Townsend, 1983). In reservoir ecosystem, methylation of Hg can occur in two ways (a) biotic methylation and (b) abiotic methylation. Biotic methylation involves the microorganisms which methylate Hg further in two ways: enzymatic methylation and non-enzymatic methylation. Enzymatic methylation involves the presence of actively metabolizing organisms like methylcobalamin a derivative of vitamin B12 for Hg methylation. In non-enzymatic methylation, the process involves non-enzymatic transfer of the methyl group of methylcobalamin to the mercuric ion (Ullrich et al., 2001). In reservoir, biotic methylation of Hg is generally thought to be high in anaerobic conditions and sulfur-reducing bacteria have been identified the main methylator of inorganic Hg in anaerobic sediment (Compeau & Bartha, 1985). However, recent studies on Hg methylation have highlighted other cases for enhancement of MeHg and also provided deep insight information about the genes of sulfur-reducing bacteria which controls the Hg methylation like Desulfobulbus propionicus and Desulfovibrio vulgaris (Shao, Kang, Wu, & Wong, 2012; Wang et al., 2018). Abiotic methylation may also enhance the Hg methylation in the reservoir due to its purely chemical-based methylation like methyl silicon on reacting with inorganic Hg to form MeHg (Nagase, Ose, Sato, & Yamada, 1986). Effluent wastes, atmospheric deposition, and sewages released by industries have also been reported to increase MeHg concentration in the reservoirs (Lehnherr, 2014; Streets, Zhang, & Wu, 2009). Both processes of Hg methylation may be responsible for the enhancement of MeHg released from sediment in the reservoir, which may get consumed by living organisms especially fish through diet or direct sorption by skin (Watras et al., 2005). The high tendency of MeHg for bioaccumulation is usually explained by its high stability, by its lipid solubility, and by its high tendency to bind with the proteins of –SH groups (Boudou & Ribeyre, 1997; R. Mason, Reinfelder, & Morel, 1995). The predominance constituent of protein in fish may appear to be its greater efficiency to bind MeHg as compared to inorganic Hg in the reservoir (King et al., 2000). Fish being a direct source of protein to human beings could be hazardous for human health in case contaminated with MeHg (Wolfe & Schwarzbach, 1998). The assessment of total Hg and MeHg in the reservoir ecosystem is an hour of need especially in two compartments sediment and fish.
Compared to temperate regions, tropical regions are less studied except Florida Everglades, Amazon basin (Watras et al., 1994). Therefore, it is very important to study the total Hg and MeHg in reservoir ecosystem since the ecosystem is under stress from hydrological regimes, seasonal changes, rapid urban, and industrial development (Wong, Duzgoren-Aydin, Aydin, & Wong, 2006).
Earlier studies have reported inorganic Hg in the Three Gorges Reservoir (TGR) before and after impoundment (Lijun & Xiaoqing, 1997; Zhang et al., 2007). The ensemble of articles determined the inorganic Hg concentration with respect to soil and sediment in the riparian along the reservoir. The toxic effect of Hg was inferred from the inorganic form of Hg, which neglected the bioavailable form of Hg and underestimated the risks to the ecosystem and human beings. Hg bioaccumulation in farmed fish was reported (Q. Xu et al., 2018). The MeHg distribution in sediment remains unknown; this baseline data is essential for long monitoring of bioavailable Hg in the TGR.
This article was motivated by understanding the linkages of total Hg and MeHg among the two key components (sediment and fish) in the TGR ecosystem. Bioavailability and methylation of Hg could be enhanced due to the deposition of sediment which could be released from sediment to biota. Therefore, we hypothesized that during the reservoir charging phase Hg would be released from sediment to biota in the reservoir. To test the hypothesis, we will ask the following: (1) What is the relationship between Hg and MeHg in sediment? (2) What is the relationship of Hg and MeHg between in fish? (3) What is the relationship between Hg and MeHg in fish and sediment in TGR?
In this article, our aims are to examine the interrelationship between the total Hg and MeHg in sediment and fish and ascertain the relative distribution of total Hg and MeHg in TGR. In addition, this study will also account for potential risk as a suggestion to indigenous people from the consumption of reservoir fish.
Materials and methods
Study area
TGR is located on the upstream of the Yangtze River and the source lies in Tibetan highlands. TGR covers (29° 16′, 31° 25′ N to 106°, 111° 50′ E) an area that ranges from Zigui to the Jiangjing city. The reservoir has a total surface area of 1080 km2 and has a capacity of 39.3 km3 at the maximum elevation of the water level. TGR was created by stepwise the impoundment of 135 m, 156 m, and 175 m above the mean sea level from 2003 to 2009 (X. Xu, Tan, & Yang, 2013). The reservoir location lies between a subtropical zone and a northern temperate zone. Approximately, the annual average rainfall of the TGR is 1100 mm and an annual temperature range of 17–19 °C. During the winter period, the average precipitation is 100 °C, 150 mm per month, while in the summer period, the reservoir receives 200 °C, 300 mm per month (Wu et al., 2004).
Sample collection
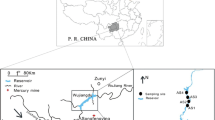
Samples of sediment and fish were collected from the water level fluctuation zone of TGR in January 2017 (Fig. 1). The water level was 175 m in the reservoir during the sampling period of the winter season. This was carried out to assess the effect of the water level fluctuation zone within 1 year on the concentration and distribution of total Hg and MeHg in sediment and fish. On the basis of the geographical characteristics of water level fluctuation, zone sampling was done in the TGR.
Sediment samples were collected from fourteen different sites of the TGR. Each site was randomly selected for sampling plot, where an area (100 m2) was sampled in the “s” shape and three samples were collected from each site (Fig. 1). A plastic spade was used to collect sediment samples with a maximum penetration depth of 15 cm. The samples were placed into a seal plastic bag using rubber gloves and a plastic bucket. Later on, the samples were placed in an icebox at 4 °C within 24 h; then, the samples were shifted to the lab for analysis. The dominant constituent found in the sediment was sand and mud.
Fish were collected with the help of local fishermen from TGR who used trawl nets and fish traps overnight to catch the fish. Then, fish were poured into the water light polythene bags and frozen at 0 °C. A total of 208 fishes were caught in the TGR. Firstly, the species of fish were identified; secondly, the length and width of fish and weight were determined, where average length, width, and weight were (17.71 cm, 5.99 cm, and 133.92 g). The fish were dissected, and the skin was removed from the body part; a fillet of 0.1 g of muscle tissue was obtained from the dorsal part of the fish for analyzation and homogenization. Analyzed fish species included the following gene types: Cyprinus carpio, Hemiculter/Wild carp, Carassius auratus, Ctenopharyngodon/grass carp, and Silurus asotus. Fish were collected from sites nearby where sediments had been taken. Both fish and sediment were dried in an oven at 60°c to estimate dry weight. All species of fish in the present study are local freshwater fish except Cyprinus carpio which is native of Europe. These species of fish prefer to use natural and man-made reservoirs. They also prefer slower moving bodies of water with soft sediment. Hemiculter/Wild carp, Carassius auratus, and Ctenopharyngodon/grass carp are omnivorous fish species; they consume grass as well as other zooplanktons, mollusks, and plant tubers and seeds. Cyprinus carpio feeds by sucking of mud from the bottom ejecting it and then selectively consuming items while they are suspended (McCrimmon, 1968). Cyprinus carpio acts as “nutrient pumps” when they consume the nutrient-rich benthic sediments and then excrete those nutrients back into the water column in a form that is available to another organism (Drenner, Smith, & Threlkeld, 1996). Silurus asotus consumes food in the open water or in the deep, lives on annelids, gastropods, and insects.
Chemical analysis
Ethics statement
The use of local fish in this study was approved by the Institutional Animal Care and Use Committee of Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences (Approval ID:ZKCQYO168).
Determination of total Hg in sediment and fish
The total Hg concentration in fish tissue and sediment were determined based on EPA 7474 (USEPA, 2007). Fish tissue 0.1 g and sediment 0.2 g were digested with 2 ml aqua regia solution (69% HNO3 and 38% HCl) at 80 °C over 12 h period and then 2 h at room temperature. Then, the sample volume was made up to 5 ml with the addition of Milli-Q water. Dilute sample was taken by 2 ml for testing by the addition of bromide-bromate solution (a combination of 11.9 g potassium bromide and 2.8 g potassium bromate in 1 l of Milli-Q water) and further addition of 1 M HCl (2.5 ml). The mixture of 50 ml was prepared for cold digestion and further 100 ml hydroxylammonium chloride (2.5 g in 50 ml Milli-Q water) solution; the final solution was tested using a Cold vapor atomic fluorescence spectroscopy (Model III Hg Analyzer Brooks Rand Laboratories®, USA). The detection limit for total Hg was 5 ng/g.
Determination of MeHg in sediment and fish
MeHg was analyzed in sediment and fish tissue following the method of (Horvat, Bloom, & Liang, 1993). The sediment sample 0.2 g and fish tissue 0.2 g were prepared. The respective sample was decanted into a 30-ml polytetrafluoroethylene (PTFE) Teflon followed by the addition of 5 ml distilled water, 0.5 ml of concentrated H2SO4, and 0.2 ml of 20% KCl solution. Ten milliliters of distilled water was used to dilute the mixture. The distillation process was aided by the addition of argon gas (50 ml/min) at 140 °C, where the distillation rate was 6 ml/h. A 30-ml solution was collected and quench cooled in a Teflon PTFE vial which was preloaded with 5 ml of Milli-Q water. Distillate with 50 ml was added to 250-ml ethylation flask preloaded with 100-ml Milli-Q water and mixture was adjusted to pH 4.9 by addition 3 ml of 2 M acetic acid-sodium acetate solution; a final aliquot of the 50 μl of 1% aqueous sodium tetraethylborate solution was decanted. The outflow was connected to a Carbotrap® and after 20 min, where adsorbed organomercury compounds were released using gas chromatography column producing Hg0 under high temperature and facilitated by argon gas. Then, outgas was detected by Cold Vapor Atomic Fluorescence Spectrometry (Model III Hg Analyzer Brooks Rand Laboratories®, USA). The detection limit was 1 ng/g.
Determination of Total organic carbon in sediment
Total organic carbon bound to sediment was determined in dried and acidified sediment LECO CHN-600 and CHN-800 elemental analyzer. The sample of sediments was burned under oxygen in a resistance furnace and carbon dioxide was measured under a selective infrared detector (Kahn, 1988). Sediment pH was measured by (Sartorius PB-10 Gottingen, Germany®) after mixing of sediment with Mille-Q water (18 MΩ cm) at a mass ratio of 1:2.5.
Quality assurance
To check the procedure validity, European reference materials of the Institute of Reference Materials and Measurements, ERM CC580 for sediment and ERM CE464 for tuna fish, were used base reference for quantification of total Hg in sediment and fish samples. The certified value for total Hg given by ERM CC580 in sediment is 132 ± 3 μg/kg and obtained value in sediment was 127.31 ± 2.35 μg/kg. The recovery of the quality assurance in case in the sediment was 96.45%. The certified value given by ERM CE464 for total Hg in tuna fish is 5.24 ± 0.10 μg/kg and obtained value in fish was 5.15 ± 0.30 μg/kg. The recovery of the quality assurance in the fish sample was 98.28%. The determination of the low concentration of the Hg was performed under ultra-clean conditions. Concentrated nitric acid (HNO3) was used to heat the PTFE vials and then rinsed with ultra-fine pure water before use. The standard calibration was maintained at > 0.99 and was analyzed after every five samples to verify the instrument calibration status.
Statistical analysis
The mean value and standard deviation were measured for the determination of total Hg and MeHg in sediment and fish. Basic statistical analysis and normality tests were applied to fish and sediment test using IBM SPSS Statistic 20® software. Fish, water, and sediment data sets from 14 respective sites in the TGR were found to be not normally distributed. Regression mod2 analysis was conducted to evaluate the relationship between total Hg and MeHg concentrations in sediment and fish using R i386 3.61. The statistical analyses were considered significant when P < 0.05.
Results and discussion
Total and methyl mercury in sediment
The minimum and maximum concentrations for total Hg in sediment samples of the winter season were ranged from 6.2 ± 0.001 to 193.3 ± 0.001 × 10−3 mg/kg, with an average of 53.76 ± 51.80 × 10−3 mg/kg and MeHg was ranged from 12.1 ± 0.04 to 348.7 ± 0.16 × 10−2 ng/g, with an average of 98.96 ± 93.07 10−2 ng/g (Table 1). The physicochemical properties of sediment are presented in Supplemental Table A. The pH varied from 5.6 to 8.6 with an average of 7.67 ± 0.83. Total organic carbon ranged from 3.63 ± 0.04 to 51.3 ± 1.2 g/kg, in all sediment with an average of 20.62 ± 14.98 g/kg. The clay and silt content varied in different sites of study location. Clay content varied from 1.05 to 33.22% with an average of 15.16 ± 10.30 in all sediment. Silt content varied from 4.80 to 42.56% with an average of 25.03 ± 13.25 in all sediment (Supplemental Table B).
In Three Gorges Reservoir, Tantai wulong 2 sediment exhibited highest total Hg average value of 193.3 ± 0.001 mg/kg, with a range of 187.1 to 199.6 mg/kg in Fig. 2a. The lowest THg concentration average value 6.2 ± 0.001 mg/kg, with a range of 4.2 to 8.3 mg/kg, was recorded at Tie jia gou. The highest concentration of sediment THg was observed at Tantai wulong 2, most likely due to traffic exhaust, industrial activities, and municipal sewage discharged from Chongqing city with a developed economy and dense population (Ye, Li, Zhang, & Zhang, 2011). Taking previously stated background value, the mean concentration for total Hg obtained in this study was lower than the earlier studies for background concentration (200 ng/g) suggested by Craig (1986) and Lindqvist, Jernelov, Johansson, & Rodhe (1984). The low-level background value for total Hg than earlier studies could be linked to the hydrology of the reservoir which is influenced by the Three Gorges Dam due to water fluctuation level in the reservoir in different seasons. The stagnant water bodies provide ideal conditions to sediment for the absorption of Hg from water (Watras et al., 2005). Other than that, it could be linked to less direct absorption of atmospheric Hg by sediment.
Table 2 shows total Hg concentrations in the sediment of reservoirs both nationally and internationally. On a national scale, TGR is the most important for the study of Hg pollution. However, on comparing with other reservoirs nationally and internationally, it is evident the level of Hg pollution is higher than the background value but lesser than Cottage Grove Reservoir of the USA.
Tantai wulong 2 sediments exhibited the highest MeHg average value 348.7 ± 0.16 ng/g, with a range of 337.9 to 359.4 ng/g in Fig. 2b. The lowest MeHg concentration average value 12.1 ± 0.04 with a range of 9.5 to 14.7 was recorded at Tie jia gou. Usually, MeHg in sediment is affected by the bioavailable form of Hg and physicochemical conditions, such as pH, temperature, and oxidation-reduction potential (Ullrich et al., 2001). The traffic exhaust at Tantai wulong 2 may have led to release the amount of elemental Hg into an atmosphere, which may get deposited into sediment and transformed into MeHg by the actions sulfur-reducing bacteria (SRB). Such newly deposited Hg is more bioavailable and ready for methylation in sediment (Compeau & Bartha, 1985).
The ratio of MeHg/THg in sediment was usually less than 21% with a range of 10.50 to 20.40% on average, and the peak value was recorded at Yuanyang. A significant regression (R2 = 0.99; P < 0.001) was observed between THg and MeHg in sediments (Fig. 3a). MeHg concentration increased with THg concentration could be the availability of the bioavailable form of Hg with an increasing amount of THg, and a negative trend regression (R2 = 0.39; P > 0.05) was observed between sediment Hg and the MeHg/THg ratio (Fig. 3b). MeHg/total Hg ratio decreased with increasing THg concentrations, likely due to dominant mercuric sulfides in sediments (Chen, Bonzongo, & Miller, 1996), whereas the ratio of MeHg/total Hg ratio decreased with increasing MeHg concentration in sediments (R2 = 0.35; P < 0.05) (Fig. 3c). That could be linked to less availability of the bioavailable form of Hg with an increasing amount of MeHg.
Generally, total organic carbon (TOC) and microbial activity in the sediment play an important role in the formation, bioavailability, and methylation of the inorganic Hg (Celo et al., 2006; Gorski et al., 2008). We examined the relation of total Hg concentration in sediment to the corresponding organic carbon content and mud (silt + clay) content (Fig. 4). The regression between total Hg and TOC concentrations of sediments resulted (R2 = 0.08; P > 0.05); the relationship of the MeHg with TOC content was also insignificant (R2 = 0.08; P > 0.05); and the insignificant relationship of TOC with total Hg and MeHg in sediment can be attributed to parameters such as pH, temperature, redox changes, availability of nutrients, and complexing agents for binding especially reduced form of sulfur thiol in natural organic matter (NOM) (Ullrich et al., 2001). Natural organic matter that exists ubiquitously in the environment contains heterogeneous organic matter substance. It has a tendency to bind trace metals and strongly affect their speciation, solubility, mobility, and toxicity. The weak regression between total Hg and MeHg with total organic carbon was also found in the previous study of Northeastern North America (Kamman et al., 2005). The relationship between total Hg and MeHg against mud was found significant (R2 = 0.41; P < 0.05). The dominant composition of mud is clay and silt. Clay has a tendency to sequestrate Hg and MeHg in the aquatic environment. The proportion of the MeHg/total Hg varied < 25 that was insignificantly correlated in organic carbon (R2 = 0.05; P > 0.05) and in the mud (R2 = 0.35; P < 0.05).
Total and methyl mercury in fish
The minimum and maximum concentrations for total Hg and MeHg in fish samples of the winter season were from 42.48 ± 6.71 to 166 ± 52.56 ng/g, with an average of 76.22 ± 31.23 ng/g, and from 21.09 ± 2.31 to 61.60 ± 13.30 ng/g, with an average of 37.89 ± 11.96 ng/g (Table 3). In Three Gorges Reservoir, Changshou fish exhibited highest THg average value of 166 ± 52.56 ng/g with a range of 116 to 231 ng/g in Fig. 5a. The lowest THg concentration average value 42.48 ± 6.71 ng/g with a range of 34 to 51 ng/g was recorded at Fuling. The highest concentration of fish THg was observed at Changshou newly developed urbanized area having many chemical industries and other factories. The released effluents enter the reservoir system and get deposited in the form of sedimentation. Later on, these effluents get consumed by fish mainly through their diet (Li, Zhou, Yuan, He, & Xie, 2015).
Changshou fish exhibited the highest MeHg average value of 61.60 ± 13.30 ng/g with a range of 50 to 78 ng/g in Fig. 5b. The lowest MeHg concentration average value 21.09 ± 2.31 with a range of 18 to 23 ng/g was recorded at Fuling. The highest concentration of fish THg was observed at Changshou; it could be linked to the high tendency of MeHg for bioaccumulation due to its high aqueous stability and lipid solubility (Malik et al., 2014). However, several scientific studies have shown that Hg concentration in fish generally tends to increase with age. Age can be assessed using scales, otoliths, or other structures (Depew et al., 2013), and therefore, the exposure for MeHg accumulation increases in fish while owing maximum time for exposure to attain the size (Stokes & Wren, 1987).
Since the samples of the fish muscle for analysis were pooled from several individuals, and the relationship was examined. The length, width, and weight of fish were measured to check the relationship and its effect on the concentration of total Hg and MeHg. The highest concentrations of total Hg and MeHg at Changshou correspond with the highest scaled length, width, and weight of fish among the different sites of the present study of TGR (Ikingura & Akagi, 1996).
Apart from the size of fish which is considered one major reason for the concentration of total and MeHg. In some other sites, the distinct concentrations of total Hg and MeHg that were observed are probably due to differing amounts of Hg by local input (Saouter, Ribeyre, Boudou, & Maury-Brachet, 1991), for example, Wanzhou had the second largest fish in terms of length, width, and weight in the present study but the second-highest concentration of total Hg and MeHg was found in the Yunyang. Total Hg concentrations were highest in C. carpio collected from the Changshou followed by C. auratus collected from Yunyang. In fish, the percent of MeHg to total Hg varied between 35 and 71% (average 53%). Reasons for the lower percentage of MeHg in C. carpio at location Fuling is not clear. However, many factors can influence the total Hg and MeHg percentage ratios like sex, age, and feeding habit of fish (Saouter et al., 1991).
In relation to sediment total Hg concentration in fish, the muscle was also proportional to MeHg concentration regression in agreement with earlier studies (Grieb et al., 1990). Total Hg and MeHg concentrations in the fish reservoir were compared with national as well as an international reservoir in Table 4. An evident level of Hg pollution was higher than the reference value given by the World Health Organization (WHO) which was 0.5 mg/kg in the year 1991.
The ratio of MeHg/THg in fish was less than 75% with a range of 36.94 to 70.27% on average, and the peak value was recorded at Zhongxian. A significant regression (R2 = 0.85; P < 0.05) was observed between THg and MeHg in fish (Fig. 6a). MeHg concentration that increased with THg concentration could be linked to the availability of the bioavailable form of Hg with an increasing amount of total Hg, and an insignificant regression (R2 = 0.05; P > 0.05) was observed between fish Hg and the MeHg/total Hg ratio (Fig. 6b). MeHg/total Hg decreased with THg concentrations, likely due to less tendency to bind with –SH groups associated with protein, whereas the ratio of MeHg/total Hg slightly increased with MeHg concentration in fish (R2 = 0.002; P > 0.05) (Fig. 6c). That could be linked to less availability of the bioavailable form of Hg with an increasing amount of MeHg.
Relation of total Hg and MeHg between sediment and fish
The relationship of total Hg concentration between sediment and fish was inferred from the examination of fish and sediment data. The concentrations of Hg in fish showed a negative trend with Hg concentrations of sediment (R2 = 0.35; P < 0.05). Similarly, total Hg in sediment was related to fish MeHg concentration (R2 = 0.44; P < 0.05) (Fig. 7a–c). Furthermore, sediment MeHg concentration was regressed with those in fish (R2 = 0.41; P < 0.05). The insignificant relationship was also found by Kamman et al. (2005). There are several factors that influence the relationship of total Hg and MeHg between sediment and fish (AAndersson et al., 1990). MeHg is accumulated and retained by fish more efficiently than total Hg (Stokes & Wren, 1987). The bioavailability of mercury in coastal sediments has been assessed, although some studies have shown that the sediments can be sequestrate for mercury (Rudd & Turner, 1983). Moreover, the availability of inorganic Hg in sediment, water column, trophic interaction, and the rate at which microflora transforms Hg into MeHg and seasonal variations influence the relationship between Hg in sediment and fish. In contrast, to the negative relationship between total Hg in sediment and fish, few studies showed a positive linear relationship between total Hg and MeHg concentrations in mussels and sediments from the Adriatic coast (Wiener, Cope, & Rada, 1990).
Interestingly, ratio of MeHg/total Hg in sediment and fish at different locations in TGR is shown in Fig. 8. The highest ratio of MeHg/total Hg in sediment found in Yunyang could be likely due to less dominance of mercuric sulfides in sediments. Other possibilities could be anthropogenic sources likely traffic exhaust (Ye et al., 2011). In the case with the fish, highest ratio of MeHg/total Hg was found in Goayang Kaizhou that could be linked to the dietary habit of fish (Li et al., 2015).
Risk from contaminated fish in TGR
The presence of a high concentration of MeHg in fish is a concern for neurotoxicity because the predominant exposure pathways for humans are the consumption of fish. The U.S. EPA has calculated one such estimate called a reference dose or RfD, which has set the action level for MeHg in fish (0.1 μg/kg bw/day) (Mahaffey, 1999).
Health risk assessments may be estimated by using reference dose value (RfD) for MeHg that was determined to be 1 × 10−4 mg/kg/day; a person could consume 0.1 μg MeHg for every kg of his/her body weight every day for a lifetime without anticipation of risk of adverse effect. The estimated dose (D) can be calculated as D = C × I/W × 1000, where C = concentration of MeHg in fish (μg/g wet weight), I = ingestion rate of fish (g/day), and W = average body weight (70 kg). Hazard index (H) for the chemical is the ratio of dose (D) to the upper level of daily chemical intake over life estimated to be without adverse effect (i.e., RfD). If the (H) value is less than 1, in that case, toxic effects are not expected to occur. Hazard value can be calculated as a function of the ingestion rate and concentration of Hg in fish. The maximum (110 g/day) and minimum (35 g/day) ingestion rates obtained through the survey were considered to represent the average consumption rate of fish by the local people of TGR. Hazard values were calculated for the ingestion of fish containing the highest (6.16 μg/g dry weight) and mean (3.79 μg/g dry weight) MeHg level for fish from TGR (Table 5). The result showed that the consumption of fish at the highest MeHg concentration found in this study is hazardous at 110 as well as 35 g/day ingestion rates. Consumption of MeHg contaminated fish containing 3.79 μg/g dry weight, the mean MeHg concentration found in this study, is hazardous to human health at a consumption rate of 1.72 g/day, which would reach an H value of 1. H value with to human intake local shows greater than > 1 in TGR.
Conclusions
Our findings do not support the proposed hypothesis; the negative relationship was found of total Hg and MeHg between sediment and fish. This might include a strong association of inorganic components of the sediments (e.g., bound to sulfides or coprecipitated with other metal oxides such as manganese and iron.
We found an insignificant relationship between total organic carbon, with total Hg and MeHg, which suggested sequestration of total and MeHg from sediment to fish is not totally influenced by the organic component of the sediment. However, the correlation between mud and total Hg/MeHg were found significant. This could be one of the reasons that total Hg and MeHg is not released from sediment to fish significantly.
The present study reveals that sediment was acting as sequestrate for total Hg and MeHg during the charging phase of the dam operation in TGR. Interestingly, ratio of MeHg/total Hg in sediment showed a decreasing trend on the increasing concentration of total Hg and MeHg, which suggests Hg was sequestrated in sediment. By comparing relative total Hg and MeHg distribution in the two key components (sediment and fish) of the reservoir ecosystem, this study implies that total Hg and MeHg showed an insignificant interrelationship between sediment and fish. In TGR, bioaccumulation of total Hg and MeHg in fish was not controlled by reservoir sediment but maybe by other external sources like the diet of fish/direct absorption of Hg from the atmosphere.
Hazard index value showed that consumption of fish at the highest MeHg concentration is a potential cause for concern at 110 as well as 35 g/day ingestion rates. Hg pollution is an emerging pollutant and causes reasonable concern for indigenous people of TGR.
References
AAndersson I, Parkman H, Jernelöv A (1990) The role of sediments as sink or source for environmental contaminants -- a case study of mercury and chlorinated organic compounds. Limnol Jena 20(2):347–359
Benoit J, Gilmour CC, Mason R, Riedel GS, Riedel GF (1998) Behavior of mercury in the Patuxent River estuary. Biogeochemistry 40(2–3):249–265
Benoit JM, Gilmour CC, Mason RP, Heyes A (1999) Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ Sci Technol 33(6):951–957
Boudou A, Ribeyre F (1997) Mercury in the food web: accumulation and transfer mechanisms. Met Ions Biol Syst 34:289–320
Celo V, Lean DR, Scott SL (2006) Abiotic methylation of mercury in the aquatic environment. Sci Total Environ 368(1):126–137
Chen Y, Bonzongo J, Miller G (1996) Levels of methylmercury and controlling factors in surface sediments of the Carson River system, Nevada. Environ Pollut 92(3):281–287
Compeau G, Bartha R (1985) Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol 50(2):498–502
Craig, P. (1986). Organomercury compounds in the environment. Organometallic compounds in the environment. Craig, PJ, Ed: Longman Essex
Depew DC, Burgess NM, Anderson MR, Baker R, Bhavsar SP, Bodaly R et al (2013) An overview of mercury concentrations in freshwater fish species: a national fish mercury dataset for Canada. Can J Fish Aquat Sci 70(3):436–451
Drenner RW, Smith JD, Threlkeld ST (1996) Lake trophic state and the limnological effects of omnivorous fish. Hydrobiologia 319(3):213–223
Durnford D, Dastoor A (2011) The behavior of mercury in the cryosphere: a review of what we know from observations. J Geophys Res-Atmos 116(D6)
Elinder, C., Curhan, G., & Sheridan, A. (2011). Epidemiology and toxicity of mercury. Uptodate. com. September, 21
Ferrara R, Mazzolai B, Lanzillotta E, Nucaro E, Pirrone N (2000) Temporal trends in gaseous mercury evasion from the Mediterranean seawaters. Sci Total Environ 259(1–3):183–190
Gorski P, Armstrong D, Hurley J, Krabbenhoft D (2008) Influence of natural dissolved organic carbon on the bioavailability of mercury to a freshwater alga. Environ Pollut 154(1):116–123
Grieb TM, Bowie GL, Driscoll CT, Gloss SP, Schofield CL, Porcella DB (1990) Factors affecting mercury accumulation in fish in the upper Michigan peninsula. Environ Toxicol Chem 9(7):919–930
Horvat M, Bloom NS, Liang L (1993) Comparison of distillation with other current isolation methods for the determination of methyl mercury compounds in low level environmental samples: part 1. Sediments. Anal Chim Acta 281(1):135–152
Ikingura JR, Akagi H (1996) Monitoring of fish and human exposure to mercury due to gold mining in the Lake Victoria goldfields, Tanzania. Sci Total Environ 191(1–2):59–68
Kahn, L. (1988). Determination of total organic carbon in sediment (Lloyd Kahn method). Standard Operating Procedures
Kamman NC, Chalmers A, Clair TA, Major A, Moore RB, Norton SA, Shanley JB (2005) Factors influencing mercury in freshwater surface sediments of northeastern North America. Ecotoxicology 14(1–2):101–111
King JK, Kostka JE, Frischer ME, Saunders FM (2000) Sulfate-reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl Environ Microbiol 66(6):2430–2437
Korthals ET, Winfrey MR (1987) Seasonal and spatial variations in mercury methylation and demethylation in an oligotrophic lake. Appl Environ Microbiol 53(10):2397–2404
Kotnik J, Horvat M, Ogrinc N, Fajon V, Žagar D, Cossa D, Sprovieri F, Pirrone N (2015) Mercury speciation in the Adriatic Sea. Mar Pollut Bull 96(1–2):136–148
Lehnherr I (2014) Methylmercury biogeochemistry: a review with special reference to Arctic aquatic ecosystems. Environ Rev 22(3):229–243
Li J, Zhou Q, Yuan G, He X, Xie P (2015) Mercury bioaccumulation in the food web of Three Gorges Reservoir (China): tempo-spatial patterns and effect of reservoir management. Sci Total Environ 527:203–210
Lijun J, Xiaoqing X (1997) Methyl mercury distribution in surface water and fish in the Three Gorges Reservoir Area. Resources Environ Yangtza Valley 4
Lindberg S, Bullock R, Ebinghaus R, Engstrom D, Feng X, Fitzgerald W, Pirrone N, Prestbo E, Seigneur C (2007) A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. AMBIO J Human Environ 36(1):19–34
Lindqvist, O., Jernelov, A., Johansson, K., & Rodhe, H. (1984). Mercury in the Swedish environment: global and local sources: National Swedish Environment Protection Board Solna, Sweden
Mahaffey KR (1999) Methylmercury: a new look at the risks. Public Health Rep 114(5):396
Mailman M, Stepnuk L, Cicek N, Bodaly RD (2006) Strategies to lower methyl mercury concentrations in hydroelectric reservoirs and lakes: a review. Sci Total Environ 368(1):224–235
Malik RN, Hashmi MZ, Huma Y (2014) Heavy metal accumulation in edible fish species from Rawal Lake Reservoir, Pakistan. Environ Sci Pollut Res 21(2):1188–1196
Martinez G, McCord S, Driscoll C, Todorova S, Wu S, Araújo J, Vega C, Fernandez L (2018) Mercury contamination in riverine sediments and fish associated with artisanal and small-scale gold mining in Madre de Dios, Peru. Int J Environ Res Public Health 15(8):1584
Mason RP, Fitzgerald WF, Morel FM (1994) The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochim Cosmochim Acta 58(15):3191–3198
Mason R, Reinfelder J, Morel FM (1995) Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut 80(1–4):915–921
McCrimmon, H. R. (1968). Carp in Canada
Meili M (1995) Pre-industrial atmospheric deposition of mercury: uncertain rates from lake sediment and peat cores. Water Air Soil Pollut 80(1–4):637–640
Nagase H, Ose Y, Sato T, Yamada M (1986) Mercury methylation by ash from refuse incineration. Sci Total Environ 53(1–2):133–138
Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S (2006) Global anthropogenic mercury emission inventory for 2000. Atmos Environ 40(22):4048–4063
Pacyna EG, Pacyna J, Sundseth K, Munthe J, Kindbom K, Wilson S et al (2010) Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos Environ 44(20):2487–2499
Ratcliffe HE, Swanson GM, Fischer LJ (1996) Human exposure to mercury: a critical assessment of the evidence of adverse health effects. J Toxicol Environ Health 49(3):221–270
Rudd JW, Turner MA (1983) The English–Wabigoon river system: II. Suppression of mercury and selenium bioaccumulation by suspended and bottom sediments. Can J Fish Aquat Sci 40(12):2218–2227
Rudd JW, Turner MA, Furutani A, Swick AL, Townsend B (1983) The English–Wabigoon River system: I. A synthesis of recent research with a view towards mercury amelioration. Can J Fish Aquat Sci 40(12):2206–2217
Saouter E, Ribeyre F, Boudou A, Maury-Brachet R (1991) Hexagenia rigida (Ephemeroptera) as a biological model in aquatic ecotoxicology: experimental studies on mercury transfers from sediment. Environ Pollut 69(1):51–67
Schroeder WH, Munthe J (1998) Atmospheric mercury—an overview. Atmos Environ 32(5):809–822
Shao D, Kang Y, Wu S, Wong MH (2012) Effects of sulfate reducing bacteria and sulfate concentrations on mercury methylation in freshwater sediments. Sci Total Environ 424:331–336
Stokes, P., & Wren, C. (1987). Bioaccumulation of mercury by aquatic biota in hydroelectric reservoirs: a review and consideration of mechanisms. Lead, mercury, cadmium and arsenic in the environment, 255–257
Streets DG, Zhang Q, Wu Y (2009) Projections of global mercury emissions in 2050. Environ Sci Technol 43(8):2983–2988
Tang J, Wang S.J, Fu SH, Sun YD, Lei JL (2008) Soil environmental quality evaluation of the Three-Gorges Reservoir Region, Acta Pedologica Sinica 45:601–607
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31(3):241–293
USEPA (2007) SW-846 test method 7474: mercury in sediment and tissues samples by atomic fluorescence spectrometry. US Environmental Protection Agency, Washington, DC
Wang K, Munson KM, Beaupré-Laperrière A, Mucci A, Macdonald RW, Wang F (2018) Subsurface seawater methylmercury maximum explains biotic mercury concentrations in the Canadian Arctic. Sci Rep 8(1):14465
Watras, C., Bloom, N., Hudson, R., Gherini, S., Munson, R., Claas, S., Wiener, J., Wiener, J., . . . (1994). Sources and fates of mercury and methylmercury in Wisconsin lakes
Watras C, Morrison K, Kent A, Price N, Regnell O, Eckley C et al (2005) Sources of methylmercury to a wetland-dominated lake in northern Wisconsin. Environ Sci Technol 39(13):4747–4758
Wiener JG, Cope WG, Rada RG (1990) Mercury accumulation in yellow perch in Wisconsin seepage lakes: relation to lake characteristics. Environ Toxicol Chem 9(7):931–940
Wolfe, M., & Schwarzbach, S. (1998). Sulaiman RA. Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem, 17, 146–160
Wong CS, Duzgoren-Aydin NS, Aydin A, Wong MH (2006) Sources and trends of environmental mercury emissions in Asia. Sci Total Environ 368(2–3):649–662
Wu J, Huang J, Han X, Gao X, He F, Jiang M, Jiang Z, Primack RB, Shen Z (2004) The three gorges dam: an ecological perspective. Front Ecol Environ 2(5):241–248
Xu X, Tan Y, Yang G (2013) Environmental impact assessments of the Three Gorges Project in China: issues and interventions. Earth Sci Rev 124:115–125
Xu Q, Zhao L, Wang Y, Xie Q, Yin D, Feng X, Wang D (2018) Bioaccumulation characteristics of mercury in fish in the Three Gorges Reservoir, China. Environ Pollut 243:115–126
Ye C, Li S, Zhang Y, Zhang Q (2011) Assessing soil heavy metal pollution in the water-level-fluctuation zone of the Three Gorges Reservoir, China. J Hazard Mater 191(1–3):366–372
Zhang L, Zang X, Xu J, Xie P, Zhu Z, Su J (2007) Mercury bioaccumulation in fishes of Three Gorges Reservoir after impoundment. Bull Environ Contam Toxicol 78(3–4):262–264
Zhou H, Hopke PK, Zhou C, Holsen TM (2019) Ambient mercury source identification at a New York State urban site: Rochester, NY. Sci Total Environ 650:1327–1337
Acknowledgments
We acknowledge the assistance of Dr. Larisa Rubel, Professor Bailian Li, and Dr. Stefan Lewandowski for correction of writing.
Funding
This study is supported by the National Natural Science Foundation of China (No. B060104), “Sponsored by CAS-TWAS President’s Fellowship for International Ph.D. students.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethical statement is appropirate approved by the institutional Animal care and use committee of Chongqing Institute of Green and Intelligent Technology Chinese Academy of Sciences (Approval ID:ZKCQYO168).
Additional information
Responsible editor: Severine Le Faucheur
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Mir, Y., Wu, S., Ma, M. et al. Mercury and its form in a dammed reservoir ecosystem during the charging phase. Environ Sci Pollut Res 27, 37099–37113 (2020). https://doi.org/10.1007/s11356-020-08935-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08935-6