Abstract
Chromium (Cr) is among the most toxic pollutants in the environment that adversely affect the living organisms and physiological processes in different plants. The present study investigated the effect of 15 mg L−1 of 5-aminolevulinic acid (ALA) on morpho-physiological attributes of cauliflower (Brassica oleracea botrytis L.) under different Cr concentrations (0, 10, 100, and 200 μM) in the growth medium. The results showed that Cr stress decreased the growth, biomass, photosynthetic, and gas exchange parameters. Chromium stress enhanced the activities of enzymatic antioxidants, catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) in response to oxidative stress caused by the elevated levels of malondialdehyde (MDA), hydrogen peroxide (H2O2), and electrolyte leakage (EL) in both roots and leaves of cauliflower. Chromium concentrations and total Cr uptake were increased in leaves, stems, and roots with increasing Cr levels in the culture medium. Foliar application of ALA increased the plant growth parameters, biomass, gas exchange parameters, and photosynthetic pigments under Cr stress compared to the treatments without ALA. Foliar application ALA decreased the levels of MDA, EL, and H2O2 while further improved the performance of antioxidant in both leaves and roots compared to only Cr-stressed plant. Chromium concentrations and total Cr uptake were decreased by the ALA application compared to treatments without ALA application. The results of the present study indicated that foliar application of ALA might be beneficial in minimizing Cr uptake and its toxic effects in cauliflower.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Under natural conditions, agricultural crops are prone to numerous biotic and abiotic stresses such as heavy metals, diseases, drought, and salinity (Ahmad et al. 2014; Rizwan et al. 2015; Adrees et al. 2015a; Khan et al. 2016). Heavy metal (HM) toxicity in plants is the most widespread problem which not only limit the crop growth and yield but also enters in the system of humans and animals through the food chain (Liu et al. 2015; Khan et al. 2015a; Rizwan et al. 2016a). Chromium, (Cr) among HMs, mainly enters in the soil through anthropogenic activities (leather tanning, metallurgical, etc.) and causes toxic effects on plant growth, development, and yield (Shakir et al. 2012; Khan et al. 2015b). The Cr concentration in soil may vary greatly depending on the composition of natural rocks and sediments as well as anthropogenic activities (Oliveira 2012; Singh et al. 2013). Many studies have witnessed that Cr stress decreased the plant growth and yield, caused leaf chlorosis and root damage, and finally, resulted in plant death (Gill et al. 2015; Dheeba et al. 2015; Gill et al. 2015; Ali et al. 2015a). Moreover, Cr toxicity severely inhibited the synthesis of protein contents and photosynthetic pigments in plants (Rodriguez et al. 2012; Singh et al. 2013; Gill et al. 2016a). Furthermore, Cr toxicity caused the overproduction of reactive oxygen species (ROS) in different plants and induced the severe oxidative stress in plants (Gill et al. 2015, 2016b). Naturally, plants have a well-developed antioxidative defense system, which is comprised of key antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), superoxide dismutase (SOD), and ascorbate peroxidase (APX) to overcome the ROS generation. However, plants fail to cope with ROS generation, especially under sever metal stress conditions (Singh et al. 2013; Jabeen et al. 2016; Ali et al. 2015b).
Different strategies have been used to reduce the HMs’ toxicity, uptake, and accumulation in crop plants (Rehman et al. 2015; Adrees et al. 2015b; Rizwan et al. 2016b; Younis et al. 2016). One of such approach is the exogenous application of plant growth regulators (PGRs) to enhance the plant tolerance against metal toxicity (Ali et al. 2013a; Xu et al. 2015; Rizwan et al. 2016c). 5-Aminolevulinic acid (ALA) is considered pivotal PGRs being used against metal stress in plants (Ali et al. 2014). It has been widely reported by many researchers that exogenous application of ALA increased the activities of antioxidant enzymes, rate of photosynthesis, gas exchange characteristics, nutrient uptake, and improved the ultrastructural changes in Brassica napus under salt stress (Naeem et al. 2010, 2011, 2012). However, little information is available about the role of ALA under heavy metal stress (Ali et al. 2013a, 2014). Exogenous application of ALA improved the Brassica napus growth, mineral nutrients, and antioxidative defense system under cadmium (Cd) and lead (Pb) stresses (Ali et al. 2013a, 2014).
Vegetables are commonly grown in pre-urban soils contaminated with HMs, including Cr, mainly due to the application of raw city effluents and tannery wastewaters (Ali et al. 2015c; Liu et al. 2015). In addition, these vegetables are mainly irrigated with city effluent which contains not only plant nutrients but also toxic HMs (Khan et al. 2015b). Overall, vegetables grown on pre-urban soils may contain higher concentrations of HMs especially Cr in edible parts and thus increase the risk of food chain contamination (Younis et al. 2016). Keeping in view the importance of Cr contamination in vegetables, this study was aimed to alleviate the Cr toxicity and uptake in cauliflower through foliar application of ALA. Our hypothesis is that exogenous ALA may alleviate Cr toxicity and play an important role in metal tolerance in cauliflower by reducing oxidative damage through improving the activities of antioxidant enzymes. Thus, this study was carried out to evaluate the potential of ALA on cauliflower growth, biomass, Cr uptake, and activities of antioxidant enzymes in Cr-stressed conditions.
Materials and methods
Plant materials and growth conditions
Seeds of cauliflower were purchased from the Ayub Agricultural Research Institute (AARI), Faisalabad, Pakistan. Healthy and uniform seeds were surface sterilized with ethanol (70%) for 1 min, dipped in sodium hypochlorite (0.01 g mL−1) for 5 min, and, finally, rinsed with distilled water. Physiochemical characteristics of soil were electrical conductivity of soil extract, 2.9 dS m−1; pH, 6.6; texture, clay loam; organic matter, available phosphorus, 2.2 mg kg−1 0.3%; and sodium adsorption ratio, 6.5 (mmol−1)1/2. Initial soil HCO3, Cl−, SO4 2−, Ca2+, Na+, and K+ concentrations (mmol L−1) were 3.2, 2.3, 6.2, 3.5, 3.7, and 0.06, respectively. Available Cu, Zn, and Cr concentrations were 0.35, 0.85, and 0.04 mg kg−1, respectively. The pot experiment was conducted in a botanical garden at 18–25 °C and about 70% humidity. To prepare seedlings, seeds were sown in sand washed with 5% hydrochloric acid (HCl) and then washed thrice with distilled water. After 4 weeks of seed sowing, seedlings of similar size were carefully uprooted from sand and transplanted in pots containing 5 kg of soil. Each pot was fertilized with a 500 mL solution containing 0.5 g L−1 P as (NH4)2HPO4, 2.19 g L−1 N as (NH2)2CO, and 2.14 g L−1 K as K2SO4. Plants were regularly watered to maintain about 70% of the soil water holding capacity, and weeds were removed manually and buried in the same pot.
Treatments
After 4 weeks of transplantation, the plants were irrigated with 0, 10, 100, and 200 μM Cr solution (500-mL pot−1) in the form of K2Cr2O7 (Sigma) in 7-day interval. These Cr concentrations were selected based on our previous study indicating that 100 μM showed slight damage to plant growth while 400 μM Cr imposed a marked damage to plant growth (Gill et al. 2015). Hexavalent chromium [Cr(VI)] was selected as it is relatively more toxic than trivalent chromium [Cr(III)] due to its higher solubility and mobility in nature as compared to the Cr(III) (Singh et al. 2013). Plants were sprayed twice (at the time of first Cr treatment and then after 7 days) without and with 15 mg L−1 ALA solution (Cosmo Oil Co. Ltd., Japan) on upper and lower surface of the leaves until runoff. The experiment was performed as a complete randomized design (CRD) having three replications.
Plant sampling and analysis
After the imposition of Cr for 4 weeks, plants were harvested and washed thoroughly with distilled water and deionized water, respectively. Growth parameters including plant height, number of leaves per plant, root length, and leaf area were measured. Fresh weights of leaf, root, stem, and flower were weighed. These were dried at 70 °C in an oven for about 72 h and then again weighed for plant dry weights.
Photosynthetic pigments and gas exchange parameters
After 3 weeks of Cr imposition, carotenoid and chlorophyll contents were determined in the uppermost fully expanded leaf. Pigments were extracted from leaves by incubating in 85% (v/v, Sigma) aqueous acetone by continuous shaking at 4 °C in the dark until the color had completely disappeared from the leaves then centrifuged at 4000×g for 10 min at 4 °C, and supernatant was taken. Spectrophotometer (Halo DB-20/DB-20S, Dynamica Company, London, UK) was used to measure the light absorbance at 663, 644, and 452.5 nm (Metzner et al. 1965). The concentration of chlorophyll and carotenoid was determined by using the adjusted extinction coefficients and equations (Lichtenthaler 1987).
For gas exchange characteristics, uppermost fully expanded leaf was taken and infrared gas analyzer (IRGA) (Analytical Development Company, Hoddesdon, England) was used for the measurement of transpiration rate (E), net photosynthetic rate (Pn), stomatal conductance (gs), and water use efficiency (WUE). These parameters were taken between 10:00 a.m. and 11:00 a.m. when the plants were fully functional.
Determination of electrolyte leakage, malondialdehyde, and hydrogen peroxide
Electrolyte leakage (EL) was measured according to the method described by Dionisio-Sese and Tobita (1998). After 4 weeks of treatments, the topmost fully expanded leaves were taken and cut into small parts of about 5 mm length and positioned in the test tubes containing 8 mL deionized water. The electrical conductivity of initial medium (EC1) was assessed after placing the tubes in an incubator in a water bath at 32 °C for 2 h. Then, the samples were again autoclaved at 121 °C for 20 min, cooled to 25 °C, and again, electrical conductivity (EC2) was measured. Finally, EL was calculated by using the following formula:
Lipid peroxidation in the leaf tissue was determined by measuring the malondialdehyde (MDA) contents of the samples. For this, thiobarbituric acid (TBA) reaction was used according the method described by Heath and Packer (1968) with minor modifications (Dhindsa et al. 1981; Zhang and Kirham 1994). Fresh sample (0.25 g) was mixed in 5 mL of 0.1% trichloroacetic acid (TCA), and then, the mixture was centrifuged at 12,000×g for 15 min. To 1.0 mL aliquot of the supernatant, 4 mL of 20% TCA comprising 0.5% TBA was added, heated at 95 °C for 30 min, and then rapidly cooled in an ice bath and centrifuged at 10,000×g for 10 min. The absorbance of the supernatant was measured at 532 nm, and value for non-specific absorbance at 600 nm was subtracted. The MDA content was calculated by means of an extinction coefficient of 155 mM−1 cm−1.
Hydrogen peroxide (H2O2) was determined by homogenizing 50 mg root/leaf soft tissue in 3.0 mL of phosphate buffer (50 mM, pH 6.5). The homogeneous mixture was centrifuged at 6000×g for 30 min. For the estimation of H2O2 content, extracted sample solution (3 mL) was mixed in 1 mL of 0.1% titanium sulfate in 20% (v/v) H2SO4. The mixture was centrifuged at 6000×g for 20 min. The strength of yellow color of the supernatant mixture was evaluated at 410 nm, and H2O2 content was calculated by using the extinction coefficient of 0.28 μmol−1 cm−1.
Determination of enzymatic antioxidants
For the determination of antioxidant enzymes, plant sampling was done after exposure of plants to different treatments for 4 weeks; antioxidant enzymes, including catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) in both roots and leaves, were measured by using a spectrophotometer. The liquid nitrogen medium was used to grind the leaves and root samples with the help of mortar and pestle. This pattern was standardized in 0.05 M phosphate buffer (maintaining pH at 7.8), and four layers of muslin cloth were used to filter the samples. All the samples were centrifuged at 12,000×g for 10 min under 4 °C, and the extract was collected for further analysis. Both SOD and POD activities were determined according to Zhang (1992), and CAT activity was measured according to Aebi (1984). In brief, for CAT activity, the assay mixture (3.0 mL) consisted of 100 μL enzyme extract, 100 μL H2O2 (300 mM), and 2.8 mL 50 mM phosphate buffer with 2 mM CA (pH 7.0). Finally, the CAT activity was determined by measuring the decrease in absorbance at 240 nm because of the H2O2 disappearance (ε = 39.4 mM−1 cm−1).
Chromium content analysis
Dry plant samples (0.5 g) were digested in a 100-mL capacity flask by adding 15 mL of concentrated HNO3 (Sigma), and then, a hot plate containing temperature up to 275 °C was used for digestion of samples and dens yellow fumes started from the samples. When the quantity of dens yellow fumes became low, then hydrogen peroxide was added until fumes disappeared. When sample color disappeared, the flasks were removed from the hot plate and 25 mL of volume was made with distilled water. Flame atomic absorption spectrometry (novA A400 Analytik Jena, Germany) was used for the determination of Cr contents in the samples. The maximum detection limit of the instrument was 4 mg L−1 for Cr.
Data quality
Data quality was maintained by using three replicated of reagent blanks during the digestion and extraction of plant samples. All the instruments were calibrated by using working solutions, which were prepared, by using standard stock solutions from certified suppliers (i.e., Sigma Aldrich, Hamburg, Germany).
Statistical analysis
All values presented in this study are mean of three replicates. Student’s t test was performed to test whether the average concentrations in the ALA-treated pots differed from those of without ALA-treated pots under the same Cr stress. Results that were significantly different were marked by (*) for p ≤ 0.05, (**) for p ≤ 0.01, and (***) for p ≤ 0.001. Statistics were performed using the XLSTAT package (Addinsoft, Paris, France).
Results
Plant growth
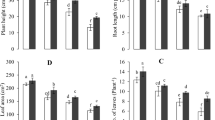
Chromium stress significantly (p ≤ 0.05) decreased the root length, plant height, leaf area, and number of leaves in comparison with control (Fig. 1). There were decreasing trend in these parameters with increasing Cr levels in the medium. At the highest Cr (200 μM) treatment, the decrease in plant height, number of leaves per plant, root length, and leaf area was about 52.3, 40.8, 42.8, and 44.4% in comparison to the control, respectively. Foliar applied ALA markedly increased the growth parameters compared to the respective Cr treatments alone. At ALA +200 μM Cr treatment, the increase in number of leaves per plant, plant height, leaf area, and root length was about 32, 32, and 11%, respectively, as in comparison to same Cr treatment separately. However, there was no significant increase that could be deducted in root length at the same Cr treatment.
Effect of ALA (15 mg L−1) on various Cr (0, 10, 100, and 200 μM) treatments on plant height (a), root length (b), number of leaves per plant (c), and leaf area (d) of cauliflower seedlings grown in soil. Values are expressed as means of three replicates with standard deviations. Single asterisk denotes significance relative to the same Cr treatment without ALA as determined by Student’s t test at a p < 0.05 and double asterisks at p < 0.01
Root, stem, leaf, and flower dry weights were markedly decreased under Cr stress as in comparison with the control (Table 1). The highest decrease in these dry weights was at the highest Cr level in the growth medium. The reduction in root, stem, leaf, and flower dry weights was about 52, 41, 53, and 74% in comparison with the control. The foliar applied ALA significantly increased the dry weights of root, stem, leaf, and flowers under Cr-stressed conditions in comparison to the respective Cr treatments alone. Foliar applied ALA + Cr stress caused an increase in the dry weights of all observed cauliflower parts in comparison with the respective Cr treatments. This increase in root, stem, leaf, and flower weights was about 19, 18, 22.5, and 39% as in comparison with the 200 μM Cr treatment, respectively.
Gas exchange characteristics and photosynthetic pigments
Under chromium stress, linear reduction in gas exchange parameters, transpiration rate, water use efficiency, net photosynthetic rate, and stomatal conductance was observed compared to the control (Fig. 2). Foliar applied ALA in cauliflower increased the gas exchange parameters in comparison with the respective Cr treatments. As compared to the untreated control, there was no significant increase in the gas exchange parameters to the ALA application without Cr stress. The highest increase (p ≤ 0.001) in water use efficiency was observed in 200 μM Cr + ALA treatment as compared to the same Cr treatment (Fig. 2d). Similarly, the highest significant increase (p ≤ 0.01) in transpiration rate, net photosynthetic rate, and stomatal conductance was observed at 100 μM Cr + ALA treatment as compared on exposure to Cr treatment alone (Fig. 2a–c).
Effect of ALA (15 mg L−1) on various Cr (0, 10, 100, and 200 μM) treatments on transpiration rate (a), stomatal conductance (b), net photosynthetic rate (c), and water use efficiency (d) of cauliflower seedlings grown in soil. Values are expressed as means of three replicates with standard deviations. Single asterisk denotes significance relative to the same Cr treatment without ALA as determined by Student’s t test at a p < 0.05, double asterisks at p < 0.01, and triple asterisks at p < 0.001
The photosynthetic pigments, total chlorophyll, carotenoid, and chlorophyll a and b, were significantly decreased in all Cr treatments (10, 100, and 200 μM) in comparison with the control (Fig. 3). Foliar applied ALA increased the photosynthetic pigments under Cr-stressed conditions as compared to the respective Cr stress separately. There was a marked increase (p ≤ 0.05) in photosynthetic pigments between with and without ALA at lower Cr stress (10 and 100 μM) while these pigments remained unaffected (p ≤ 0.05) at the higher Cr treatment (200 μM).
Effect of ALA (15 mg L−1) on various Cr (0, 10, 100, and 200 μM) treatments on chlorophyll a (a), chlorophyll b (b), total chlorophyll (c), and carotenoids (d) of cauliflower seedlings grown in soil. Values are expressed as means of three replicates with standard deviations. Single asterisk denotes significance relative to the same Cr treatment without ALA as determined by Student’s t test at a p < 0.05
Oxidative stress and antioxidative enzyme activities
The contents of EL, H2O2, and MDA were examined in leaves and roots of cauliflower plants under different chromium levels in the presence or absence of ALA (Fig. 4). Electrolyte leakage, MDA, and H2O2 contents were gradually increased in both cauliflower parts viz. roots and leaves with increasing Cr levels as compared to the control. Foliar application of ALA decreased oxidative stress in comparison to the respective Cr treatments. The significant decrease in root EL was noted at 100 μM Cr treated with ALA as compared to without ALA. However, the significant (p ≤ 0.01) decrease in both roots and leaves MDA contents was observed at 200 μM Cr + ALA treatment in comparison with the same Cr treatment. Similarly, the significant decrease (p ≤ 0.001) in H2O2 contents in leaves was observed in 200 μM Cr + ALA-treated plants as compared the same Cr treatment alone.
Effect of ALA (15 mg L−1) on various Cr (0, 10, 100, and 200 μM) treatments on EL in roots (a), EL in leaves (b), MDA concentration in roots (c), MDA concentration in leaves (d), H2O2 in roots (e), and H2O2 in leaves (f) of cauliflower seedlings grown in soil. Values are expressed as means of three replicates with standard deviations. Single asterisk denotes significance relative to the same Cr treatment without ALA as determined by Student’s t test at a p < 0.05, double asterisks at p < 0.01, and triple asterisks at p < 0.001
Chromium stress markedly disturbed the activities of antioxidant enzymes in leaves and roots (Fig. 5). In both cauliflower parts viz. leaves and roots, the activities of key antioxidant enzymes (CAT, SOD and POD) were significantly increased under Cr stress in comparison with respective untreated control. The application of 10 and 100 μM Cr treatments gradually and markedly increased the activities of antioxidant enzymes as compared to the control. However, 200 μM Cr treatment decreased the activities of the estimated antioxidant enzymes as compared to 100 μM Cr treatment, but there were observed higher activities as compared to other Cr treatments as well as untreated control except activity of POD enzyme in leaves. Foliar applied ALA further increased the activities of antioxidant enzymes in comparison with the respective Cr treatments alone. The highest significant increase in CAT activity was noted in root treated with 100 μM Cr + ALA in comparison with the same Cr treatment when applied separately. Similarly, the highest significant increase in SOD activity was found in leaves treated with 10 μM Cr + ALA as compared to the same Cr treatment alone.
Effect of ALA (15 mg L−1) on various Cr (0, 10, 100, and 200 μM) treatments on CAT in leaves (a), CAT in roots (b), SOD in roots (c), SOD in leaves (d), POD in roots (e), and POD in leaves (f) of cauliflower seedlings grown in soil. Values are expressed as means of three replicates with standard deviations. Single asterisk denotes significance relative to the same Cr treatment without ALA as determined by Student’s t test at a p < 0.05 and double asterisks at p < 0.01
Chromium contents
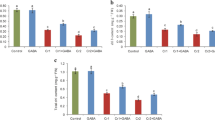
The contents of Cr in root, stem, leaf, and flower of cauliflower plants are presented in Table 2. The Cr contents significantly enhanced in all plant parts with the increasing levels of Cr in the growth medium. Chromium contents were highest in the roots followed by stem, leaf, and flower, respectively. Foliar applied ALA significantly reduced the Cr contents in all observed plant parts as compared to the respective Cr treatments alone. Total Cr uptake increased with increasing Cr levels in the soil while ALA application markedly reduced the total Cr uptake by the plants in comparison with the respective Cr treatments when applied individually except in flowers treated with 200 μM Cr (Fig. 6). In flowers, the highest significant decrease (p ≤ 0.05) in total Cr uptake was observed at 100 μM Cr + ALA as compared to the same Cr treatment (Fig. 6a). Similarly, the significant decrease (p ≤ 0.01) in total Cr uptake was observed at both 10 and 100 μM Cr + ALA in comparison with the respective Cr treatments (Fig. 6b).
Effect of ALA (15 mg L−1) on various Cr (0, 10, 100, and 200 μM) treatments on total Cr uptake in flower (a), total Cr uptake in leaves (b), total Cr uptake in stem (c), and total Cr uptake in roots (d) of cauliflower seedlings grown in soil. Values are expressed as means of three replicates with standard deviations. Single asterisk denotes significance relative to the same Cr treatment without ALA as determined by Student’s t test at a p < 0.05 and double asterisks at p < 0.01
Discussion
In the present investigation, application of Cr in the growth medium influenced negatively on the plant growth, development, and biomass. The reduction in growth trend was higher at higher Cr levels in the medium solution (Fig. 1; Table 1). Several studies have shown the toxic impacts of Cr on plant growth, development, and biomass including B. napus (Afshan et al. 2015), mung bean (Jabeen et al. 2016), and wheat (Ali et al. 2015a, 2015b). Chromium-mediated decrease in different characteristics of plant growth, development, and biomass might be due to the lower uptake and accumulation of nutrients by plants as Cr interaction with nutrients occurs and limit their uptake by plants (Pradas-del-Real et al. 2013). It was reported that Cr toxicity damaged ultrastructure of different parts of B. napus (Gill et al. 2015). Ali et al. (2013b) reported that Cr stress caused damage in the root tip cells of barley. However, Cr toxic effects on plant depend and vary markedly with different growth conditions and plant species (Diwan et al. 2012; Gill et al. 2015). Foliar application of ALA increased different characteristics of plant growth, development, and biomass under Cr stress compared to the respective Cr treatments alone (Fig. 1, Table 1). The promotive role of ALA has also been reported in B. napus under Pb and Cd stresses (Ali et al. 2013a, 2014). Akram et al. (2012) reported that ALA could regulate a number of metabolic processes in sunflower under salt stress, thereby improving plant growth and biomass. Ameliorative effects of ALA and different parameters of plant growth, development, and biomass might be due to the ameliorative effect of ALA on cauliflower ultrastructure under Cr stress as was found in B. napus under Cd stress (Ali et al. 2013a).
Gas exchange parameters and chlorophyll contents decreased with increasing Cr levels (Figs. 2 and 3). These results are in accordance with recent findings indicating that Cr is toxic to plants and decreased chlorophyll contents (Afshan et al. 2015; Jabeen et al. 2016). Chromium-induced reduction in photosynthesis might be due to reduction in iron contents in leaves of cauliflower and Cr-mediated toxic effects on chlorophyll metabolism. Rodriguez et al. (2012) reported that Cr stress altered the morphology of chloroplasts and severely affected the Rubisco activity of pea (Pisum sativum L.) seedlings. Reduction of chlorophyll contents might also be due to the production of enzymes involving in chlorophyll degradation or inhibition of its biosynthesis under Cr stress (Singh et al. 2013). Photosynthetic pigments were damaged by Cr through oxidative stress. Similar reasons might also be involved in reduction of photosynthesis in cauliflower under Cr-stressed conditions. Foliar applied ALA increased the photosynthesis of plants in Cr applied treatments in comparison to the respective Cr treatments applied separately (Figs. 2 and 3). Several studies have reported that the addition of ALA caused the increase in photosynthetic parameters in B. napus under salt (Naeem et al. 2010, 2011, 2012) and HM stresses (Ali et al. 2013a, 2014). ALA-mediated increase in chlorophyll and photosynthetic parameters in Cr applied plants might be due to the preventive role of ALA in reducing chloroplast ultrastructural damage as was observed in B. napus under Cd-stressed conditions (Ali et al. 2013a). Our findings in this study showed that ALA foliar application markedly increased the photosynthetic pigments at lower Cr (10 and 100 μM) stress while these were non-significantly improved at higher Cr stress (200 μM) as in comparison with the respective Cr applied treatments separately (Fig. 3). Chromium toxic effects on the photosynthetic pigments depend upon the duration of the Cr stress applied (Oliveira 2012; Singh et al. 2013). Furthermore, the ALA-mediated non-significant increase in chlorophyll a content has also been found in B. napus under 500 μM Cd level in hydroponics for 15 days (Ali et al. 2013a). Thus, it can be suggested that there might be ALA-mediated significant increase in photosynthetic pigments under Cr stress during the long-term exposure to Cr stress applied than the present study.
In the present study, Cr toxicity caused oxidative stress, EL, MDA, and H2O2 and caused significant increase in antioxidant enzymes activities at lower Cr (10 and 100 μM) while decrease at higher Cr levels (200 μM) in both leaves and roots of cauliflower (Figs. 4 and 5). Our present results are in accordance with the recent published studies that Cr stress affected the activities of antioxidant enzymes in B. napus (Afshan et al. 2015; Ali et al. 2015a, b). It was observed that Cr-stressed treatments at 50 and 100 ppm levels caused a reduction in the CAT activity in hydroponically grown wheat for 7 days (Dey et al. 2009). Gill et al. (2015) reported that Cr increased the activity of CAT enzyme while it decreased activity of APX enzyme in B. napus. The increase in oxidative stress and decrease in antioxidant enzyme activities at higher concentrations of Cr (200 μM) might be due to higher Cr uptake and accumulation in cauliflower which deteriorated the defense mechanism of cauliflower against Cr stress. The decrease in antioxidant enzyme activities, in plants under Cr stress, suggests the lower efficiency of antioxidants enzymes to scavenge ROS, and as a result, decrease in the plant growth and biomass was observed under higher Cr stress. Exogenous application of ALA decreased the oxidative stress and enhanced the antioxidant enzyme activities under Cr stress (Figs. 4 and 5). ALA at lower concentration increased the H2O2 production in spinach leaves and increased the antioxidant enzyme activities (Nishihara et al. 2001). ALA decreased the MDA contents in B. napus plants under herbicide stress conditions (Zhang et al. 2008) which is in agreement with our observations that ALA at lower Cr concentration declined the MDA content as compared to ALA alone at higher Cr concentrations in cauliflower. ALA-mediated reduction in oxidative stress and enhancement in the activities of antioxidant enzymes might be a very successful strategy of plants against metal stress.
Chromium concentrations and total Cr uptake increased in all observed plant parts with an increase in Cr levels in the growth medium (Fig. 6; Table 2). The Cr contents were highest in the roots followed by stem, leaves, and flowers. These results indicated the ability of the plants to avoid Cr stress by depositing in the roots and less translocation towards shoots (Afshan et al. 2015; Ali et al. 2015). The application of ALA decreased the Cr concentrations and Cr uptake by the cauliflower as compared to the same treatments without the ALA supply except Cr uptake by flowers at the highest Cr treatment + ALA as compared to the same Cr treatment without ALA (Fig. 6, Table 2). The decrease in Cr uptake by foliar applied ALA might be due to the ALA-mediated activation of antioxidant machinery and reduction in the ultrastructural root tip damages (Ali et al. 2014). The increase in total Cr uptake (concentration × dry biomass) by flowers treated with 200 μM Cr + ALA as in comparison with same Cr treatment alone (Fig. 6a) might be due to the so-called dilution effect (lower uptake but larger biomass) as suggested previously (Rizwan et al. 2012). Thus, it could be suggested that ALA might be applied for phytoremediation purpose of Cr and other metal contaminated soils. However, field studies still needed to validate these results on a large scale.
Conclusion
The cultivation of vegetables on Cr contaminated soils can lead to Cr accumulation in humans via the food chain. Foliar application of ALA might be effective in reducing Cr concentration in vegetables such as cauliflower. The results of the present investigation found that Cr stress had negative effects on the biomass, plant growth, and photosynthesis in cauliflower, and results were more significant at higher Cr applied treatments. Chromium application significantly increased the oxidative stress as indicated by EL, H2O2, and MDA and increased the activities of antioxidant enzymes. Foliar applied ALA might have a role in improving the activities of the antioxidant system and reducing the oxidative stress under Cr toxicity. The ALA alleviated the Cr-mediated decrease in growth, gas exchange parameters, and chlorophyll contents. The ALA decreased the Cr concentrations in different parts of plants and increased the plant growth and biomass. Overall, the results of the present investigation revealed that ALA might have the ability to reduce the toxic effects of Cr in cauliflower. Further work is needed to evaluate the role of foliar applied ALA on Cr and other metal uptake by different vegetables grown in texturally different soils.
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Rehman MZ, Irshad MK, Bharwana SA (2015a) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Adrees M, Ali S, Iqbal M, Bharwana SA, Siddiqi Z, Farid M, Ali Q, Saeed R, Rizwan M (2015b) Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of antioxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol Environ Saf 122:1–8
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689
Ahmad P, Wani MR, Azooz MM, Tran LSP (eds) (2014) Improvement of crops in the era of climatic changes. Springer
Akram NA, Ashraf M, Al-Qurainy F (2012) Aminolevulinic acid induced regulation in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) under saline regimes. Sci Hort 142:143–148
Ali B, Wang B, Ali S, Ghani MA, Hayat MT, Yang C, Xu L, Zhou WJ (2013a) 5-aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regul 32:604–614
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang GP (2013b) The influence of silicon on barley growth, photosynthesis and ultrastructure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W (2014) Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Indus Crops Prod 52:617–626
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015a) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015b) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res 22:10669–10678
Ali Z, Malik RN, Shinwari ZK, Qadir A (2015c) Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Int J Environ Sci Technol 12:537–550
Dey SK, Jena PP, Kundu S (2009) Antioxidative efficiency of Triticum aestivum L. exposed to chromium stress. J Environ Biol 30:539–544
Dheeba B, Sampathkumar P, Kannan K (2015) Fertilizers and mixed crop cultivation of chromium tolerant and sensitive plants under chromium toxicity. J Toxicol article ID 367217:1–9
Dhindsa RS, Dhindsa PP, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Diwan H, Ahmad A, Iqbal M (2012) Characterization of chromium toxicity in food crops and their role in phytoremediation. J Bioremed Biodeg 3:1–7
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Gill RA, Ali B, Cui P, Shen E, Farooq MA, Islam F, Ali S, Mao B, Zhou W (2016a) Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genom 17:1–25
Gill RA, Zhang N, Ali B, Farooq MA, Xu J, Gill MB, Mao B, Zhou W (2016b) Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ Sci Pollut Res 23:20483–20496
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015a) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22:13772–13799
Khan MU, Malik RN, Muhammad S, Ullah F, Qadir A (2015b) Health risk assessment of consumption of heavy metals in market food crops from Sialkot and Gujranwala Districts, Pakistan. Human Ecol Risk Assess 21:327–337
Khan MU, Shahbaz N, Waheed S, Mahmood A, Shinwari ZK, Malik RN (2016) Comparative health risk surveillance of heavy metals via dietary foodstuff consumption in different land-use types of Pakistan. Human Ecol Risk Assess 22:168–186
Lichtenthaler HK (1987) Chlorophylls and carotenoids pigments of photosynthetic biomembranes. In: Colowick SP, Kaplan NO (eds) Methods in Enzymology, vol 148, pp 350–382
Liu L, Zhang X, Zhong T (2015) Pollution and health risk assessment of heavy metals in urban soil in China. Human Ecol Risk Assess. doi:10.1080/10807039.2015.1078226
Metzner H, Rau H, Senger H (1965) UntersuchungenzurSynchronisierbakeiteinzelnerPigmentmangel-Mutation von Chlorella. Planta 65:186–194 (in German)
Naeem MS, Jin ZL, Wan GL, Liu D, Liu HB, Yoneyama K, Zhou WJ (2010) 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oil seed rape (Brassica napus L.). Plant Soil 332:405–415
Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K, Takeuchi Y, Zhou WJ (2011) 5-aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant 33:517–528
Naeem MS, Warusawitharana H, Liu H, Liu D, Ahmad R, Waraich EA, Xu L, Zhou W (2012) 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol Biochem 57:84–92
Nishihara E, Takahashi K, Nakata N, Tanaka K, Watanabe K (2001) Effect of 5-aminolevulinic acid (ALA) on photosynthetic rate, hydrogen peroxide content, antioxidant level and active oxygen-scavenging enzymes in spinach (Spinacia oleracea L.). J Jpn Soc Hort Sci 70:346–352
Oliveira H (2012) Chromium as an environmental pollutant: insights on induced plant toxicity. J Bot. doi:10.1155/2012/375843
Pradas-del-Real AE, García-Gonzalo P, Alarcón R, González-Rodríguez A, Lobo MC, Pérez-Sanz A (2013) Effect of genotype, Cr (III) and Cr (VI) on plant growth and micronutrient status in Silene vulgaris (Moench). Spanish J Agri Res 11:685–694
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilizationof cadmium in contaminated soil and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rizwan M, Meunier JD, Hélène M, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209-210:326–334
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Rehman MZ, Qayyum MF, Abbas F (2015) Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut Res 22:15416–15431
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2016a) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23:1414–1427
Rizwan M, Ali S, Qayyum MF, Ibrahim M, Rehman MZ, Abbas T, Ok YS (2016b) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248
Rizwan M, Ali S, Rizvi H, Rinklebe J, Tsang DCW, Meers E, Ok YS, Ishaque W (2016c) Phytomanagement of heavy metals in contaminated soils using sunflower—a review. Criti Rev Environ Sci Technol. doi:10.1080/10643389.2016.1248199
Rodriguez E, Santos C, Azevedo R, Moutinho-Pereira J, Correia C, Dias MC (2012) Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem 53:94–100
Shakir L, Ejaz S, Ashraf M, Qureshi NA, Anjum AA, Iltaf I, Javeed A (2012) Ecotoxicological risks associated with tannery effluent wastewater. Environ Toxicol Pharmacol 34:180–191
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254
Xu L, Ali B, Gill RA, Li L, Zhou W (2015) Alleviation of cadmium toxicity by 5-aminolevulinic acid is related to improved nutrients uptake and lowered oxidative stress in Brassica napus. Int J Agric Biol 18:557–564
Younis U, Malik SA, Rizwan M, Qayyum MF, Ok YS, Shah MHR, Rehman RA, Ahmad N (2016) Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ Sci Pollut Res 23:21385–21394
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD POD and CAT activities in biological system. In: Zhang XZ (ed) Research Methodology of Crop Physiology, Beijing Agriculture Press, pp 208–211
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhang WF, Zhang F, Raziuddin R, Gong HJ, Yang ZM, Lu L, Ye QF, Zhou WJ (2008) Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul 27:159–169
Acknowledgments
We are highly thankful to the Higher Education Commission (HEC), Pakistan, and Government College University, Faisalabad, Pakistan, for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yi-ping Chen
Rights and permissions
About this article
Cite this article
Ahmad, R., Ali, S., Hannan, F. et al. Promotive role of 5-aminolevulinic acid on chromium-induced morphological, photosynthetic, and oxidative changes in cauliflower (Brassica oleracea botrytis L.). Environ Sci Pollut Res 24, 8814–8824 (2017). https://doi.org/10.1007/s11356-017-8603-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8603-7