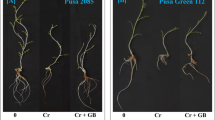
Abstract
Little information is available on the role of glycinebetaine (GB) in chromium (Cr) tolerance while Cr toxicity is widespread problem in crops grown on Cr-contaminated soils. In this study, we investigated the influence of GB on Cr tolerance in wheat (Triticum aestivum L.) grown in sand and soil mediums. Three concentrations of chromium (0, 0.25, and 0.5 mM) were tested with and without foliar application of GB (0.1 M). Chromium alone led to a significant growth inhibition and content of chlorophyll a, b, proteins and enhanced the activity of antioxidant enzymes. Glycinebetaine foliar application successfully alleviated the toxic effects of Cr on wheat plants and enhanced growth characteristics, biomass, proteins, and chlorophyll contents. Glycinebetaine also reduced Cr accumulation in wheat plants especially in grains and enhanced the activity of antioxidant enzymes in both shoots and roots. This study provides evidence that GB application contributes to decreased Cr concentrations in wheat plants and its importance in the detoxification of heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Number of leather tanning industries has rapidly increased in Pakistan and worldwide. Salts containing Cr are frequently used in tanning industries ( Anwaar et al. 2014). Without proper management, tanning waste has become a major source of Cr accumulation in soils (Rafique et al. 2010; Shakir et al. 2012; Ali et al. 2013a) along with other anthropogenic activities such as mining and electroplating (Oliveira 2012; Singh et al. 2002). Among heavy metals, Cr is considered to have high toxicity in plants, animals, and humans. Cr accumulation in agricultural soils is of great concern due to the adverse effects on crop growth and food safety (Oliveira 2012; Farid et al. 2013a, b). In many plant species, Cr toxicity decreased plant growth, caused chlorosis in leaves, damaged roots, and decreased grain yield (Rodriguez et al. 2012; Ali et al. 2013a; Gill et al. 2014; Singh et al. 2002). In addition, Cr is phytotoxic due to the generation of reactive oxygen species (ROS), which could lead to severe oxidative damage in plants. Thus, Cr-induced oxidative stress in plants can harm the production of photosynthetic pigments and protein contents (Shanker et al. 2005; Dey et al. 2009; Rodriguez et al. 2012). To scavenge ROS production, plants may develop an antioxidative system through enhancing the activity of antioxidant enzymes, e.g., superoxide dismutase (SOD), peroxidase (POD), and ascorbate peroxidase (APX) under metal stress (Mittler 2002). However, under severe stress conditions, the antioxidant capacity of plants may not be sufficient to prevent toxic effects of metal stress which decreased plant growth and yield (Gill et al. 2015; Farooq et al. 2013).
A large number of approaches have been used to reduce the Cr uptake and toxicity in crop plants. One such approach is the exogenous application of osmoprotectants to reduce the metal toxicity in crop plants under stressful environmental conditions. Glycinebetaine (N,N,N-trimethylglycine, abbreviated as GB) is a quaternary ammonium compound that is found in plants and mammals, etc. (Chen and Murata 2011). It is a very important osmoregulation substance, and its level varies considerably among plant species. Glycinebetaine is environmentally safe, nontoxic, and water-soluble (Mäkelä et al. 1996). It has been reported that level of GB increased in plants subjected to abiotic stresses (Dhir et al. 2012). However, in many other plant species, no GB is detectable under either normal or stressful conditions. The natural accumulation of GB, however, is not enough to protect plants from abiotic stresses. Under such condition, exogenous application of GB may help to reduce the adverse effects of various environmental stresses (Hossain et al. 2010; Islam et al. 2010). Recently, there is strong evidence that GB plays an important role in plants against tolerance to abiotic stresses (Giri 2011). Glycinebetaine increased salt tolerance in rice seedlings by increasing the activities of antioxidant enzymes such as CAT and APX (Hasanuzzaman et al. 2014). Exogenous GB enhanced drought tolerance in wheat plants by improving transpiration rate and photosynthesis and uptake of nutrients (Raza et al. 2014) and in Lentil decreased the oxidative stress by reducing H2O2 levels (Molla et al. 2014). Recently, GB application reduced Cd toxic effects in rice plants grown hydroponically by increasing growth and chlorophyll contents (Cao et al. 2013). Moreover, Pb toxicity was reduced by GB foliar application in cotton plants (Bharwana et al. 2014; Farid et al. 2013b). However, the mechanisms of GB-mediated reduction of metal toxicity in plants are still unknown. More precisely, little information is available on the effect of GB under Cr toxicity in plants.
Wheat (Triticum aestivum L.) is one of the main staple foods consumed in Pakistan and worldwide with the production of 24.21 million tons from an area of 8.66 million hectares in 2013 in Pakistan (Siddique et al. 2000). Wheat is the third most produced crop after maize and rice worldwide (FAO 2013). Wheat is more sensitive to Cr toxicity as compared to other crops (Dey et al. 2009; Diwan et al. 2012). Pakistan is facing land crisis on one hand and increase in population on the other hand. Therefore, cultivation of nonfood crops just for the sake of remediation purposes ends up as a futile practice. Thus, preference should be given to food crops for phytoremediation along with different techniques to counteract metal toxicities.
Although there are several reports on the physiological role and biosynthetic pathway of GB in many plant species, few investigations have been reported on the effects of exogenous GB on both growth and antioxidant defense system in wheat under Cr stress. We hypothesized that exogenous application of GB could enhance the capacity of wheat plants by affecting growth and antioxidant activity under Cr stress. Hence, present study was carried out to analyze the potential of foliar applied GB against Cr stress in wheat plants grown in soil and sand medium and its effects on plant growth, photosynthetic pigments, Cr uptake, and antioxidant activities in protecting the plants from Cr toxicity.
Materials and methods
Plant materials and growth conditions
Two types, soil and sand, of growth mediums were used in the study. Sand and soil were collected from botanical garden of Government College University, Faisalabad, Pakistan. The soil was homogenized, air-dried under room temperature, and passed through 2-mm mesh. Physicochemical properties of soil are given in Table 1. Sand was washed thoroughly with distilled water, air-dried, and passed through 2-mm mesh.
The wheat (T. aestivum L. cv. Lasani 2008) seeds were surface sterilized with 3 % H2O2 for 10 min then thoroughly washed with double distilled water. Seeds were sown in plastic pots each containing 5 kg of sand or soil. All treatments were performed in three replicates. The pot experiment was conducted in a botanical garden at 18–25 °C and 70 % humidity at the time of sowing and 30–35 °C and 85 % humidity at the time of harvesting. Wheat seedlings were thinned to 15 individuals per pot after 15 days of germination, and the pulled up plants were crushed carefully into the same pot. Each soil pot was fertilized with a 500-ml solution containing 2.19 g l−1 N (as (NH2)2CO), 0.5 g l−1 P (as (NH4)2HPO4), and 2.14 g l−1 K (as K2SO4). Half of the fertilizer solution was applied after 15 days of germination and remaining half after 30 days of germination. Each sand pot was fertilized with modified Hoagland solution (Sigma) containing following macroelements: 0.2 M Ca(NO3)2, 0.09 M MgSO4, 0.4 M KH2PO4, 0.01 M FeSO4, 0.3 M KNO3, and microelements: 0.4 mM CuSO4, 1.4 mM ZnSO4, 0.5 mM H3BO3, 10 mM H2MoO4 when required (Kanwal et al. 2014). All plastic and glass wares were rinsed with 10 % HNO3 and then washed with distilled water till neutral pH.
Treatments
After 30 days of germination, both soil and sand pots were irrigated with Cr as K2Cr2O7 solution (500 ml pot−1) of 0, 0.25, and 0.5 mM in 7-day interval, respectively, with three replications. The GB in 0.1 % Tween-20 solution was applied at 0 and 100 mM on the leaves until runoff at both tillering and booting vegetative stages. The experiment was arranged as a complete randomized design (CRD). Pots were regularly rotated, and weeds were removed manually.
Plant sampling and analysis
Plants were harvested after 120 days of treatments by cutting the shoots approximately 1 cm above the soil and sand surface. Growth parameters including plant height, root length, kernel length, and number of tillers per plants were measured. Plant samples were separated into grains, husk, leaves, shoots, and roots. All samples were washed with tap water then with 2 % HCl and then with double distilled water thoroughly to remove any aerial deposition. Samples were oven-dried at 70 °C until a constant weight was reached and then weighed.
Pigment content assay
After 90 days of treatments, chlorophylls and carotenoid contents were determined in the uppermost fully extended fresh leaves. Pigments were extracted by incubating leaves in 85 % (v/v, Sigma) aqueous acetone by continuous shaking at 4 °C under darkness until color had completely disappeared from the leaves. Samples were centrifuged at 4000 rpm for 10 min at 4 °C, and supernatant was taken. Light absorbance at 663, 644, and 452.5 nm was determined by spectrophotometer (Halo DB-20/DB-20S, Dynamica Company, London, UK) (Metzner et al. 1965). The concentrations of chlorophylls and carotenoids were calculated by using the adjusted extinction coefficients and equations (Lichtenthaler 1987).
Chromium content analysis
Plant samples (0.5 g) were digested by adding 15 ml of concentrated HNO3 (Sigma) in a 100-ml flask; then, mixture was placed on a hot plate containing temperature up to 275 °C and dens yellow fumes appeared from the samples. When quantity of dens yellow fumes become low, then hydrogen peroxide was added until fumes disappeared. Flasks were removed from the hot plate when sample color disappeared and the volume was made up to 25 ml by using distilled water. Cr contents in samples were determined by using flame atomic absorption spectrometry (AAS) made of novA A400 Analytik Jena, Germany, followed by Ehsan et al. (2013).
The concentration of Cr in plant samples was measured by the following formula:
Cr concentration (mg kg−1 DW) = reading of AAS × dilution factor / dry wt. of plant part.
Evaluation of antioxidant enzymes and protein content
After 90 days of treatment, the content of soluble protein was analyzed by using coomassie brilliant blue G-250 as dye and bovine serum albumin as a standard (Bradford 1976). In brief, frozen samples were ground in liquid nitrogen with mortar and pestle and then homogenized in 10 ml of 50-mm sodium phosphate buffer of pH 7.0 having 1 mm EDTA-Na2 and 2 % (w/v) polyvinyl pyrolidine-40 (PVP-40). Homogenate was centrifuged at 4 °C for 15 min at 11,000 rpm. The supernatant was collected and used for the analysis of the activities of antioxidant enzymes. One milliliter (1 ml) of Bradford solution was further added to 100-μl crude extract plus absorbance documented at the wavelength of 595 nm for the evaluation of entire protein content.
Catalase (CAT) (EC 1.11.1.6) activity was determined according to Aebi (1984). The assay mixture (3.0 ml) was composed of 100-μl enzyme extract, 100 μl H2O2 (300 mM), and 2.8-ml 50 mM phosphate buffer with 2 mM CA (pH 7.0). The CAT activity was assayed by monitoring the decrease in the absorbance at 240 nm as a consequence of H2O2 disappearance (ε = 39.4 mM−1 cm−1).
Ascorbate peroxidase (APX) (EC 1.11.1.11) activity was assayed according to the method of Nakano and Asada (1981). The reaction mixture consisted of 100-μl enzyme extract, 100 μl ascorbate (7.5 mM), 100 μl H2O2 (300 mM), and 2.7-ml 25 mM potassium phosphate buffer with 2 mM CA (pH 7.0). The oxidation activity of ascorbate was determined by the change in wavelength at 290 nm (ε = 2.8 mM−1 cm−1).
Statistical analysis
Data presented are means of three replicates. Analysis of variance (ANOVA) was done by using a statistical package, SPSS version 16.0 (SPSS, Chicago, IL) followed by Tukey’s post hoc test between the means of treatments to determine the significant difference.
Results
Effect of exogenous glycinebetaine on plant growth and biomass
Wheat plant height, root length, kernel length, and number of tillers per plant significantly decreased with increasing Cr levels as compared with control in both sand and soil mediums (Fig. 1). Furthermore, the reduction was more obvious in sand culture and at higher Cr (0.5 mM) treatment as compared to soil culture. Foliar application of GB significantly increased these plant parameters as comparative to respective Cr treatments alone. In control plants, application of GB slightly decreased plant growth characteristics as compared to without GB application both in sand and soil cultures except number of tillers in sand culture where we observed opposite trend.
Effects of various chromium (Cr) concentrations and glycine betaine on plant height (a), root length (b), kernel length (c), and number of tillers (d) of wheat plants grown in sand and soil medium separately. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05according to the Tukey’s test for sand and soil medium separately
Shoot dry weight significantly decreased with Cr addition in soil culture while shoot dry slightly increased in sand culture (Fig. 2a). Foliar GB application significantly increased shoot dry weight as compared to the respective Cr treatment alone except in control plant where we observed opposite trend. Root dry weight significantly decreased with Cr application in both cultures (Fig. 2b). Foliar GB application significantly increased root dry weight as compared to the respective Cr treatment alone except in control plant in which root dry weight decreased. Husk and kernel weight significantly decreased under Cr stress alone as compared to control (Fig. 2c, d). Exposure of wheat seedlings to GB significantly increased husk and kernel weights as compared to respective Cr treatment alone except in control plants where husk and kernel weights were decreased with GB as compared to without GB.
Shoot weight (a), root weight (b), husk weight (c), and kernel weight (d) of wheat plants grown in sand and soil medium separately and exposed to various Cr concentrations without and with foliar applied glycine betaine. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05 according to the Tukey’s test for sand and soil medium separately
Effect of exogenous glycinebetaine on photosynthetic pigments and protein contents
Total chlorophyll and carotenoid concentrations significantly decreased with increasing Cr stress as compared to control in both cultures (Fig. 3a, b). There was decreasing trend in both chlorophylls and carotenoid concentrations with increasing Cr levels in both mediums. Total chlorophyll concentration significantly increased with foliar applied GB under Cr stress as compared to Cr treatments alone. Carotenoid concentration significantly increased with GB foliar application under Cr stress as compared to the respective Cr alone treatments except control plants (Fig. 3b). Foliar applied GB decreased carotenoid concentration in control plants as compared to without GB.
Total chlorophyll (a), carotenoids (b), leaf and root protein (c, d) concentrations of wheat plants grown in sand and soil medium separately and exposed to various Cr concentrations with and without foliar applied glycine betaine. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05according to the Tukey test for sand and soil medium separately
The evaluation of protein contents also indicated significant effects of GB treatment on Cr stressed and unstressed wheat plants (Fig. 3c, d). Leaf and root protein concentration significantly decreased with increasing Cr levels in both culture mediums. Foliar applied GB under Cr stress significantly increased protein concentrations in both leaves and roots relative to the Cr alone treatments.
Effect of exogenous glycinebetaine on chromium concentration
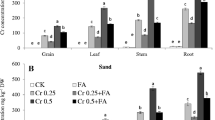
As shown in Fig. 4, exposure to Cr alone caused significant increase in Cr concentration in grains, leaves, stem, and roots of wheat plants with increasing Cr levels in both culture mediums. Increase in Cr levels also increased Cr concentrations in all plant parts. Roots accumulated largest Cr concentration followed by stem, leaf, and grains in soil-cultured wheat plants. In sand culture, largest Cr concentration was also in roots followed by leaf, stem, and grains. GB decreased Cr concentration in all plant parts as compared to respective Cr treatments alone.
Chromium concentration in grain, leaf, stem, and root of wheat plants grown in soil (a) and sand (b) medium separately and exposed to various Cr concentrations with and without foliar applied glycine betaine. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05 according to the Tukey’s test for sand and soil medium separately
Effect of exogenous glycinebetaine on antioxidant enzyme activities
Chromium stress led significant alteration in antioxidant defense in leaves and roots of wheat plants (Fig. 5). In comparison with control, leaf and root CAT and APX activities significantly decreased in response to Cr stress in both soil and sand cultures (Fig. 5). Seedlings treated with foliar GB under Cr stress had significantly higher CAT and APX activities as compared to the seedlings with respective Cr alone treatment in both cultures. It means that foliar applied GB caused an increase in antioxidant enzyme activity in Cr stressed wheat plants.
Leaf and root catalase (a, b) and ascorbate peroxidase (b, c) activities of wheat plants grown in sand and soil medium separately and exposed to various Cr concentrations without and with foliar applied glycine betaine. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05 according to the Tukey’s test for sand and soil medium separately
Discussion
Recently, Cr contamination in agricultural soils has become an environmental issue worldwide. In present study, we analyzed the possible role of exogenous GB on growth and antioxidant defense system and Cr uptake against Cr stress in wheat.
Chromium effect on plant morphology and composition
In the current study, plant growth characteristics and biomass were severely decreased with increasing Cr levels in both cultures (Figs. 1 and 2). Chromium stress-induced plant growth inhibition has already been well documented by many researchers in many plant species including wheat (Dey et al. 2009; Diwan et al. 2012; Ali et al. 2013b). It has been observed that as the Cr uptake increased, plant growth was affected because Cr is known to interact with essential micronutrients for plant growth and limit their availability by the decreased uptake (Pradas-del-Real et al. 2013). Chromium affects plant growth adversely which depends upon its concentration and plant species (Diwan et al. 2012; Gill et al. 2015). In present study, reduction in root length could be due to the accumulation of Cr in roots (Fig. 4) and/or due to damage of root tip cells (Ali et al. 2013c). Decrease in shoot growth due to Cr might be due to ultrastructural damages of leaf mesophyll cells and oxidative stress (Gill et al. 2015).
In addition to growth and biomass decrease, other parameters were affected: total chlorophyll concentrations decreased with increasing Cr concentrations in plants (Fig. 3), indicating that chlorophyll content may give early signal of Cr toxicity in wheat. Although the general agreement in literature is that excess Cr induces a decrease in photosynthetic pigments in plants including wheat (Dey et al. 2009). Chromium-induced reduction in photosynthetic pigments has been attributed to alteration of chloroplast ultrastructure and inhibition of photosynthetic pigments, gas exchange parameters due to decomposition of chl a, b, and carotenoids by increasing chlorophyllase activities (Gill et al. 2015; Ali et al. 2011; Hegedus et al. 2001). Reduction in chlorophylls may also be due to the ROS generation in plants under metal stress (Ehsan et al. 2014). Carotenoid concentration also decreased under Cr stress (Fig. 3b). Decrease in carotenoid under metal stress has already been observed in wheat (Yadav and Singh 2013). Decrease in carotenoid content is harmful to plants because carotenoid protects the chlorophyll from photooxidative destruction (Middleton and Teramura 1993). Total soluble protein concentrations decreased in leaves and roots under Cr stress as compared to their respective controls (Fig. 3c, d). These results are in line with the findings of Gill et al. (2015). Metal stress is known to produce ROS (Habiba et al. 2015) which caused modification/degradation of proteins. In present study, the decrease in protein concentration may be due to negative effect of Cr on photosynthetic pigments (Fig. 3a, b) or due to increased Cr uptake (Fig. 4).
Chromium concentration increased in wheat plants irrespective of culture medium (Fig. 4). Chromium concentration in roots was larger followed by stem, leaves, and grains indicating that the root is the main part for Cr accumulation in a wheat plant. Larger Cr concentration in roots has also been observed in many plant species such as barley (Ali et al. 2013a) and Brassica napus (Gill et al. 2015). Increased Cr sequestration in roots may be due to precipitation of Cr as insoluble salts or due to immobilization with other molecules such as sugar, pectins, celluloses, and hemicelluloses as proposed by Shahid et al. (2013) or due to compartmentalization in vacuoles of root cell (Ali et al. 2013b).
Plants grown in sand culture accumulated larger Cr concentration in all plant parts as compared with soil culture (Fig. 4). Rizwan (2012) studied the effect of different minerals, sand, clay, and amorphous silica, mixtures on the availability of Cd and Cu to wheat plants. Author reported that bioavailable (DTPA-extracted) Cd and Cu concentrations increased with increasing sand proportion in the mineral mixtures. Metal concentrations also increased in wheat shoots and roots while other elements such as silicon decreased with increasing sand proportion in the mineral mixtures that resulted decrease in biomass. In present study, high Cr concentrations in sand culture might be due to larger availability of Cr while in soil culture, Cr may be immobilized, and as a result, less Cr was accumulated by the plants. Other possible explanation of increased Cr in sand culture is that in soil culture, there was more competition between Cr and other nutrients as compared to sand culture. Moreover, larger Cr uptake by the plants grown in sand might be due to lower cation exchange capacity of sand as compared to soil (Suganya and Sivasamy 2006) or due to other abiotic factors such as pH, organic matter, and texture (Sherene 2010). This higher Cr concentration in all plant parts might resulted decrease in growth characteristics, biomass, and photosynthetic pigments in sand-grown plants as compared to soil-grown plants (Figs. 1, 2, and 3).
In the present study, the activities of CAT and APX were decreased in both shoots and roots under Cr concentrations (Fig. 5). These results are in line with the findings of Dey et al. (2009) who also reported that Cr, 50 and 100 ppm, stress decreased the activity of CAT in 7-day-old wheat plants grown in hydroponics. However, Gill et al. (2015) reported that CAT activity increased while APX activity decreased in B. napus under Cr stress. Contrary to our findings, it has been reported that leaf APX activity increased under Cd stress in wheat (Khan et al. 2008) and Indian mustard (Mobin and Khan 2007). The reduction in antioxidant enzyme activities might be due to higher Cr concentration in wheat plants which declined the plant defense mechanism against abiotic stress. The decline in CAT and APX activities in Cr stressed plants suggests the declining efficiency of antioxidants to scavenge ROS, and thereby increasing the chances of their accumulation.
The decrease in growth and biomass, loss of total chlorophyll, alteration in the activities of CAT and APX, and the increase in Cr content in wheat subjected to Cr stress suggests the possibility of oxidative stress situation in the plant.
Mitigation of Cr toxicity in wheat by GB foliar application
In our present study, it was found that GB markedly alleviated Cr-induced reduction in growth and biomass (Figs. 1 and 2). Exogenous application of GB enhanced tolerance to abiotic stresses in many plant species such as drought stress in wheat and lentil (Raza et al. 2014; Molla et al. 2014) and salinity stress in rice (Hasanuzzaman et al. 2014). Chen and Murata (2011) summarized that foliar applied GB is efficiently translocated from source to sink tissues via the phloem. Increase in biomass with GB might be due to enhanced uptake of nutrients, such as nitrogen, phosphorus, and potassium, by plants under stressful conditions (Shahbaz et al. 2011). Moreover, the increase in growth and biomass might be due to positive effects of GB on net CO2 assimilation rate, transpiration rate, and substomatal CO2 concentration of wheat plants as reported in sunflower lines with foliar applied GB under water limited conditions (Iqbal et al. 2009). Under abiotic stress, GB may activate the expression of genes for ROS-scavenging enzymes, and as result, photosynthetic machinery is protected which positively affected plant growth and biomass of wheat plants (Einset et al. 2008). Additionally, GB may protect CO2-fixing enzymes such as Rubisco and Rubisco activase under abiotic stress which, in turn, depresses the production of ROS (Chen and Murata 2011), and as a result, plant growth may increase under Cr stress.
To best of our knowledge, studies are scarce about the role of GB on plant growth under metal stress. Increase in growth and biomass with foliar applied GB might be due to reduction of oxidative stress and enhanced activity of antioxidant enzymes under metal stress (Bharwana et al. 2014). In our study, the increase in plant growth under GB application may be due to lower Cr uptake and translocation in wheat plants (Fig. 4) which reduced the toxic effects on plants and as a results wheat growth enhanced (Fig. 2). Total chlorophylls and carotenoid concentration increased with GB application under Cr stress (Fig. 3a, b). It has been reported that under heat and drought stress in wheat plants, overaccumulation of GB alleviated the damage of chloroplast ultrastructure, thylakoid lamellae and improved the photosynthetic capacity by increasing stomatal conductance (Wang et al. 2010a). In another study, they also reported GB-mediated increase in the chlorophyll contents and gas exchange characteristics of wheat under drought and heat stress (Wang et al. 2010b). In present study, increase in photosynthetic pigments may be due to the reduced Cr concentration in leaves of GB applied plants (Fig. 1) and due to enhanced activities of antioxidant enzymes (Fig. 5). Application of GB increased the protein contents in leaves and roots of wheat plants (Fig. 3c, d). It has been reported that GB reduced protein carbonylation and increased the level of the reduced form of glutathione under salt stress (Hoque et al. 2008). Glycinebetaine maintain protein activity in the chloroplast under stress conditions (Nomura et al. 1998). In present study, increase in protein production might be due to positive effect of GB on chloroplast and plant growth.
Foliar application of GB decreased Cr uptake by different plant parts as compared to Cr alone treatment (Fig. 4). GB-mediated reduction in heavy metals such as Cd and Pb has already been reported in other plant species (Islam et al. 2010; Hossain et al. 2010; Cao et al. 2013; Bharwana et al. 2014). Decrease in Cr uptake with GB application might be due to the protective role of GB in the cell membranes, and as a result, less Cr enters the cytoplasm (Giri 2011). Another possible explanation of decreased Cr uptake with GB might be due to competition of Cr with other nutrients. The increased uptake of nutrients with foliar applied GB may compete with Cr uptake. Shahbaz et al. (2011) reported that foliar applied GB increased nutrients, NPK, in wheat under drought stress. Contrarily, Cao et al. (2013) reported that GB 24-h pretreatment decreased the Fe and Mn in shoots/roots of rice plants. Thus, the varying uptake and distribution of nutrients may involve in the plant tolerance against Cr stress, but these mechanisms are remained to be explored. However, the increase and/or decrease in nutrients with GB might be due to difference of plant species or GB application methods.
Under stressful conditions, such as Cr, plants have evolved antioxidant enzyme systems including CAT and APX. Antioxidant enzymes play a key role in plant defense against oxidative stress by H2O2 scavenging. In present study, GB supplemented Cr-stressed seedlings showed a sharp increase in CAT and APX activities as compared to the control as well as Cr alone treated seedlings (Fig. 5). Similar effects of GB on CAT activity were also observed in response to various stresses (Islam et al. 2010; Cao et al. 2013; Bharwana et al. 2014). Park et al. (2004) reported that in tomato plants, GB enhanced expression of CAT genes under chilling stress and as a result enhanced activity of catalase which scavenge ROS. Contrarily, Hossain et al. (2010) reported that CAT activity was decreased in Vigna radiata under Cd stress. This decrease and/or increase in antioxidant activites might be due to the difference of methods, metals, plant species, and growth condition. Increase in APX activity with GB under Cd stress has been reported in V. radiata (Hossain et al. 2010) and under Pb stress in cotton (Bharwana et al. 2014). It has been reported that under severe stress, the activities of antioxidant enzymes reduced due to overproduction of ROS (Chen and Murata 2011). In the presence of GB, increase in CAT and APX activities might be due to reduction of ROS generation. In present study, increase in CAT and APX activities with GB might also be due to lower Cr uptake by the plants (Fig. 4). Our results suggest that exogenous GB could contribute in the detoxification of ROS by enhancing APX and CAT activities under Cr stress. Increase in antioxidant enzyme activity with GB application under Cr stress might be one of the possible mechanisms of GB-induced metal tolerance in plants.
Conclusion
In conclusion, our results indicated that Cr affected wheat growth, photosynthetic pigments, and protein contents which were accompanied by a significant increase in Cr concentration in plant and decrease in antioxidant enzyme activity. By contrast, foliar application of GB alleviated Cr toxicity and thus increased growth and biomass of wheat plants by preventing Cr accumulation and enhancing antioxidant capacity when compared with the Cr treatment alone. Thus, the data indicate that exogenous application of GB can minimize health risks by preventing Cr accumulation in important grain crops such as wheat. However, information about the effect of exogenous GB application on metal uptake by different plant species and the mechanisms of tolerance are still limited and must be further studied.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ali S, Zeng F, Cai S, Qiu B, Zhang GP (2011) The interaction of salinity and chromium in the influence of barley growth and oxidative stress. Plant Soil Environ 57:153–159
Ali S, Farooq MA, Jahangir MM, Abbas F, Bharwana SA, Zhang GP (2013a) Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biol Plant 57:785–791
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang GP (2013b) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang G (2013c) Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem 32:2234–2239
Ali B, Huang CR, Qi ZY, Ali S, Daud MK, Geng XX, Zhou WJ (2013d) 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Env Sci Pollut Res 20:7256–7267
Anwaar SA, Ali S, Ali S, Ishaque W, Farid M, Farooq MA, Sharif M (2014) Silicon (Si) alleviates cotton (Gossypiumhirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ Sci Pollut Res 22: 3441-3450
Bharwana SA, Ali S, Farooq MA, Iqbal N, Hameed A, Abbas F, Ahmad MSA (2014) Glycinebetaine-induced lead toxicity tolerance related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. Turk J Bot 38:281–292
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao F, Liu L, Ibrahim W, Cai Y, Wu F (2013) Alleviating effects of exogenous glutathione, glycinebetaine, brassinosteroids and salicylic acid on cadmium toxicity in rice seedlings (OryzaSativa). Agrotechnology 2:1
Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Dey SK, Jena PP, Kundu S (2009) Antioxidative efficiency of TriticumaestivumL. exposed to chromium stress. J Environ Biol 30:539–544
Dhir B, Nasim SA, Samantary S, Sarivastva S (2012) Assessment of osmolyte accumulation in heavy metal exposed salivinanatans. Int J Bot 8:153–158
Diwan H, Ahmad A, Iqbal M (2012) Characterization of Chromium Toxicity in Food Crops and their Role in Phytoremediation. J Biorem Biodegrad 3:159
Ehsan S, Ali S, Noureen S, Farid M, Shakoor MB, Aslam A, Bharwana SA, Tauqeer HM (2013) Comparative assessment of different heavy metals in urban soil and vegetables irrigated with sewage/industrial waste water. Ecoterra 35:37–53
Ehsan S, Ali S, Noureen S, Mehmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of Cd by Brassica napus L. Ecotoxicol Environ Saf 106:164–172
Einset J, Winge P, Bones AM, Connolly EL (2008) The FRO2 ferric reductase is required for glycine betaine’s effect on chilling tolerance in Arabidopsis roots. Physiol Plant 134:334–341
Farid M, Ali S, Shakoor MB, Bharwana SA, Rizvi H, Ehsan S, Tauqeer HM, Iftikhar U, Hannan F (2013a) EDTA assisted phytoremediation of cadmium, lead and zinc. Int Agron Plant Prod 4:2833–2846
Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M, Hanif MS (2013b) Morphological, physiological and biochemical responses of different plant species to Cd stress. Int J ChemBio chem Sci 3:53–60
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249
Food and Agriculture Organisation of the United Nations (2013) http://faostat.fao.org/site/567/
Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I, Pereira, Tuteja N (2013) Glutathione reductase and glutathione: a boon in disguise for plant abiotic stress defense operations. Plant PhysiolBiochem 70:20–212
Gill RA, Hu XQ, Ali B, Yang C, Shou JY, Wu YY, Zhou WJ (2014) Genotypic variation of the responses to chromium toxicity in four oilseed rape cultivars. Biol Plant 58:539–550
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Giri J (2011) Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav 6:1746–1751
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22:1534–1544
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous Proline and Glycine Betaine Mediated Upregulation of Antioxidant Defense and Glyoxalase Systems Provides Better Protection against Salt-Induced Oxidative Stress in Two Rice (Oryzasativa L.) Varieties. Bio Med Res Int. doi:10.1155/2014/757219
Hegedus A, Erdel S, Horvath G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barely seedlings under Cd stress. Plant Sci 160:1085–1093
Hoque MA, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y (2008) Proline and glycinebetaine enhance antioxidant defence and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J Plant Physiol 165:813–824
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272
Iqbal N, Ashraf M, Ashraf MY (2009) Influence of exogenous glycinebetaine on gas exchange and biomass production in sunflower (Helianthus annuus L.) under water limited conditions. J Agron Crop Sci 195:420–426
Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y (2010) Exogenousproline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166:1587–1597
Kanwal U, Ali S, Shakoor MB, Farid M, Hussain S, Yasmeen T, Abbas F (2014) EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ SciPollut Res 21:9899–9910
Khan NA, Samiullah, Singh S, Nazar R (2007) Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticumaestivum) cultivars differing in yield potential under cadmium stress. J Agron Crop Sci 193:435–444
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152:686–692
Lichtenthaler HK (1987) Chlorophylls and carotenoids pigments of photosynthetic biomembranes In: Colowick SP, Kaplan NO (ed.): Methods Enzymol 148:350–382
Mäkelä P, Peltonen-Sainio P, Jokinen K, Pehu E, Setälä H, Hinkkanen R, Somersalo S (1996) Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Sci 121:221–230
Mäkelä P, Jokinen K, Kontturi M, Peltonen-Sainio P, Pehu E, Somersalo S (1998) Foliar application of glycinebetaine a novel product from sugar beet as an approach to increase tomato yield. Indian Crop Prod 7:139–148
Metzner H, Rau H, Senger H (1965) UntersuchungenzurSynchronisierbakeiteinzelnerPigmentmangel-Mutation von Chlorella. Planta 65:186–194 (in German)
Middleton EM, Teramura AH (1993) The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol 103: 741–752
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–10
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Molla MR, Ali MR, Hasanuzzaman M, Al-Mamun MH, Ahmed A, Nazim-ud-Dowla MAN, Rohman MM (2014) Exogenous Proline and Betaine-induced Upregulation of Glutathione Transferase and Glyoxalase I in Lentil (Lens culinaris) under Drought Stress. NotulaeBotanicaeHortiAgrobotanici Cluj-Napoca 42:73–80
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nomura M, Hibino T, Takabe T, Sugiyama T, Yokota A, Miyake H, Takabe T (1998) Transgenically produced glycinebetaine protects ribulose 1,5-bisphosphate carboxylase/oxygenase from inactivation in Synechococcus sp. PCC7942 under salt stress. Plant Cell Physiol 39:425–432
Oliveira H (2012) Chromium as an environmental pollutant: insights on induced plant toxicity. J Bot. doi:10.1155/2012/375843
Park EJ, Jeknic Z, Sakamoto A, Denoma J, Yuwansiri R, Murata N, Chen TH (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40:474–487
Pradas-del-Real AE, García-Gonzalo P, Alarcón R, González-Rodríguez A, Lobo MC, Pérez-Sanz A (2013) Effect of genotype, Cr (III) and Cr (VI) on plant growth and micronutrient status in Silene vulgaris (Moench). Span J Agri Res 11:685–694
Rafique U, Ashraf A, Khan AK, Nasreen S, Rashid R (2010) Toxic chromium from tanneries pollute water resources and soils of Sialkot (Pakistan). J Chem SocPak 32:644–649
Raza MAS, Saleem MF, Moazzam JM, Khan I (2014) Impact of foliar applied glycinebetaine on growth andPhysiology of wheat (triticumaestivum L.) Under drought. Pak J Agri Sci 51:327–334
Rizwan (2012) Silicon-mediated heavy metal tolerance in durum wheat: evidences of combined effects at the plant and soil levels. PhD thesis University of Aix-Marseille France
Rodriguez E, Santos C, Azevedo R, Moutinho-Pereira J, Correia C, Dias MC (2012) Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem 53:94–100
Shahbaz M, Masood Y, Perveen S, Ashraf M (2011) Is foliar-applied glycinebetaine effective in mitigating the adverse effects of drought stress on wheat (Triticumaestivum L.). J Appl Bot Food Qual 84:192–199
Shahid M, Ferrand E, Schreck E, Dumat C (2013) Behavior and impact of zirconium in the soil–plant system: plant uptake and phytotoxicity. Rev Environ ContamToxicol 221:107–127
Shakir L, Ejaz S, Ashraf M, Qureshi NA, Anjum AA, Iltaf I, Javeed A (2012) Ecotoxicological risks associated with tannery effluent wastewater. Environ Toxicol Pharmacol 34:180–191
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Inter 31:739–753
Sherene T (2010) Mobility and transport of heavy metals in polluted soil environment Cotton Research Station, Veppanthattai, Perambalur, Tamilnadu Biological Forum. An Int J 2:112–121
Siddique MRB, Hamid A, Islam MS (2000) Drought stress effects on water relations of wheat. Bot Bull Acad Sin 4i:35–39
Singh DB, Varma S, Mishra SN (2002) Putrescine effect on nitrate reductase activity, organic nitrogen, protein, and growth in heavy metal and salinity stressed mustard seedlings. Biol Plant 45:605–608
Suganya S, Sivasamy R (2006) Moisture retention and cation exchange capacity of sandy soil as influenced by soil additives. J Appl Sci Res 2:949–951
Tamas L, Dudikova J, Durcekova K, Haluskova L, Huttova J, Mistrik I, Olle M (2008) Alteration of the gene expression, lipid peroxidation, proline and thiol content along the barley root exposed to cadmium. J Plant Physiol 165:1193–1203
Wang GP, Li F, Zhang J, Zhao MR, Hui Z, Wang W (2010a) Over accumulation of glycinebetaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 48:30–41
Wang GP, Zhang XY, Li F, Luo Y, Wang W (2010b) Over accumulation of glycinebetaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica 48:117–126
Winge P, Einset J, Bones AM, Connolly EL (2008) The FRO2 ferric reductase is required for glycine betaine’s effect on chilling tolerance in Arabidopsis roots. Physiol Plant 134:334–341
Yadav K, Singh NB (2013) Effects of benzoic acid and cadmium toxicity on wheat seedlings. Chilean J Agric Res 73:168–174
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579:6265–6271
Acknowledgments
Thanks to Higher Education Commission of Pakistan for financial support. The results presented in this paper are a part of MSc studies of Tania Anwar.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Ali, S., Chaudhary, A., Rizwan, M. et al. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res 22, 10669–10678 (2015). https://doi.org/10.1007/s11356-015-4193-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4193-4