Abstract
A survey was conducted in a freshwater reservoir located in Guizhou province, south China, to investigate the influence of fish aquaculture activities on mercury (Hg) distribution, speciation change, and bioaccumulation. Water, sediment, and aquaculture fish samples were collected from aquaculture sites (AS) and the corresponding reference sites (RS). The results showed that total mercury (THg) concentration in overlying water in Wujiangdu reservoir reached 6.87 ± 14.9 ng L−1. THg concentration in a different layer follows the sequence: surface layer > bottom layer > three intermediate layers with significant difference. In addition, the total methylmercury (TMeHg) concentration in overlying water was 0.113 ± 0.211 ng L−1. The highest TMeHg was observed in the bottom layer. Both dissolved Hg (DHg) and dissolved methylmercury (DMeHg) accounted for more than 50% of THg and TMeHg in the top four layers of overlying water, respectively. In contrast, particulate Hg (PHg) and particulate methylmercury were the major portion of THg and TMeHg in bottom layer and reached to 67 and 58.7% of THg and TMeHg, respectively. Aquaculture activities were estimated to contribute an annual loading of approximately 69.8 ng g−1of THg yearly in the top 1 cm of sediment underneath the rafts due to unconsumed fish feed. The extra loading of THg in sediment may have potential to stimulate the release of Hg to the overlying water. Both DMeHg and DHg in sediment pore water and organic matter (OM) contents in the top 4 cm of AS were noticeably higher than RS, which indicated that the accumulation of OM due to aquaculture activities promoted MeHg production in the top surface of sediment pore water. No significant difference was noted between THg in RS (296 ± 104 ng g−1) and AS (274 ± 132 ng g−1) in the solid phase. In addition, the sediments were net sources of both MeHg and inorganic Hg. For Hg in fish tissues, THg and inorganic Hg (IHg) in the liver were significantly higher (THg: p < 0.001, F = 9.98; IHg: p < 0.001, F = 13.1) than those in the other organs. In contrast, MeHg concentration in the muscle was significantly higher than (p < 0.01, F = 4.83) that in the other organs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in seafood production using aquaculture provides a valuable source of high-quality protein and is an important cash crop in many parts of the world (Sapkota et al. 2008). An estimated 56% of the world’s population obtains at least 20% of their animal protein intake from finfish and shellfish (FAO 2004). Global fish production from aquaculture has therefore increased rapidly over the past four decades, contributing significant quantities to the world’s supply of fish for human consumption (Subasinghe et al. 2009). In 2013, 97.2 million tonnes of seafood were produced (FAO 2014). This is the first time that aquaculture production has exceeded the capture from the worldwide. China has the largest amount of aquaculture products, contributing 59% of the world total (FAO 2014).
However, the majority of aquaculture facilities rely heavily on the input of trash fish and feed pellets, in addition to the application of agrochemicals and antibiotics. The organic matter (OM) accumulated in sediment beneath aquaculture facilities consisted of unconsumed feed, excreta from fish, and many chemical and biological contaminants derived from aquaculture operations (Liang et al. 2011; Sapkota et al. 2008). Increased oxygen consumption during OM degradation leads to progressively more anoxic conditions at the sediment/water interface, which may lead to mobilization and speciation change of various contaminants.
Mercury (Hg) contamination is globally ubiquitous in aquatic ecosystems (Driscoll et al. 2013). Direct and indirect sources of Hg contribute continuously to the increasing loading of Hg to the aquatic environment (UNEP 2013). Inorganic Hg is readily transformed into methylmercury (MeHg), a highly toxic and bioaccumulative form in the anoxic benthic environment. The deposition and depletion of OM may therefore be benefit for the potential methylation of inorganic Hg (Khwaja et al. 2006; Yin et al. 2013). However, extremely high contents of OM may inhibit MeHg production in sediment since the binding of OM with Hg will lower the bioavailability of Hg to sulfur-reducing bacteria or iron-reducing bacteria (Hammerschmidt et al. 2008; Liang et al. 2011, 2013). Therefore, the role of OM on MeHg production in the aquatic system is still unclear. In addition, aquaculture activities may also result in the suspension of particles, which may alter the partition of Hg between solid and liquid phase, the transportation of Hg between sediment and overlying water, leading to further effects on the bioavailability of Hg to aquatic biota (Liang et al. 2012).
MeHg is accumulated in aquatic biota and biomagnified through aquatic food chains, so that consumption of Hg-contaminated fish is the primary route of this toxin to reach top-level predators including human beings (Mergler et al. 2007; Oken et al. 2005). It was reported that Hg concentration in wild fish mainly depends on the trophic level with high contents normally observed in carnivorous fish than herbivorous ones (Mason et al. 1995a, 2000). High contents of MeHg were observed in wild-fish muscle since it is accumulated mainly under cysteine thiol complexes (Harris et al. 2003) and is excreted slowly with a half-life time of approximately 400 days (Downs et al. 1998). Changes in the speciation, transport, and bioaccumulation of Hg in fish aquaculture zones are therefore extremely important and merit investigation.
The influence of fish aquaculture on changes in Hg speciation has been studied in the estuaries (Liang et al. 2011), fish ponds (Shao et al. 2011), and reservoirs (Meng et al. 2016). However, there is a lack of overall information concerning Hg concentration, speciation change, transportation, and bioaccumulation in overlying water, sediment, fish, and fish feed of the aquaculture sites. Therefore, the objectives of this study were to investigate the effects of aquaculture activities on the following: (1) Hg speciation change in water, sediment, and pore water; (2) Hg distribution between solid and liquid phase; and (3) the accumulation characteristics of Hg in different organs of aquaculture fish.
Materials and methods
Study areas
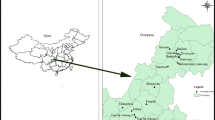
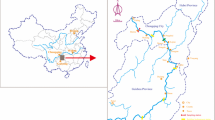
The sampling sites were located in Wujiangdu (WJD) reservoir, at the lower Wujiang River basin, Guizhou province, south China (Fig. 1). The WJD reservoir was built in 1979 and has a watershed of 27,790 km2 and a volume of 23 × 108 m3 water. It is located in the lower Wujiang river basin. The average water residence time in the WJD is 53.0 days (Meng et al. 2010) and cage aquaculture activities started in 1999.
Sampling procedures
All the overlying water samples were collected from a depth of 0.5 m (surface layer), and depths of 15, 30, 45, and 70 m (bottom layer, the inter-surface between sediment and water) from the aquaculture sites (AS) and reference sites (RS), without aquaculture activities. Four replicate sampling points were selected from both AS and RS.
Overlying water samples were collected by using an acid-purified water sampler (QC-15, Pulite, Beijing, China). Water samples were transferred from the sampler into acid-cleaned borosilicate glass bottles. These bottles had been rigorously cleaned before use by immersion in 10% (v/v) HNO3, followed by rinsing with ultrapure deionized water (18.2 MΩ Milli-Q) and heating for 1 h in a furnace at 500 °C; The samples were then double-bagged and stored in a sealed box.
Filtered samples were collected on-site using a 0.45-μm filter (Millipore) and subsequently analyzed for dissolved Hg (DHg) and dissolved MeHg (DMeHg). Total Hg (THg) and total MeHg (TMeHg) concentrations were analyzed in each of the unfiltered samples.
Sediment core samples were collected by using customer sediment sampler in each site. Core samples were divided into 1-cm increments for the top 20 cm and 2 cm below 20 cm. Pore water was separated by centrifuging from sediment. Both sediment and pore water samples for MeHg analysis were kept in an anaerobic condition from sample collection by using the anaerobic bag to sample separating on anaerobic operation table.
Four replicates of cultured fish including catfish (CF) (Silurus asotus), grass carp (GC) (Ctenopharyngodon idellus), crucian carp (CRC) (Carassius auratus), common carp (COC) (Cyprinus carpio), and Wuchang bream (WCB) (Megalobrama amblycephala) were collected. All fish samples were dissected in the laboratory. In addition, two types of fish feeds, trash fish, and feed pellet were also collected.
Sample analysis
Analysis of THg
THg and DHg in water samples were oxidized with BrCl, and reduced by SnCl2 as well as NH4OH·HCl. An aliquot of fish samples (~ 0.2 g) was digested with 4 mL concentrated nitric acid (65% Suprapur®, Merck) in a 25-ml glass vial at 95 °C for 4 h, while sediment samples with 4 ml mix acid (HCl:HNO3 = 3:1) at 95 °C for 2 h. After cooling, Milli-Q water (18.2 Ω) was added to volume, and the solution was filtered through 0.45-μm filter paper. The digest solutions were then oxidized with BrCl and reduced with SnCl2 as well as NH4OH·HCl (Bloom and Fitzgerald 1988, USEPA 2002). All samples for THg analysis were quantified using dual amalgamation cold-vapor atomic fluorescence spectroscopy (CVAFS) (Brooks Rand, Seattle, USA). The difference in value between THg and DHg was represented as particulate Hg (PHg).
Analysis of MeHg
A 45-mL aliquot of acidified water sample was distilled at 125 °C for 3 h. The distillate was adjusted to pH 4.9 with 2 M sodium acetate and ethylated by addition of 80 μL 1% (v/v) NaB(C2H5)4.
Approximately 1.0 g of dry sediment sample was accurately weighed into a clean 50-mL centrifuge tube. The 25% HNO3 solution (4 mL) and 1 mL of 1 M CuSO4 solution were added to the sample and allowed to leach for 4 h. After leaching, approximately 10 mL of dichloromethane was added. The sample was intermittently shaken over the next hour and then centrifuged for 20 min at 3000 rpm to assist in the separation of the aqueous layer from the organic layer. The aqueous layer was removed by a vacuum air pump so that only the organic layer was collected directly in a centrifuge tube. Approximately 35 mL of Milli-Q water was added to the bottle along with a bamboo skewer to prevent the dichloromethane from “bumping” during the back extraction. The bottle was heated in a water bath at 45 °C for solvent evaporation until no visible solvent was left. The temperature of the water bath was increased to 75 °C, and the sample was purged with nitrogen for 8 min to remove solvent residue. The volume of the sample was adjusted to 40 mL with double distilled water (Liang et al. 1994).
About 0.1 g (dry and homogenous) of fish tissue samples was placed into a 40-mL amber glass vial, with KOH methanol solution (25%, 2.5 mL) added before capping the vial tightly. The sample was digested in an oven at 65 °C for 3–4 h. After digestion, the final volume was topped up to 40 mL with methanol prior to analysis.
Concentrations of MeHg in all of samples were quantified by using GC-CVAFS (Brooks Rand, Seattle, USA) following distillation and aqueous phase ethylation (Liang et al. 1994; USEPA 2001).Particulate MeHg (PMeHg) in water samples was calculated as the difference between the concentrations of TMeHg and DMeHg.
Determination of dissolved organic carbon and total suspended solid
Dissolved organic carbon (DOC) was determined by TOC-VCPH (Shimadzu, Japan). Total suspended solid (TSS) was determined by filtering an aliquot of water (typically 1500 mL) through a pre-weighed polycarbonate membrane filter with 0.45-μm pore size and 47 mm in diameter. The filter was dried at 103–105 °C at 2 h and stored in the drying basin. It was weighed after cool down to room temperature.
Diffusive flux of inorganic and MeHg from sediment pore water
The diffusion fluxes of inorganic (IHg) and MeHg from sediments pore water were estimated using Fick’s first law:
Where F is the diffusive flux of IHg or MeHg at the sediment–water interface, θ is the tortuosity (dimensionless), and ϕ is the sediment porosity. A relationship between tortuosity and porosity has recently been proposed (Boudreau 1996) and will be used for all flux calculations:
Dw is the diffusion coefficient of the solute in the absence of sediment. Dw values of IHg and MeHg were 9.5 × 10−6 and 1.3 × 10−5 cm2 s−1 at 25 °C as previously described (Covelli et al. 2008). Cw and Cpw are the concentrations of solute in the overlying water and in pore water, respectively, and Δx is the distance (cm) separating Cw and Cpw.
Temperature corrections that were made to the diffusion coefficients at 25 °C were made when necessary using the relationship (Lerman 1979):
Where Δt is the temperature difference in degree centigrade.
Quality control of sample analysis
Field blanks, matrix spikes, certified reference materials (CRMS) (CC580 for sediment and TORT-3 for fish), and 10% of duplicate samples were conducted for the quality control of THg and TMeHg determination. The method detection limit was 0.10 pM for THg and 0.05 pM for TMeHg. Field blanks of water samples were 2.10 pM for THg and 0.11 pM for TMeHg. The relative standard deviations for duplicate sample analyses were < 12.5% for MeHg and THg analyzing. Recoveries for CRMS were within the acceptable ranges (96.4–104% for THg and 93.1–99.4% for MeHg) In addition, recoveries for matrix spikes in water samples ranged from 90.2 to 102% for TMeHg analysis, and from 96.8 to 103% for THg analysis.
Data analyses
All statistical results were analyzed by SPSS 16.0 for Windows. Differences in Hg concentrations in all sampling sites were performed by one-way ANOVA (S-N-K test). Prior to one-way ANOVA, the data was log-transformed to obtain equal variance.
Results
Variation change of Hg species, DOC, and SS in overlying water bodies
Table 1 shows the concentrations of THg, DHg, and PHg in different layers of overlying water from AS to RS in WJD reservoir. THg concentrations in overlying water collected from AS and RS were 36.4 ± 89.7 and 29.0 ± 55.2 pM, respectively, without significant difference (p > 0.05, F = 0.079). Comparing different layers, the THg concentrations in the surface and bottom layers were significantly higher (p < 0.01, F = 3.75) than those in the other three intermediate layers (15, 30, and 45 m). There were also no significant differences in DHg (p > 0.05, F = 0.194) or PHg (p > 0.05, F = 0.120) between AS and RS for all of samples. However, it was noted that DHg concentrations in the surface and bottom layer of AS were significantly higher (p < 0.05, F = 4.01) than those in RS.
Table 2 shows the concentrations of TMeHg, DMeHg, and PMeHg in different layers of overlying water. TMeHg concentrations in overlying water collected from AS and RS were 0.30 ± 0.28 and 0.52 ± 0.82 pM, respectively, without significant differences. However, TMeHg concentrations in the bottom layers of AS (0.87 ± 1.02 pM) were significantly lower (p < 0.05, F = 4.08) than those in RS (2.11 ± 0.21 pM). In terms of vertical distribution, TMeHg concentrations in the bottom layer were significantly higher (p < 0.01, F = 48.7) than those in the other layers.
Table 3 shows the results of DOC and total SS at different depths. No significant differences were found between AS and RS in either DOC or SS. Again, SS in the bottom layer was significantly higher than in the other four depths.
Hg concentration in sediment core and pore water of sediment
Figure 2 shows THg and TMeHg concentrations in sediment cores and the DHg and DMeHg concentrations in sediment pore water. No significant difference (p > 0.05, F = 1.264) was found between THg at RS (296 ± 104 ng g−1) and THg at AS (274 ± 132 ng g−1). High concentrations of THg were observed in the top 3 cm at AS, and samples from 15 to 17-cm depth had relatively higher THg at RS. MeHg in sediment decreased from the top to the bottom subsequently. THg concentrations at RS from 10 to 20 cm were higher than those at AS.
In the liquid phase, DHg concentrations both in AS and RS decreased gradually from the surface to 5 cm in depth. No significant difference of DHg concentration was observed between AS (11.4 ± 4.66 pM) and RS (11.4 ± 2.18 pM). DMeHg concentrations for AS and RS were 1.27 ± 2.60 and 0.77 ± 1.07 pM, respectively. Although there was no significant difference for DMeHg in pore water between AS and RS for the whole sediment core, DMeHg in the top 5 cm of AS was obviously higher than that of RS (Fig. 2). Relatively higher DMeHg concentration was noted on the surface layer of pore water, especially for AS, which was consistent with the distribution of MeHg in sediment profiles. DMeHg concentrations in pore water were generally higher than those in interface water, implying that the sediment was an important source of MeHg to water column. Moreover, the vertical distribution pattern of the DHg was as same with DMeHg in pore water, indicating that positive correlation between DHg and DMeHg.
Hg concentration in fish organs and fish feeds
Figure 3 shows the THg, MeHg, and IHg concentrations in different organs from five species of aquaculture fish. THg and IHg in the liver were significantly higher (THg: p < 0.001, F = 9.98; IHg: p < 0.001, F = 13.1) than those in the other organs. In contrast, MeHg concentration in the muscle was significantly higher (p < 0.01, F = 4.83) than that in the other organs.
The diets for cultured fish in WJD is commonly comprised of trash fish and feed pellets. THg levels in trash fish and feed pellets were 47.9 ± 3.96 and 25.0 ± 7.45 ng g−1, respectively. MeHg levels in trash fish and feed pellets were 38.2 ± 1.88 and 7.44 ± 1.00 ng g−1, respectively.
Discussion
Aquaculture activities increasing THg loading in the aquatic environment
The environmental impact of intensive cage aquaculture results mainly from the accumulation of organic waste generated from fish excretion and the food supply (unconsumed feed). THg concentrations in fish feed reached 34.2 ng g−1 in WJD reservoir. The annual average fish feeds used for cage aquaculture is about 9409 t (Sun et al. 2005). Approximately 40% of fish feed is not consumed and is deposited into the sediment (HKAFCD 2009). The fish aquaculture area in WJD is 131,600 m2 (Luo et al. 2011). Based on this estimation, the THg loadings from the unconsumed fish feeds in the top surface sediment (1 cm) in WJD were about 69.8 ng g−1 annually. However, no significant differences were observed in THg concentrations in the surface sediment between AS and RS. This might be attributable to the large inputs of Hg from other sources in WJD reservoir. Feng et al. (2009b) calculated that the amount of Hg inputted into WJD reservoir of the following amounts: river and the other reservoir catchment (134,679 g), ground water (17,869 g), direct runoff (8517 g), and precipitation (5845 g). Thus, the input of Hg, especially from the river and the other catchment contributed more than the aquaculture activities in WJD.
However, THg and DHg concentrations in the surface layer of overlying water of AS were higher than those of RS (Table 1). These results indicate that aquaculture activities resulted in Hg contamination of the aquatic environment at AS in the surface layers. Higher THg and DHg concentrations in the surface layer of AS than RS might be attributable to discharges of domestic sewage by the fish farmer on the aquaculture raft. Domestic wastewater from kitchens and toilets were discharged directly into the water bodies since fish farmers live on the raft. Consequently, the THg and DHg concentrations in the surface layer of AS were higher than those of RS.
Aquaculture activities promoted MeHg formation and transportation in benthic environment
Formation of MeHg is affected by parameters such as sulfate reducing bacteria, sulfide concentration, OM content, and DHg concentration in pore water (Ullrich et al. 2001). Deposition of fish excreta and fish feed in the benthic environment results in the accumulation of OM at AS (Liang et al. 2011, 2016). The degradation of OM causes progressively more anoxic conditions at the sediment/water interface, which may lead to the mobilization and potential methylation of Hg2+ (Cossa and Gobeil 2000). Therefore, the observed increase in MeHg production in AS may be generally attributable to the degradation of OM from unconsumed fish feed and fish excretion. In addition, direct abiotic methylation by humic and fulvic acids is also generally considered to be a factor affecting MeHg production (Khwaja et al. 2006; Yin et al. 2013). Humic acid concentrations from AS are a benefit for MeHg formation in sediment (Zhao 2016). Thus, the humic or fulvic acids derived from organic wastes at AS may also contribute to the abiotic methylation of Hg. These results were consistent with our previous finding that high primary productivity in the reservoir produced elevated OM content that would favor the MeHg production in the sediment (Feng et al. 2009a; Liang et al. 2016). Moreover, the percentage of DMeHg to DHg (%MeHg) in sediment pore water followed a similar trend to the OM contents of the sediment (Fig. 4). The values of both %MeHg and OM in the top 4 cm of AS were clearly higher than those of RS. Their results indicate that the accumulation of OM due to aquaculture activities promoted MeHg production in the top layer of sediment pore water.
High concentration of MeHg in AS also promotes the transportation of MeHg from pore water to overlying water as the diffusion fluxes of MeHg in AS was higher than those in RS (Table 4). Therefore, the accumulation of OM from aquaculture activities promotes the MeHg formation and transportation of MeHg from sediment to overlying water.
Accumulation of Hg in aquaculture fish
It is well known that Hg, and especially MeHg, accumulates in aquatic organisms from their food resources (i.e., tropic transfer) (Mason et al. 1995b). In addition, Hg accumulates in some organs and tissues, e.g., through gills and scales mainly from the water bodies. The liver is a detoxification and storage organ and is able to accumulate large quantities of pollutants, such as, Hg, through active involvement in pollutants metabolism (Gonzalez et al. 2005). Relatively low MeHg concentrations and %MeHg were found in liver samples in this study (9.41 ± 16.1% across all samples), and this might be related to detoxification processes that demethylate MeHg to IHg in the liver (Eagles-Smith et al. 2009).
The present results show that the %MeHg in the muscle of all the aquaculture fish samples averaged 54.7 ± 22.3%. MeHg accumulates in the muscle mainly in cysteine thiol complexes (Harris et al. 2003) and is excreted slowly with a half-life of approximately 400 days (Downs et al. 1998). Thus, MeHg is the predominant species of Hg in fish muscle. However, this ratio was lower than in other studies about the ratio of MeHg to THg in fish muscle, generally ranging from 80 to 95% (Bloom 1992). This is not surprising considering they are cultured fish with relatively high growth rates. A rapid increase in weight might predispose aquaculture fish to biological accumulation of Hg.
The gills are considered to be the main entry point for Hg(II) present in the aqueous phase as they possess a wide surface area and are in continuous contact with the external medium (Mieiro et al. 2009). The present data show that the fish gills contained relatively high concentrations of IHg compared with MeHg, and this may be attributed to the majority of the Hg in the water existing as IHg (Klinck et al. 2005), as well as the fact that gills function as organs of toxicant excretion.
In all species of fish, the skin and scales have relatively low concentrations of both THg and MeHg, indicating that Hg cannot accumulate in these organs.
Conclusions
Aquaculture activities in the WJD reservoir resulted in loadings of THg from lost fish feed about 69.8 ng g−1 annually in the top 1 cm of sediment. The accumulation of OM from unconsumed fish feeds and fish excretion in AS favored MeHg formation and MeHg transportation from sediment to overlying water. Higher contents of IHg than MeHg in aquaculture fish samples were observed which were likely due to the relatively high growth rates of the aquaculture fish species.
References
Bloom N, Fitzgerald WF (1988) Determination of volatile mercury species at the picogram level by low-temperature gas chromatography with cold-vapour atomic fluorescence detection. Anal Chim Acta 208:151–161
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017
Boudreau BP (1996) The diffusive tortuosity of fine-grained unlithified sediments. Geochim Cosmochim Acta 60:3139–3142
Cossa D, Gobeil C (2000) Mercury speciation in the Lower St. Lawrence Estuary. Can J Fish Aquat 57:138–147
Covelli S, Faganeli J, De Vittor C, Predonzani S, Acquavita A, Horvat M (2008) Benthic fluxes of mercury species in a lagoon environment (Grado Lagoon, Northern Adriatic Sea, Italy). Appl Geochem 23:529–546
Downs SG, MacLeod CL, Lester JN (1998) Mercury in precipitation and its relation to bioaccumulation in fish: a literature review. Water Air Soil Pollut 108:149–187
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983
Eagles-Smith CA, Ackerman JT, Yee J, Adelsbach TL (2009) Mercury demethylation in waterbird livers: dose–response thresholds and differences among species. Environ Toxicol Chem 28, 568–577
FAO (2014): Fishery and aquaculture statistics. Food and agriculture organization of the United Nations Rome
FAO (2004) The state of world fisheries and aquaculture. In: Department F (ed) Food and Agriculture Organization of the United Nation, Rome: Italy, pp 1–153
Feng X, Jiang H, Qiu G, Yan H, Li G, Li Z (2009a) Geochemical processes of mercury in Wujiangdu and Dongfeng reservoirs, Guizhou, China. Environ Pollut 157:2970–2984
Feng X, Jiang H, Qiu G, Yan H, Li G, Li Z (2009b) Mercury mass balance study in Wujiangdu and Dongfeng reservoirs, Guizhou, China. Environ Pollut 157:2594–2603
Gonzalez P, Dominique Y, Massabuau JC, Boudou A, Bourdineaud JP (2005) Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio). Environ Sci Technol 39, 3972–3980
Hammerschmidt CR, Fitzgerald WF, Balcom PH, Visscher PT (2008) Organic matter and sulfide inhibit methylmercury production in sediments of New York/New Jersey Harbor. Mar Chem 109:165–182
Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301:1203
HKAFCD (2009) Fish feed management, good aquaculture practices series 1. In: Hong Kong Agriculture FaCD (ed) Hong Kong
Khwaja AR, Bloom PR, Brezonik PL (2006) Binding constants of divalent mercury (Hg2+) in soil humic acids and soil organic matter. Environ Sci Technol 40:844–849
Klinck J, Dunbar M, Brown S, Nichols J, Winter A, Hughes C, Playle RC (2005) Influence of water chemistry and natural organic matter on active and passive uptake of inorganic mercury by gills of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 72:161–175
Lerman A (1979) Geochemical Processes –Water and Sediment Environments. John Wiley and Sons, New York
Liang L, Horvat M, Bloom NS (1994) An improved speciation method for mercury by GC/CVAFS after aqueous phase ethylation and room temperature precollection. Talanta 41:371–379
Liang P, Shao D-D, Wu S-C, Shi J-B, Sun X-l WF-Y, Lo SCL, Wang W-X, Wong MH (2011) The influence of mariculture on mercury distribution in sediments and fish around Hong Kong and adjacent mainland China waters. Chemosphere 82:1038–1043
Liang P, Wu S-C, Li Y-C, Li H-B, Yu G-b YS, Wong MH (2012) The effects of mariculture activities on the adsorption/desorption and chemical fractionations of mercury on sediments. Mar Pollut Bull 64:836–843
Liang P, Lam C-L, Chen Z, Wang H-S, Shi J-B, Wu S-C, Wang W-X, Zhang J, Wang H, Wong M-H (2013) Formation and distribution of methylmercury in sediments at a mariculture site: a mesocosm study. J Soils Sediments 13:1301–1308
Liang P, Gao X-F, You Q-Z, Zhang J, Cao Y-C, Zhang C, Wong M-H, Wu S-C (2016) Role of mariculture in the loading and speciation of mercury at the coast of the East China Sea. Environ Pollut 218:1037–1044
Luo, J-X, Xu H-Y, Sun X-C, Tang Z-B, Li N, Lu B (2011): Impact of Reservoir Cage Culture on Environment & Countermeasures Research at Wujiangdu Hydropower Station. Guizhou Water Power 25, 36–38 (in Chinese)
Mason PR, Laporte J-M, Andres S (2000) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297
Mason RP, Reinfelder JR, Morel FMM (1995a) Bioaccumulation of mercury and methylmercury. In: Porcella DB, Huckabee JW, Wheatley B (Eds) Mercury as a Global Pollutant: Proceedings of the Third International Conference held in Whistler, British Columbia, July 10–14, 1994. Springer Netherlands, Dordrecht, pp 915–921
Mason RP, Reinfelder JR, Morel FMM (1995b) Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut 80:915–921
Meng B, Feng XB, Chen CX, Qiu GL, Sommar J, Guo YN, Liang P, Wan Q (2010) Influence of eutrophication on the distribution of total mercury and methylmercury in hydroelectric reservoirs. J Environ Qual 39
Meng B, Feng X, Qiu G, Li Z, Yao H, Shang L, Yan H (2016) The impacts of organic matter on the distribution and methylation of mercury in a hydroelectric reservoir in Wujiang River, Southwest China. Environ Toxicol Chem 35:191–199
Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH (2007) Methylmercury exposure and health effects in humans: a worldwide concern. Ambio 36:3–11
Mieiro CL, Pacheco M, Pereira ME, Duarte AC (2009) Mercury distribution in key tissues of fish (Liza aurata) inhabiting a contaminated estuary-implications for human and ecosystem health risk assessment. J Environ Monit 11:1004–1012
Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW (2005) Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Health Perspect 113:1376–1380
Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, Lawrence R (2008) Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ Int 34:1215–1226
Shao D, Liang P, Kang Y, Wang H, Cheng Z, Wu S, Shi J, Lo SCL, Wang W, Wong MH (2011) Mercury species of sediment and fish in freshwater fish ponds around the Pearl River Delta, PR China: human health risk assessment. Chemosphere 83:443–448
Subasinghe R, Soto D, Jia J (2009) Global aquaculture and its role in sustainable development. Rev Aquac 1:2–9
Sun JL, Zhong X, Liu YX, Fu J (2005) Th e status analysis of cage culture fi shing of reservoir and lake in Guizhou province. Guizhou Environ Protect Sci Technol 11:30–34 (in Chinese)
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
UNEP (2013) Sources, emissions. Releases and Environmental Transport, Geneva
USEPA (2001) Methyl mercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS (Method 1630). In: USEPA (ed) Washington, DC
USEPA (2002) Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry (Method 1631, revision E). In: USEPA (ed) Washington, DC
Yin YG, Li YB, Ma X, Liu JF, Jiang GB (2013) Role of natural organic matter in the biogeochemical cycle of mercury : binding and molecular transformation. Prog Chem 25:2169–2177
Zhao K-Y (2016): Study on mercury pollution in mariculture areas around the Pearl River Estuary and risk assessment of mercury exposure. Thesis. Peking University. 52-53
Acknowledgements
Financial support from the Innovative Team Foundation of Zhejiang Province (2013TD12), the National Natural Science Foundation of China (No. 21577130), and Zhejiang Provincial Public Techniques Research and Social Development Project (2015C33050) are gratefully acknowledged. We would also like to thank the support of the Innovation Technology Fund (ITS/174/14FX) for visiting the Education University of Hong Kong.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Liang, P., Feng, X., You, Q. et al. The effects of aquaculture on mercury distribution, changing speciation, and bioaccumulation in a reservoir ecosystem. Environ Sci Pollut Res 24, 25923–25932 (2017). https://doi.org/10.1007/s11356-017-0189-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0189-6





 liver;
liver;  muscle;
muscle;  gills;
gills;  fish roe;
fish roe;  skin;
skin;  swim bladder;
swim bladder;  scale
scale