Abstract
Anthropogenic activities are a major source for contaminating the agricultural soil with heavy metals, which can affect physiological and metabolic processes in plants. Among the heavy metals, chromium (Cr) is the most toxic pollutant that negatively affects plants’ metabolic activities, growth, and yield. Chromium reduces the plant growth and development by influencing the photosynthetic performance and antioxidant enzyme activities. This study was designed to examine the promotive role of exogenously applied glycinebetaine (GB) on plant morphophysiological and biochemical attributes in cauliflower (Brassica oleracea botrytis L.) under Cr toxicity. Four levels (0, 10, 100, and 200 μM) of Cr were tested under the application of GB (1 mM). The results delineated that Cr stress caused a considerable reduction in plant growth, photosynthetic pigment, gas exchange parameters, and biomass production. At high concentration (200 μM), chromium stress decreased the plant height (57%), root length (32%), number of leaves (45%), and leaf area (29%) as compared with controls. Due to Cr stress, the electrolyte leakage and accumulation of malondialdehyde and hydrogen peroxide increased both in the roots and leaves of cauliflower, whereas antioxidative enzyme activities (SOD, CAT, and POD) decreased both in the roots and leaves of cauliflower due to Cr stress. At 200 μM of chromium treatment, root dry weight, stem dry weight, leaf dry weight, and flower dry weight declined up to 43%, 40%, 53%, and 72%, respectively. With the application of GB, dry biomass of plant increased significantly as compared with no GB treatment under chromium stress. As Cr level increased in growth media, its concentration also increased in all plant parts including roots, stem, leaves, and flowers. However, GB application efficiently alleviated the Cr toxic effects on cauliflower and maintained higher plant growth, biomass production, photosynthetic attributes, and gas exchange traits as compared with their respective controls. Exogenously applied GB decreased oxidative stress and improved antioxidative enzyme activities as compared with treatments without GB application. Furthermore, Cr concentrations taken by plants were decreased due to GB application. These findings suggest that GB can play a positive role to maintain plant morphology and photosynthetic attributes under Cr toxic conditions in cauliflower.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasingly growing industrial development has become a major source of heavy metal emission and accumulation into the environment, which ultimately pollute the agricultural ecosystem (Hu et al. 2013). Leather industries are a major source of chromium (Cr) pollution in Pakistan and the world due its presence in the tannery effluents. These leather industries are mainly located in Sialkot, Kasur, Lahore, Sheikhupura, and Faisalabad (Anwaar et al. 2014). Cr addition to agricultural soils shows a number of undesirable effects upon soil health, plant growth, and production (Singh et al. 2013; Dheeba et al. 2015). Different anthropogenic activities, tannery industry, mining practices, electroplating, and volcanic eruption are also the sources of Cr production and addition to the soil environment (Ali et al. 2015).
Crops grown in urban industrial soils are usually irrigated with raw tannery effluents which contain toxic heavy metals like Cr in addition to plant nutrients (Khan et al. 2015). Then, these Cr-contaminated crops become part of our food chain causing health hazards to the livings. Cr is strongly recommended as non-essential metal in the biological functions of plants. Contaminated soils with Cr are a key source of Cr entrance towards our food chain (Samantaray et al. 2015; Rebhi et al. 2019). It was found that plant growth, biomass, photosynthetic parameters, and enzyme activities were strongly affected by Cr stress (Ahmad et al. 2019; Ali et al. 2015; Gill et al. 2015). Cr showed phytotoxic effects because of overproduction of reactive oxygen species (ROS), leading to the oxidative stress in brassica plant (Gill et al. 2015). Similarly, Cr toxicity disturbed the production of protein and photosynthetic pigment content in plants of wheat and peas (Dey et al. 2009; Rodriguez et al. 2012). In addition, severe metal toxicity may prevent the initiation and efficiency of the antioxidant system of plants that ultimately reduces plant growth and production. However, the toxicity due to heavy metals (Cr and Cd) varies from species to species and also dose-dependent (Gill et al. 2015; Farooq et al. 2013). Chromium toxicity decreases the availability of nutrient elements such as sodium, iron, manganese, copper, zinc, and calcium and affects negatively the plant metabolic process, which resulted in significant reduction in plant growth and yield (Zhao et al. 2019)
Under different types of stresses, plants enhance their self-defense system by enhancing the activities of the antioxidant enzymes. Plants under environmental stress conditions can develop the antioxidant defense system to cope with ROS toxic effect (Mittler 2002). Superoxide dismutase catalyzes the conversion of superoxide (O2•−) to the less reactive hydrogen peroxide (H2O2). This H2O2 is further detoxified to O−2 and H2O through CAT and POD activities. Combined use of these three enzymes revealed the above ensures lower intracellular levels of O2− and H2O2 (Abid et al. 2016a). The practice of enhancing plants’ ability to sustain various physiological processes under stressed conditions is considered an important tool for ensuring the sustainable production of crops under the scenarios of increasingly growing environmental stress conditions in the agricultural system (Abid et al. 2016b). One such approach is the application of glycinebetaine (N,N,N-trimethylglycine; GB) which is known as an ameliorating agent for the reduction of environmental stresses (Giri 2011). The main function of GB is to improve plant osmotic potential and to maintain plant biochemical reactions under stressed environment (Giri 2011).
The application of GB can be anticipatory and economical for the agriculture as it ameliorates toxic environmental stresses and enhances crop production. GB improves antioxidant enzyme activities under salt stress in rice seedlings (Hasanuzzaman et al. 2014), while improved growth and photosynthetic processes in maize (Yang and Lu 2005). GB maintains the higher nutrient uptake, photosynthetic, and transpiration rates under drought condition in wheat (Raza et al. 2014). In another study, Tisarum et al. (2019) reported that exogenous foliar application of GB modulated the physiological adaptions in sweet potato under water deficit condition by accumulation of soluble sugar, stabilizing the photosynthetic pigment, net photosynthetic rate, and chlorophyll florescence, and improved the overall growth performance, e.g., shoot height, number, and length of leaves. Khan et al. (2015) observed that GB application reduced the adverse effects of salinity in Brassica napus by improving chlorophyll a, chlorophyll b, and total chlorophyll in the plant which was decreased by salinity stress. Similarly, GB reduced the effect of lead stress in cotton plants (Bharwana et al. 2014; Farid et al. 2013) and ameliorated Cd stress in rice by improving growth traits (Cao et al. 2013). However, so far, a little is known whether GB application prevents chromium-induced oxidative, photosynthetic, and morphological changes in cauliflower. In the present study, we tested the hypothesis whether GB ameliorates Cr toxic effects and improves the plant growth in cauliflower. Outcomes of our study would possibly introduce a potential way to prevent Cr toxicity in cauliflower.
Materials and methods
Plant material and growth conditions
Pot experiments were conducted in the Botanical Garden, Government College University Faisalabad, Pakistan. Selected seeds of cauliflower were sterilized by dipping in ethanol (70%) for 1 min followed by sodium hypochlorite for 5 min and finally rinsed with distilled water five times. Sand was washed with HCL (5%) and with distilled water to grow the seedlings. Sterilized seeds were sown at 1.5 cm depth in plastic pots filled with washed sand. Pots were applied half-strength Hoagland solution 2 times after a 10-day interval. Experiment was carried out under 18–25 °C and 70% humidity condition. Four-week-old seedlings of the same size were transplanted in other plastic pots containing 10 kg soil. Physiochemical soil properties are mentioned in Table 1. Inorganic fertilizers were supplied properly at the rate of 2.14 g L−1 of K as potassium sulfate, 2.19 g L−1 of N as urea, and 0.5 g L−1 of P as diammonium phosphate.
Chromium stress application
On the basis of previous experiments (Gill et al. 2015), four different levels (0, 10, 100, and 200 μM) of K2Cr2O7 (Sigma Pvt. Ltd.) prepared with distilled water were used as Cr treatments. Three replicates were used for all treatments and arranged in complete randomized design. After 4 weeks of transplantation, plants were irrigated with 500 mL Cr solution within the 7-day interval. Afterwards, the GB (1 mM) was applied within the 1-week interval.
Plant sampling and analysis
After 4 weeks of the treatments’ imposition, cauliflower seedlings were harvested and carefully washed firstly with tap water followed by distilled water. Growth attributes like number of leaves, root length, and leaf area were measured. Plant parts, e.g., flower, leaves, root, and stem, were separated to calculate the fresh weights. Plant materials were oven-dried for 72 h at 70 °C and then dry weights were recorded.
Photosynthetic pigment contents
After 3 weeks of treatment, the uppermost fully extended leaves were used to determine chlorophyll (Chl) and carotenoid contents. Pigment was extracted in 85% (v/v) acetone and kept under 4 °C in darkness until leaf color disappeared, then centrifuged at 4000×g for time duration of 10 min; the supernatant absorbance was recorded at 663, 644, and 452.5 nm for Chl a, Chl b, and carotenoids, respectively, by a spectrophotometer (Metzner et al. 1965). Chl and carotenoid contents were calculated by adjusting extinction coefficients and equations (Lichtenthaler 1987). Finally, the contents of the pigments were shown as mg g−1 fresh weights (FW).
Gas exchange parameters
Gas exchange parameters, e.g., stomatal conductance (Gs), transpiration rate (E), photosynthetic rate (Pn), water use efficiency (WUE), were measured by Infrared Gas Analyzer (IRGA) (LI-6200, Li-Cor Inc., Lincoln, NE, USA) during daytime between 09:00 am to 11:00 am.
Detection of hydrogen peroxide, malondialdehyde, and electrolyte leakage
Malondialdehyde (MDA) content was determined to estimate the extent of lipid peroxidation in plant leaves. The method of Heath and Packer (1968) was followed to estimate MDA further modified by others. The H2O2 and MDA were recorded by previously established procedures (Dhindsa et al. 1981; Zhang and Kirkham 1994; Dionisio-Sese and Tobita 1998).
Determination of enzymatic antioxidants
After 4 weeks of treatments, fresh leaves and roots were collected from plants for enzymatic analysis. Root and leave samples (1.0 g) were ground in precooled mortar and pestle. Then, the samples were homogenized by using phosphate buffer (0.5 M, pH 7.8), filtered through muslin cloth and then centrifuged at 12,000×g for time duration of 10 min at 4 °C temperature. The supernatant was stored for further estimation of SOD and POD activities. Antioxidant enzymes, including CAT, SOD, and POD, were evaluated spectrophotometrically both in roots and leaves. The SOD and POD were recorded through the given method of Zhang (1992), and CAT reading was noted by the given protocol of Aebi (1984). Mixture containing 2.8 mL of phosphate buffer (50 mM), 100 μL enzyme extract, and 100 μL of 300 mM H2O2 was used, and CAT was recorded by measuring the reduction in absorbance at 240 nm due to H2O2 disappearance.
Determination of chromium content
In order to measure the Cr contents, the samples were kept overnight in HNO3 and HClO4 solution with 3:1 (v/v), respectively. Afterwards, the digestion process was completed on hot plate and absorbance was checked by the atomic absorption spectrophotometer (NOVA-A400 Analytik Jena, Germany). Concentration and accumulation of chromium in plant parts were calculated according to Monni et al. (2000)
Statistical analysis
Data was statistically analyzed through SPSS for windows. For mean comparison, a post hoc test followed by a Tukey test was run. The value for P was perceived significant at ≤ 0.05.
Results
In this study, four chromium (Cr) concentrations (0, 10, 100, and 200 μM) were applied with and without GB foliar treatment. Different morphological, physiological, and biochemical parameters such as change in plant growth, gas exchange process, photosynthetic pigments, lipid peroxidation, antioxidative system, and Cr uptake and accumulation by plants were studied.
Changes in plant morphological traits
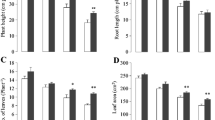
To observe the effect of GB on morphological traits of cauliflower under Cr stress, plant height, root length, number of leaves, and leaf area were measured. The results showed that all these parameters decreased by chromium stress as compared with controls (Fig. 1). As the level of Cr increased in growth media, further reduction in these plants’ growth traits was observed. However, exogenously applied GB improved the plant height, root length, number of leaves, and leaf area in both controlled and Cr-stressed conditions. Without GB, plant height decreased by 25%, 39%, and 57%, while with GB application, it was reduced by 18%, 30%, and 43% under Cr10, Cr100, and Cr200, respectively, as compared with controls (Fig. 1A). Root length reduced up to 13%, 23%, and 32% without GB, and it declined up to 8%, 15%, and 25% with GB application under Cr10, Cr100, and Cr200, respectively, as compared with controls (Fig. 1B). Leaf area decreased by 22%, 27%, and 29% with no GB, while under GB application, it decreased by 19%, 23%, and 25% under Cr10, Cr100, and Cr200, respectively, as compared with controls (Fig. 1C). Similarly, without GB application, the number of leaves decreased by 26%, 35%, and 45%, while with GB application, it decreased by 18%, 29%, and 33% under Cr10, Cr100, and Cr200, respectively, as compared with controls (Fig. 1D).
Effect of different treatments of glycinebetaine (GB) (1 mM) and chromium (Cr) (0, 10, 100, and 200 μM) on plant height (A), root length (B), number of leaves plant−1 (C), and leaf area (D) of cauliflower seedlings grown. Values are expressed as means of 10 replicates with standard deviations. Different letters indicate that values are significantly different at P < 0.05
Data showed that dry weights of these parts were decreased due to chromium stress at all levels (Table 1). As the level of Cr stress increased, a significant reduction in matter production was observed. Exogenously applied GB decreased the effects of Cr stress and showed higher dry weights as compared with Cr-stressed plants without GB application. Without GB, dry weight of roots decreased by 23%, 31%, and 43%, while with GB application, it decreased by 17%, 25%, and 34% under Cr10, Cr100, and Cr200, respectively, as compared with controls. Stem dry weight reduced by 5%, 22%, and 40% without GB, and it declined by 3%, 19%, and 32% with GB application under Cr10, Cr100, and Cr200, respectively, as compared with controls. Leaf dry weight decreased by 25%, 31%, and 53% without GB, while with GB application, it decreased by 11%, 27%, and 44% under Cr10, Cr100, and Cr200, respectively, as compared with controls. Similarly, without GB application, dry weight of flower decreased by 26%, 50%, and 72%, while with GB application, it decreased by 12%, 35%, and 64% under Cr10, Cr100, and Cr200, respectively, as compared with controls. This shows that Cr reduced plant growth and development in cauliflower and GB resulted in less of a decrease (Table 1), which might be associated with the effect of GB to maintain the photosynthetic process of the plants.
Changes in gas exchange parameters and photosynthetic pigments
To determine changes in photosynthetic activity, different parameters such as stomatal conductance (Gs), carbon dioxide fixation (Pn), transpiration rates (Tr), and water use efficiency (WUE) in the leaves were analyzed (Fig. 2). These traits were decreased by Cr stress and higher reduction was observed with increasing Cr level in soil. However, GB application improved these traits. The lowest values of Tr, Gs, Pn, and WUE were recorded at Cr 200 μM as 68%, 71%, 59%, and 55%, respectively, in plants without GB application, whereas in plants with GB application, decline in Tr, Gs, Pn, and WUE was 60%, 68%, 48%, and 35%, respectively, as compared with control plants.
Effect of different treatments of glycinebetaine (GB) (1 mM) and chromium (Cr) (0, 10, 100, and 200 μM) on transpiration rate (A), stomatal conductance (B), net photosynthetic rate (C), and water use efficiency (D) of cauliflower seedlings. Values are expressed as means of five replicates with standard deviations. Different letters indicate that values are significantly different at P < 0.05
Similarly, Cr stress significantly decreased the photosynthetic pigments, including chlorophyll a and chlorophyll b, total chlorophylls, and carotenoids as compared with controls (Fig. 3). Decreasing trends were observed at all Cr levels (10,100, and 200 μM), but the maximum decrease in these traits was observed at Cr 200 μM. Application of GB improved the photosynthetic activity under Cr stress. At Cr 200 μM, chlorophyll pigment contents declined significantly as compared with controls, whereas with GB application, chlorophyll pigments were improved significantly as other stressed plants.
Effect of different treatments of glycinebetaine (GB) (1 mM) and chromium (Cr) (0, 10, 100, and 200 μM) on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and carotenoids (D) of cauliflower seedlings. Values are expressed as means of five replicates with standard deviations. Different letters indicate that values are significantly different at P < 0.05
Changes in oxidative stress and antioxidant enzyme activities
To observe effects of GB on the physical qualities of the membrane and oxidative stress in response to Cr stress, electrolyte leakage (EL) and contents of MDA and H2O2 were examined (Fig. 4). An increase in EL, MDA, and H2O2 contents were observed both in leaves and roots under Cr stress as compared with controls. However, GB application declined EL, MDA, and H2O2 as compared with Cr-alone treatment. Maximum enhancements in these traits were found when Cr was applied at 200 μM. At this level, EL, MDA, and H2O2 were increased by 68%, 49%, and 48% in roots without GB, while with GB application, the increase in these parameters was 55%, 41%, and 39%, respectively, as compared with controls. Similar trends were observed from the analysis of leaves.
Effect of glycinebetaine (GB) on EC in roots (A), EC in leaves (B), MDA concentration in roots (C), MDA concentration in leaves (D), H2O2 in roots (E), and H2O2 in leaves (F) of cauliflower seedlings grown under various Cr (0, 10, 100, and 200 μM) levels. Values are expressed as means of five replicates with standard deviations. Different letters indicate that values are significantly different at P < 0.05
Furthermore, Cr stress increased antioxidant enzyme activities in leaves and roots (Fig. 5). Antioxidative enzyme activities increased as the level of Cr stress increased; it was maximum at 100 μM and tended to decrease afterwards. The GB application further augmented the SOD, POD, and CAT activities under Cr stress. At 100 μM, the SOD, POD, and CAT activities increased in roots up to 42%, 39%, and 30% in treatments without GB, whereas in treatments with GB, these increased up to 49%, 51%, and 32%, respectively, as compared with controls. Similar results were obtained in the leaves for the enzymatic activities. These findings exemplify the role of GB for regulating the membrane stability and generation of ROS in cells under the conditions of Cr stress.
Effect of glycinebetaine (GB) on CAT in leaves (A), CAT in roots (B), SOD in roots (C), SOD in leaves (D), POD in roots (E), and POD in leaves (F) of cauliflower seedlings grown under various Cr (0, 10, 100, and 200 μM) levels. Values are expressed as means of five replicates with standard deviations. Different letters indicate that values are significantly different at P < 0.05
Changes in chromium uptake and accumulation in different plant parts
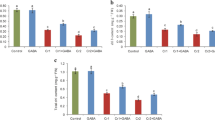
Chromium concentration in flower, stem, leaves, and roots of cauliflower were measured (Fig. 6). Cr uptake in all these parts increased due to Cr stress. The maximum uptake of Cr in flower and leaves was observed at Cr 100 μM, whereas, in stem and roots, it increased non-significantly at 200 μM. However, the GB application reduced Cr uptake in all plant parts. At 200 μM, Cr uptake in flower, leaves, stem, and roots was 35, 13, 15, and 14 mg plant−1 without GB application, whereas, with GB application, Cr uptake in these parts was 22, 11, 13, and 13 mg plant−1, respectively.
Effect of glycinebetaine (GB) on total Cr uptake in flower(A), total Cr uptake in leaves (B), total Cr uptake in stem (C), and total Cr uptake in roots (D) of cauliflower seedlings grown under various Cr (0, 10, 100, and 200 μM) levels. Values are expressed as means of three replicates with standard deviations. Different letters indicate that values are significantly different at P < 0.05
Similarly, Cr stress caused an accumulation of the Cr content in root, stem, leaves, and flower of the plant (Table 3). Maximum Cr content in all these parts was present at 200 μM Cr stress level. The highest Cr concentrations were observed in plant root, followed by stem, leaves, and flower, respectively. However, the plants with GB application showed lower Cr concentrations in all plant parts as compared with plants without GB application. At 200 μM Cr level in the growth medium, Cr content was 2370, 1800, 1558, and 725 mg kg−1 in roots, stem, leaves, and flower of the plants without GB application, while Cr content was 1900, 1366, 1258, and 635 mg kg−1in these parts of plants with GB application, respectively.
Discussion
Cr contamination in cultivated lands has become a serious issue globally. In the present study, we examined the effects of Cr toxicity on morphophysiological and biochemical attributes of cauliflower plant and role of GB in alleviating Cr toxic effects. It was observed that the plant growth characteristics and biomass decreased under Cr stress. An increase in Cr level in growth medium further declined plant growth traits (Table 1, Fig. 1). Chromium-induced stress inhibits the plant growth and biomass as documented by several researchers (Jabeen et al. 2015; Zhao et al. 2019; Ali et al. 2015a, 2015b). This reduction in growth and biomass might be attributed to the Cr interaction with essential nutrients and reduced their uptake by plants (Pradas-del-Real et al. 2013). In our study, decrease in root length under Cr stress might be due to the accumulation of Cr in root cells or damage of root tip cells (Ali et al. 2013). Reduction in shoot lengths might be due to Cr-induced ultrastructural damages to leaf mesophyll cells (Ma et al. 2016; Gill et al. 2015), which ultimately resulted in decline of shoot growth. However, foliar application of GB improved plant growth and biomass by ameliorating Cr stress in cauliflower seedlings (Fig. 1, Table 2). Various studies have reported the damage preventive role of GB under various environmental stresses (Estaji et al. 2019; Tisarum et al. 2019). Previously, it has been found that foliar GB application improved plant growth under Cr stress in mung bean (Jabeen et al. 2015) and wheat (Ali et al. 2015b). Moreover, Bharwana et al. (2014) also documented positive effects of GB in cotton under Pb stress and in wheat under Cd stress (Rasheed et al. 2014). The enhanced plant growth might be due to GB enhancing nutrient uptake in stressed plants (Shahbaz et al. 2011). An enhanced photosynthetic rate accompanied by improved stomatal conductance and Rubisco activity has been reported by the application of GB under drought stress (Nomura et al. 1998).
In addition to plant growth reduction, the concentration of photosynthetic pigments decreased with increasing level of Cr in the growth medium (Figs. 2 and 3), suggesting that chlorophyll pigments may give the former signals to Cr stress (Dey et al. 2009). The decrease in the photosynthetic pigments might be due to ultrastructural changes in chloroplasts (Gill et al. 2015) or production of ROS under Cr stress (Ahmad et al. 2019; Habiba et al. 2015). The inhibition in biosynthesis or higher enzymatic activities that degrade chlorophyll may reduce the chlorophyll contents under Cr stress (Singh et al. 2013). Reduction of photosynthesis in cauliflower under Cr stress might be due to similar reasons. Foliar GB application improved photosynthetic pigments under Cr stress (Figs. 2 and 3). Reduction in carotenoids is perilous to plants, as carotenoids play a vital role in protection of chlorophyll from photooxidative destruction (Middleton and Teramura 1993; Yadav and Singh 2013).
Previous studies have stated that GB application improved gas exchange characteristics under stress conditions (Shahbaz et al. 2011; Shahbaz and Zia 2012). Recently, various studies have shown the promotive role of GB in gas exchange characteristics and photosynthetic parameters of different plants under various heavy metals (Chen and Murata 2011; Jabeen et al. 2015; Ali et al. 2015b). Further, Einset et al. (2008) revealed that GB application may actuate the expression of gene related to ROS cleaning enzymes, which resulted in preservation of photosynthetic apparatus from oxidative damages and ultimately enhanced plant growth and biomass.
In this study, higher Cr levels resulted in enhanced Cr concentration and total Cr uptake plants (Fig. 6, Table 3). The Cr concentration was maximum in root and minimum in flowers which indicates that cauliflower plant might have great resistance against Cr stress as documented by Ali et al. (2015b). The reduction in uptake of Cr may be due to GB protecting the cell membrane from damages that resulted in low amount of Cr entering the cytoplasm (Giri 2011). The other possible explanation is that the application of GB might have enhanced nutrient uptake by plant under stress condition (Shahbaz et al. 2011); therefore, the uptake of Cr decreased because Cr competes with other nutrients.
It was noticed that Cr increased ROS production, EL, MDA, and H2O2 contents. Interestingly, antioxidant enzyme activities were increased at lower Cr level, while decreased at higher Cr doses in the leaves and roots of cauliflower (Fig. 4). Higher Cr concentration might reduce cauliflower defense mechanism, resulting in decreased antioxidant enzyme activities and increased oxidative stress. Similar findings were reported by many other researchers where Cr produced excess ROS in wheat (Ali et al. 2015a), mung bean (Jabeen et al. 2015), and oilseed rape (Gill et al. 2015). A decrease in antioxidant enzyme activities under Cr stress in our study is in line with the previous observations (Ali et al. 2015a, 2015b). However, information about positive effect of GB on the antioxidant activities under Cr stress is not available in the previous studies. A promotive role of foliar GB was observed on the antioxidant enzyme activities and oxidative damage under Cr stress (Figs. 4 and 5). The GB reduced ROS production in cotton under Pb stress (Raza et al. 2014) and in lettuce (Lactuca sativa L.) under salt stress (Yildirim et al. 2015).
Plants scavenge from oxidative damage by evolving antioxidant enzyme activities (SOD, POD, and CAT). The mild and moderate Cr stress increased the SOD, POD, and CAT activities; however, severe Cr stress reduced the activities of all enzymes (Fig. 5). Exogenous GB application enhanced antioxidant enzyme activities in stressed and non-stressed plants. Under Cd stress, GB application markedly enhanced the antioxidant enzyme activities in rice and mung bean (Cao et al. 2013; Hossain et al. 2010). It was also reported that under drought stress, the GB application increased the activities of antioxidant enzymes in maize (Xin et al. 2011). The decrease in antioxidant enzyme activities might be ascribed to overproduction of ROS or excessive electrolyte leakage (Farid et al. 2017; Farid et al. 2018; Hussain et al. 2018).
In conclusion, our findings revealed that Cr stress significantly reduced plant biomass and growth, photosynthesis, and antioxidant enzyme activities. Oxidative stress was dominant due to Cr toxicity. However, the application of GB significantly enhanced the plant growth and biomass and reduced the oxidative stress by improving antioxidant enzymes under Cr stress. The GB application also reduced the Cr uptake and translocation. Less is known about the mechanism of GB in cauliflower under Cr stress. Hence, further studies are needed at field level in order to see the GB role and its mechanism towards various plant species under heavy metal stress.
Data availability
The data used to support the findings of this study are included within the article.
References
Abid M, Tian Z, Ata-UI-Karim ST, Liu Y, Cui Y, Rizwan Z, Dong J, Dai T (2016a) Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and -sensitive wheat cultivars. Plant Physiol Biochem 106:218–227
Abid M, Tian Z, Tahir AS, Wang F, Yang L, Rizwan Z, Dong J, Dai T (2016b) Adaptation to and recovery from drought stress at vegetative stages in wheat (Triticum aestivum ) cultivars. Funct Plant Biol 43:1159–1169
Aebi H (1984) Catalase in vitro. In Methods Enzymol 105:121–126
Ahmad R, Ali S, Rizwan M, Dawood M, Farid M, Hussain A, Wijaya L, Alyemeni MN, Ahmad P (2019) Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant. https://doi.org/10.1111/ppl.13001
Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang G (2013) Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem 32:2234–2239
Ali Z, Malik RN, Shinwari ZK, Qadir A (2015) Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Int J Environ Sci Technol 12:537–550
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015a) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015b) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res 22:10669–10678
Anwaar SA, Ali S, Ali S, Ishaque W, Farid M, Farooq MA, Sharif M (2014) Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ Sci Pollut. Res 22:3441–3450
Bharwana SA, Ali S, Farooq MA, Iqbal N, Hameed A, Abbas F, Ahmad MSA (2014)
Cao F, Liu L, Ibrahim W, Cai Y, Wu F (2013) Alleviating effects of exogenous glutathione, glycinebetaine, brassinosteroids and salicylic acid on cadmium toxicity in rice seedlings (Oryza sativa). Agro Technol 2:1–7
Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Dey SK, Jena PP, Kundu S (2009) Antioxidative efficiency of wheat (Triticum aestivum L.) exposed to chromium stress. J Environ Biol 30:539–544
Dheeba B, Sampathkumar P, Kannan K (2015) Fertilizers and mixed crop cultivation of chromium tolerant and sensitive plants under chromium toxicity. J Toxicol 2015:367217
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Einset J, Winge P, Bones AM, Connolly EL (2008) The FRO2 ferric reductase is required for glycine betaine’s effect on chilling tolerance in Arabidopsis roots. Physiol Plant 134:334–341
Estaji A, Kalaji HM, Karimi HR, Roosta HR, Moosavi-Nezhad SM (2019) How glycine betaine induces tolerance of cucumber plants to salinity stress? Photosynthetica 57(3):753–761
Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M, Hanif MS et al (2013) Morphological, physiological and biochemical responses of different plant species to Cd stress. Int J Chem Bio Chem Sci 3:53–60
Farid M, Ali S, Rizwan M, Ali Q, Abbas F, Bukhari SAH, Wu L (2017) Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotox Environ Safe 145:90–102
Farid M, Ali S, Rizwan M, Ali Q, Saeed R, Nasir T et al (2018) Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotox Environ Safe 151:255–265
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z et al (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotox Environ Safe 96:242–249
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Zhou WJ (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Giri J (2011) Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behave 6:1746–1751
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22:1534–1544
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous proline and glycine betaine mediated up regulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Bio Med Res Int 2014:757219
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophy 125:189–198
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272
Hu YA, Liu XP, Bai JM, Shih KM, Zeng EY, Cheng HF (2013) Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environ Sci Pollut Res 20:6150–6159
Hussain A, Ali S, Rizwan M, Rehman MZ, Hameed A, Hafeez F, Wijaya (2018) Role of zinc–lysine on growth and chromium uptake in rice plants under Cr stress. J Plant Growth Regul 37:1413–1422
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2015) Glycinebetaine mediates chromium tolerance in mung bean through lowering of C uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662
Khan MU, Malik RN, Muhammad S, Ullah F, Qadir A (2015) Health risk assessment of consumption of heavy metals in market food crops from Sialkot and Gujranwala districts, Pakistan. Human Ecol Risk Assess Int J 21:327–337
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic bio-membranes. In Methods Enzymol 148:350–382
Ma J, Lv C, Xu M, Chen G, Lv C, Gao Z (2016) Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ Sci Pollut Res 23(2):1768–1778
Metzner H, Rau H, Senger H (1965) Untersuchungen zur Synchronisierbakeit einzelner Pigmentmangel-Mutanten von Chlorella. Planta 65:186–194
Middleton EM, Teramura AH (1993) The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol 103:741–752
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Monni S, Salemaa M, Millar N (2000) The tolerance of Empetrum nigrum to copper and nickel. Environ Pollut 109(2):221–229
Nomura T, Iwadare M, Serizawa M, Ozawa K, (1998) A bitrate and band width scalable CELP Coder. Proceeding of the IEEE International Conference on Acoustics Speech and Signal Processing May 12–15. IEEE Xplore Press, Seattle WA, 1998, 341–344
Pradas-del-Real AE, Garcia-Gonzalo P, Alarcon R, Gonzalez-Rodriguez A, Lobo MC, Perez Sanz A (2013) Effect of genotype, Cr (III) and Cr (VI) on plant growth and micronutrient status in Silene vulgaris (Moench). Spanish J Agric Res 11:685–694
Rasheed R, Ashraf AF, Hussain I, Haider MZ, Kanwal U, Iqbal M (2014) Exogenous proline and glycinebetaine mitigate cadmium stress in two genetically different spring wheat (Triticum aestivum L.) cultivars. Braz J Bot 37:399–406
Raza MAS, Saleem MF, Moazzam JM, Khan I (2014) Impact of foliar applied glycinebetaine on growth and physiology of wheat (Triticum aestivum L.) under drought. Pak J Agric Sci 51:327–334
Rebhi AE, Lounici H, Lahrech MB, Morel JL (2019) Response of Artemisia herba alba to hexavalent chromium pollution under arid and semi-arid conditions. Int J Phytoremed 21(3):224–229
Rodriguez E, Santos C, Azevedo R, Moutinho-Pereira J, Correia C, Dias MC (2012) Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem 53:94–100
Samantaray S, Das S, Mohanty RC, Mohanty M, Pradhan C (2015) Altered germination index and chlorophyll biosynthesis in seedlings of wild rice cultivars in response to hexavalent chromium stress. Discovery 27:27–35
Shahbaz M, Zia B (2012) Does exogenous application of glycinebetaine through rooting medium alter rice (Oryza sativa L.) mineral nutrient status under saline conditions? J Appl Bot Food Qual 84:54–60
Shahbaz M, Masood Y, Perveen S, Ashraf M (2011) Is foliar-applied glycinebetaine effective in mitigating the adverse effects of drought stress on wheat (Triticum aestivum L.)? J Appl Bot Food Qual 84:192–199
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK et al (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254
Tisarum R, Theerawitaya C, Samphumphuang T, Singh HP, Chaum S (2019) Foliar application of glycinebetaine regulates soluble sugars and modulates physiological adaptations in sweet potato (Ipomoea batatas) under water deficit. Protoplasma:1–15
Xin ZL, Mei G, Shiqing L, Shengxiu L, Zongsuo L (2011) Modulation of plant growth, water status and antioxidantive system of two maize (Zea may L.) cultivars induced by exogenous glycinebetaine under long term mild drought stress. Pak J Bot 43:1587–1594
Yadav K, Singh NB (2013) Effects of benzoic acid and cadmium toxicity on wheat seedlings. Chilean J Agric Res 73:168–174
Yang X, Lu C (2005) Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol Plant 124:343–352
Yildirim E, Ekinci M, Turan M, Dursun A, Kul R, Parlakova F (2015) Roles of glycine betaine in mitigating deleterious effect of salt stress on lettuce (Lactuca sativa L.). Arch Agron Soil Sci 61:1673–1689
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. - In: Zhang XZ (ed.): Research methodology of crop physiology. Pp. 208-211. Agriculture Press, Beijing
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhao Y, Hu C, Wang X, Qing X, Wang P, Zhang Y, Zhang X, Zhao X (2019) Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotox Environ Safe 173:314–321
Funding
The authors are thankful for the financial support from the Government College University, Faisalabad, and Higher Education Commission, Pakistan, under HEC Project No. 20-3653/NRPU/R&D/HEC/14/437 and NRPU Project No. 5634/Punjab/NRPU/R&D/HEC/2016. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group no. RGP-199.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, R., Ali, S., Abid, M. et al. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ Sci Pollut Res 27, 1101–1111 (2020). https://doi.org/10.1007/s11356-019-06761-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06761-z