Abstract
Production of the greenhouse gas nitrous oxide (N2O) from the completely autotrophic nitrogen removal over nitrite (CANON) process is of growing concern. In this study, the effect of added hydrazine (N2H4) on N2O production during the CANON process was investigated. Long-term trace N2H4 addition minimized N2O production (0.018% ± 0.013% per unit total nitrogen removed) and maintaining high nitrogen removal capacity of CANON process (nitrogen removal rate and TN removal efficiency was 450 ± 60 mg N/L/day and 71 ± 8%, respectively). Ammonium oxidizing bacteria (AOB) was the main N2O producer. AOB activity inhibition by N2H4 decreased N2O production during aeration, and the N2H4 concentration was negatively correlated with N2O production rate in NH4+ oxidation via AOB, whereas N2O production was facilitated under anaerobic conditions because hydroxylamine (NH2OH) production was accelerated due to anammox bacteria (AnAOB) activity strengthen via N2H4. Added N2H4 completely degraded in the initial aeration phases of the CANON SBR, during which some N2H4 intensified anammox for total nitrogen removal to eliminate N2O production from nitrifier denitrification (ND) by anammox-associated, while the remaining N2H4 competed with NH2OH for hydroxylamine oxidoreductase (HAO) in AOB to inhibit intermediates formation that result in N2O production via NH2OH oxidation (HO) pathway, consequently decreasing total N2O production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The completely autotrophic nitrogen removal over nitrite (CANON) (Sliekers et al. 2002) process combines nitritation and anaerobic ammonium (NH4+) oxidization (anammox) into a single process, in which aerobic ammonium oxidizing bacteria (AOB) partially oxidize NH4+ to nitrite (NO2−), while anammox bacteria (AnAOB) convert the resulting NO2− and any remaining NH4+ into nitrogen gas (N2). The CANON process is a cost-effective and energy-efficient alternative to the conventional nitrogen removal process because of its reduced oxygen and carbon source demands (Sliekers et al. 2002; De Clippeleir et al. 2013; Joss et al. 2009), and it has been applied in full-scale wastewater treatment projects (Joss et al. 2009, 2011; Vázquez-Padín et al. 2009). Nitrous oxide (N2O) is easily produced as an intermediate from incomplete nitrification and denitrification (Rassamee et al. 2011). Because N2O is an ozone-depleting agent (Ravishankara et al. 2009) and a potent greenhouse gas with a global warming potential (GWP100) of 298 (IPCC 2011), N2O produced by the CANON process is a subject of concern. Many researchers have assessed N2O production and emission using different types of CANON reactors (Sliekers et al. 2002; Kampschreur et al. 2008, 2009a; Okabe et al. 2011; Xiao et al. 2014; Wang et al. 2017; Blum et al. 2018). These studies show that, irrespective of the operation mode or process conditions, controlling N2O production and/or emissions during aerobic periods based on nitrification by AOB is advantageous because of reductions in the total N2O emission from the system (Wunderlin et al. 2013a). A better understanding of the biological turnover processes of nitrogen compounds during N2O production via aeration, as well as reactor operation parameters that can be adjusted to reduce N2O production, is needed to improve process sustainability.
Hydroxylamine (NH2OH) oxidation (HO) and nitrifier denitrification (ND) driven by AOB are the main biological pathways for N2O production during aeration in the CANON process (Wunderlin et al. 2012, 2013a, b; Harris et al. 2015; Pocquet et al. 2016; Ali et al. 2016). The concentrations of NH4+, NO2− and dissolved oxygen (DO) in the reactor have direct effects on the two pathways by which N2O is produced (Xiao et al. 2014; Schreiber et al. 2012; Peng et al. 2015, 2016). N2O production by ND is favored at high NO2− concentrations and low DO concentrations (Wunderlin et al. 2012; Harris et al. 2015; Peng et al. 2015), whereas high NH4+ concentrations and a high NH4+ removal rate favor N2O production by HO (Wunderlin et al. 2013a, b). When the NH4+ or DO concentrations in the reactor are elevated beyond a threshold, N2O production by ND is enhanced and becomes the dominant source of N2O. Moreover, N2O production via HO is a relatively unimportant source of N2O in CANON reactors under all conditions (Harris et al. 2015). Although N2O is not a side-product of AnAOB metabolism (Kartal et al. 2011), AnAOB play an important role in N2O production by providing NH2OH as an electron donor for AOB, which reduce NO2− by ND in the CANON process (Xiao et al. 2014), and the existence of an unclear N2O production pathway associated with anammox metabolism merits further study (Harris et al. 2015; Ali et al. 2016). Until recently, distinguishing the contributions of the HO, ND- and anammox-associated pathways to N2O production during the CANON process has been difficult, and N2O production has been regarded as either a single or synergistic effect of those pathways, depending on the specific operational state of the process.
The focus of aeration strategies, especially during NH4+ oxidation, is to mitigate N2O emission. In an earlier study, optimization of the DO concentration and dynamic co-optimization of DO and pH were found to be effective methods of controlling N2O production (Kampschreur et al. 2009a, b). Nevertheless, a single change in either of these operating conditions did not significantly reduce the amount of N2O emission from the CANON process (Weissenbacher et al. 2011). Later, the NO2− concentration and NH2OH oxidation activity were both found to be positively correlated with N2O production (Okabe et al. 2011; Wunderlin et al. 2012). Maintaining a low concentration of dissolved NO2− and a low level of NH2OH oxidation activity are key requirements for minimizing N2O production during the CANON process (Wunderlin et al. 2013a). Aerobic NH4+ oxidation activity influences NH2OH oxidation and the NO2− concentration (Xiao et al. 2014; Wunderlin et al. 2013a, b). Increasing the frequency of aeration cycling to stimulate aerobic NH4+ oxidation activity significantly minimizes N2O production while maintaining maximum liquid phase nitrogen removal efficiency (Domingo-Félez et al. 2014). However, operating conditions are very difficult to control during frequent aeration cycling because long-term adjustments and fine control of the process (Domingo-Félez et al. 2014) are required. Maintaining a low rate of NH4+ oxidation has become the central focus of strategies aimed at reducing N2O production from the CANON process. Taken together, the previous study has shown that, although a low NH4+ oxidation rate can negatively influence denitrification activity, high-frequency intermittent aeration with short cycle times, low volumetric N loading, and maintaining an extremely low NH4+ oxidation rate are effective strategies for reducing N2O emission by the CANON process (Blum et al. 2018). Methods of rapidly and efficiently minimizing N2O production and emissions while maintaining the stability and performance of the CANON process are an important research focus.
The role of hydrazine (N2H4) as an intermediate product in anammox (Kartal et al. 2011) has received a significant attention. The addition of N2H4 to a CANON reactor can simultaneously enhance the total nitrogen (TN) removal performance and reactor stability (Yao et al. 2013; Xiao et al. 2015). However, the development of an improved “green” CANON process with a reduced environmental impact is hindered by a lack of knowledge regarding the effect of N2H4 on N2O production. In the present work, a lab-scale CANON granular sequencing batch reactor (SBR) with alternating oxygen-limited/anaerobic conditions was implemented to study the effect of exogenous N2H4 on N2O production. The influence of exogenous N2H4 on sources of N2O was investigated using batch experiments, and the mechanism of N2O reduction was studied during one cycle of SBR operation. The results of this study provide a strategy for tracing the effects of added N2H4 in a CANON reactor to rapidly achieve minimal N2O production while maintaining stable and effective nitrogen removal.
Materials and methods
Operation of the CANON SBR
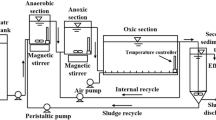
A continuously running CANON SBR with enriched granular sludge was utilized in this study. A schematic diagram of the CANON SBR, as well as its working volume, operating parameters, and components, with related concentrations of synthetic wastewater and the method of long-term N2H4 (4 mg/L) addition was reported by Xiao et al. (2014, 2015).
Batch tests
Batch tests were conducted to investigate the influence of exogenous N2H4 (in the form of N2H4 × H2SO4) on sources of N2O in the CANON process. How the batch tests are prepared and conducted has been reported by Xiao et al. (2014) .The experimental conditions are shown in Table 1.
Under oxygen-limited conditions, batch tests A and B focused on the influence of exogenous N2H4 on N2O production in the CANON process. Different concentrations of N2H4 were added in batch tests C, D, and E (with methanol added to inhibit AnAOB activity) to investigate the effect of exogenous N2H4 on N2O production during aerobic NH4+ oxidation. Batch tests H, I, and J were conducted to distinguish the contributions of HO and ND during aerobic NH4+ oxidation. Allylthiourea (ATU) and methanol were added in batch tests H and I to impede oxidation of NH4+ to form NH2OH (Xiao et al. 2014). Diethyldithiocarbamate (DDC), an inhibitor of copper-containing nitrite reductase (NirK), was added in batch test I to block reduction of NO2− to form N2O by the ND pathway (Ravishankara et al. 2009). It has been reported that a small amount of N2O may be formed from chemical oxidation of NH2OH (Shapleigh and Payne 1985). Therefore, batch test J (aerobic conditions without sludge) was designed to investigate N2O production via chemical oxidation of NH2OH in the CANON process. Batch tests K and L were conducted to investigate the influence of the addition of a trace amount of N2H4 on N2O production under anaerobic conditions. Batch tests F, G, and M were conducted to investigate the production of N2O via heterotrophic denitrification (HD).
Granular sludge taken from the CANON SBR was washed three times with nutrient solution before the batch tests. The concentrations of trace elements and NaHCO3 in the batch tests were consistent with those observed in the operation of the CANON SBR. Mineral medium and inhibitors were added into 500-mL batch reactors according to the experimental conditions shown in Table 1. Initial concentrations of NH4+-N, NO2−-N, NH2OH-N, and N2H4-N were measured. DO was measured with a DO meter (BANTE820, Shanghai Bant Instrument Company, China). A gas flow meter (95 mL air/min) was used to control the aeration rate and maintain the DO level at 0.35 ± 0.05 mg/L under oxygen-limited conditions. The batch reactors were flushed with nitrogen gas (99.99%) at 30 mL N2/min to create anaerobic conditions in batch tests K, L, and M. The pH was measured using a pH electrode (HI991002, Hanna Instruments, Italy) and adjusted to 7.8 by adding 1 mol/L HCl and 1 mol/L NaOH. The temperature of the batch reactors was maintained at 31 ± 1 °C using a water bath.
Sampling and analysis
Batch test samples (10 mL) were taken from the reactors every hour using syringes. According to previously reported protocols (APHA 1998), NH4+-N, NO2−-N, and chemical oxygen demand (COD) were analyzed using colorimetric methods, whereas nitrate (NO3−) was measured spectrophotometrically. Mixed liquor suspended solids (MLSS) and mixed liquor volatile suspended solids (MLVSS) were measured using standard methods (2540). The NH2OH concentration was measured using a spectrophotometric method as previously described (Frear and Burrell 1955). The N2H4 concentration was measured using a spectrophotometric method as previously described (Watt and Chrisp 1952), and the interference of NO2− was eliminated by the addition of 0.5% sulfamic acid (George et al. 2008). Total N2O, consisting of the N2O in the emission gas and the N2O dissolved in the mixed liquid, was analyzed in triplicate using gas chromatography (2010-plus, Shimadzu Scientific Instruments, Japan). The experimental conditions for the GC measurements and the methods used for measurement and calculation of the total concentration and production rate of N2O-N were described (Xiao et al. 2014).
Results
CANON SBR performance and N2O production
The N2O production and nitrogen removal performance of the CANON SBR used in this study (with N2H4 addition) from days 505 to 525 are shown in Fig. 1.
From days 505 to 509, the total nitrogen removal rate (NRR) and TN removal efficiency of the reactor gradually increased (Fig. 1 panel a). In contrast, from days 505 to 509, the average N2O production rate sharply decreased from 0.20 to 0.064 mg N/L/day (Fig. 1 panel b). The NRR and TN removal efficiencies reached 450 ± 60 mg N/L/day and 71 ± 8% (average value from days 509 to 525), respectively, which were nearly equal to the results (420 ± 40 mg N/L/day and 72 ± 9%, respectively) previously reported for the reactor (Xiao et al. 2015). From days 509 to 525, the average N2O production rate was relatively low (0.066 ± 0.047 mg N/L/day) and represented only approximately 0.018 ± 0.013% of the NRR.
Batch tests
The nitrogen conversion and N2O average production rates of the batch tests are shown in Table 2.
Under oxygen-limited conditions
In batch test A (without N2H4 addition), the NRR was 213.30 mg N/g VSS/day, and the average N2O production rate (0.078 mg N/g VSS/day) was only 0.037% of the NRR. With the addition of 3.2 mg N/L N2H4 in batch test B, the NRR increased slightly to 286.86 mg N/g VSS/day, whereas the average N2O production rate decreased to 0.032 mg N/g VSS/day, which was only 0.012% of the NRR and 41% of that in batch test A.
In batch test C, with methanol added to inhibit AnAOB activity and without the addition of N2H4, the NRR and average N2O production rate were 24.85 mg N/g VSS/day and 0.06 mg N/g VSS/day, respectively, which were 11.7% and 76.9%, respectively, of the values measured in batch test A. In batch test D, with the addition of 3.04 mg N/L N2H4, the TN removal and average N2O production rate decreased to 19.09 mg N/g VSS/day and 0.02 mg N/g VSS/day, respectively. The average N2O production rate of batch test D was 33% of that of batch test C, whereas it was 62.5% of that of batch test B. In batch test E, with the addition of 7.16 mg N/L N2H4, the NRR decreased to 9.59 mg N/g VSS/day, and the average N2O production rate was lower than the detection limit. In batch tests C, D, and E, N2O production decreased in parallel with the added concentrations of N2H4 during aerobic NH4+ oxidation. The conversion rates at different concentrations of N2H4 in batch tests D and E were nearly equivalent, which indicated that the maximum aerobic degradation rate of N2H4 was reached when approximately 3 mg N/L N2H4 was added to the reactor. It has been reported that N2H4 might have the capacity to impact the growth of AOB (Choi et al. 2013), and AOB can degrade N2H4 (Yao et al. 2013) .Therefore, the half-saturation coefficient of N2H4 in aerobic NH4+ oxidation was likely less than 3 mg N/L, which was lower than that previously reported for anammox (10.42 mg N/L) (Yao et al. 2015).
On the basis of the results of batch tests A and C without N2H4 addition, the contributions of AOB and AnAOB to the total N2O production were determined to be 76.9% and 23.1%, respectively. When a similar concentration of exogenous N2H4 was added in batch tests B and D, the contributions of AOB and AnAOB to total N2O production were 62.5% and 38.5%, respectively. These results demonstrate that exogenous N2H4 slightly increased the contribution of AnAOB to total N2O production during aeration.
In the presence of only NO2− in batch test F, very little TN was removed, and only a small amount of NO3− was produced by nitrite oxidization bacteria (NOB). With the addition of NO2− and methanol together in batch test G, very little TN was removed and only slight variation in COD was observed, while only a small amount of NO3− was produced (Fig. 2). N2O production was not detected in batch test F or batch test G. These results indicate that HD did not contribute to nitrogen removal and N2O production under oxygen-limited conditions.
In the presence of NH2OH only, no significant change in the concentration of NH2OH or N2O was observed after 4 h of continuous aeration under aerobic conditions without sludge in batch test J. Therefore, the N2O production pathway from NH2OH chemical oxidation in the CANON process could be ignored. With the addition of 20 mg/L ATU to inhibit NH4+ oxidation and 10 mM methanol to restrict AnAOB activity in batch test H, the NH2OH removal rate was 175.68 mg N/g VSS/day, and the average N2O production rate was 0.074 mg N/g VSS/day, which was greater than that of batch test C. With the addition of 20 mg/L ATU, 10 mM methanol, and 10 mM DDC in batch test I, the NH2OH removal rate marginally decreased to 170.10 mg N/g VSS/day, while the average N2O production rate was 0.046 mg N/g VSS/day, which was 62.1% of that of batch test H. In batch test I, NirK activity was restrained by DDC to block N2O formation from NO2− reduction by ND. HO and ND are pathways for N2O production in the CANON process under oxygen-limited conditions. The contributions of HO and ND to total N2O production during aeration were 62.1% and 37.9%, respectively.
Under anaerobic conditions
In batch test K without N2H4 addition, the NRR was 305.06 mg N/g VSS/day and the N2O average production rate was 0.024 mg N/g VSS/day, which was 0.008% of the NRR. With the addition of N2H4 in batch test L, the NRR slightly increased to 322.67 mg N/g VSS/day, and the average N2O production rate was 0.094 mg N/g VSS/day, which was 0.029% of the NRR and 3.9 times that of batch test K. Exogenous N2H4 effectively increased N2O production via anammox under anaerobic conditions in the CANON process.
With the addition of both NO2− and methanol in batch test M, very little COD variation or nitrogen removal was observed, and no N2O production was detected (Fig. 2). These results demonstrate that HD was still not observable under anaerobic conditions.
Dynamic N2O production in one cycle of the CANON SBR operation
The dynamic concentration profiles during one cycle of CANON SBR operation (day 518) are illustrated in Fig. 3. NH4+-N decreased, whereas NO3−-N production by NOB increased slightly at the beginning of the aeration phase and then remained unchanged. Exogenous N2H4 decreased during the first hour of the cycle. In aeration phase I (0–1 h, shown in Fig. 3), NO2− gradually accumulated during the first half hour, after which the amount of NO2− decreased. The N2O concentration and average production rate peaked at 0.84 mg N2O-N/L and 0.15 mg N2O-N/L/day during the first half hour and then decreased. During aeration phase II (1–2.5 h, shown in Fig. 3), the NO2− concentration rapidly increased, and peak NO2− concentration occurred at 1.25 h. The N2O concentration and average production rate slightly increased in aeration phase II. In anaerobic phase III (2.5–4 h, shown in Fig. 3), the NO2− concentration rapidly decreased, and the N2O concentration and average production rate decreased (to 0.009 mg N/L/day) at the end of the anaerobic period.
Discussion
Minimized N2O production with long-term N2H4 addition
Information regarding N2O production observed in various studies during autotrophic nitrogen removal over nitrite is summarized in Table 3. In this study, the enhanced lab-scale CANON granular system with exogenous N2H4 was maintained stable, high-rate nitrogen removal performance as previously reported (Rassamee et al. 2011). The average N2O production rate was 0.066 ± 0.047 mg N/L/day, which accounted for only 0.018% ± 0.013% of the NRR, which was a minimal contribution in comparison with the corresponding results from other reactors (Table 3).
Alternatively, with regard to aeration control strategies aimed at achieving minimal N2O production (0.011% ± 0.004% per unit NH4+ removed) during autotrophic nitrogen removal over nitrite, shorter cycle times (160 min), lower volumetric N loading (less than 100 mg NH4+-N/L), and a low rate of aerobic NH4+ oxidation (5 mg N/g VSS/h) (Blum et al. 2018) have been reported to effective. As shown in Table 2, the results obtained in the batch experiments in this study regarding N2O production with the addition of trace N2H4 (0.011% per unit NH4+removed) are in agreement with previously reported results from experiments conducted with a similar cycle time (240 min) and volumetric N loading (85.9 mg NH4+-N/L), but with a higher NH4+ oxidation rate (11.95 mgN/gVSS/h). Consequently, these results indicate that exogenous N2H4 minimized N2O production by the CANON process while retaining stable, high-rate nitrogen removal performance.
Effect of N2H4 on N2O production pathways
Under oxygen-limited conditions in the CANON system, HD had no impact on TN removal or N2O production in batch tests F and G. The activity level of heterotrophic bacteria was extremely low, as previously reported (Xiao et al. 2014). Whether anammox activity was inhibited or not (in batch tests A and C), the average N2O production rates of batch tests B and D (with the same added N2H4 concentration) were reduced by 67% and 59%, respectively, in comparison with those of batch tests A and C (without N2H4 addition), respectively. Hydroxylamine oxidoreductase (HAO) plays a key role in production of NO and NO2− (Poughon et al. 2001), which play an important role in the HO and ND pathways of N2O production by AOB (Wunderlin et al. 2012). N2H4 competes strongly with NH2OH for HAO in AOB (Hollocher et al. 1981); therefore, the reduction in total N2O production in the CANON process under oxygen-limited conditions was primarily caused by inhibition of AOB activity by exogenous N2H4, which likely influenced the HO and/or ND pathways of N2O formation.
Moreover, the total N2O production rate was reduced by 67% with inhibition of AnAOB activity, whereas it was reduced by 59% without inhibition of AnAOB activity, suggesting that the inhibitory effect of by N2H4 on N2O production from AOB coexisting with AnAOB was less severe than its effect on N2O production from AOB only. N2H4 significantly increases the specific growth rate (Yao et al. 2013) and abundance of AnAOB (Xiao et al. 2015), as well as specific anammox activity (Ma et al. 2018). Thus, exogenous N2H4 was likely utilized by AnAOB to accelerate anammox activity for TN removal during aeration, which weakened the inhibitory effect on N2O formation from ND by AOB.
In this research, batch tests H, I, and J in the presence of NH2OH under oxygen-limited conditions showed that N2O is produced by the HO and ND pathways during aeration in the CANON process. The contributions of HO and ND to total N2O production during aeration with inhibition of AnAOB activity were 62.1% and 37.9%, respectively. Similar results have been reported in a partial nitrification granular reactor in which the HO pathway accounted for 65% of total N2O production (Rathnayake et al. 2013). These findings demonstrate that N2O production mechanisms are complex and could involve multiple N2O production pathways in CANON process. The variation in the N2O production rate in batch tests H and I is shown in Fig. 4. The N2O production rate in batch test I, in which the ND pathway was inhibited, was lower than that of batch test H during the reaction. HO and ND were both involved in N2O production, as NH2OH was the sole electron donor. With the addition of methanol to inhibit AnAOB activity, the N2O production rate in the presence of NH4+ (batch test C) was slower than that of NH2OH (batch test H). When NH2OH replaces NH4+ as the only substrate, more electrons are released from NH2OH oxidation to NO2− for the respiratory chain of AOB because of a lack of ammonia monooxygenase (AMO) activity (Chandran and Smets 2008).
Under anaerobic conditions, HD by heterotrophic bacteria (Ali et al. 2016; Li et al. 2017) and ND by AOB in the anammox process (Blum et al. 2018; Xiao et al. 2014) were primary pathways for N2O production in the one-stage autotrophic nitrogen removal process. The results of batch tests K, L, and M showed that the contribution of heterotrophic bacteria to N2O production in non-aerated conditions could be ignored. ND via AOB in the anammox process was primarily responsible for N2O generation as previously reported, which NH4+ had no impact on N2O production, yet NH2OH was the pivotal electron donor involved in the reduction of NO2− to N2 and N2O via ND in the anammox process (Xiao et al. 2014). N2H4 was synthesized from NH4+ and NO by hydrazine synthase (HZS) (Kartal et al. 2011) and could convert to NH2OH by disproportionation during anammox metabolism (van der Star et al. 2008). The added N2H4 can be oxidized by AnAOB using hydrazine dehydrogenase (HDH) to release electrons (Yao et al. 2013, 2015, 2016), which are passed to HZS through the electron transport chain to facilitate N2H4 synthesis (Ma et al. 2018). In addition, the conversion of N2H4 to NH2OH accelerated N2O production because NH2OH enhanced N2O emission (Ritchie and Nicholas 1972). Furthermore, some other unknown pathways associated with anammox metabolism might be involved in N2O production in the CANON process (Harris et al. 2015; Ali et al. 2016). The metabolic diversity of AnAOB (Kartal et al. 2011; Oshiki et al. 2016) suggests that different species could have different effects on N2O production. The community structure of AnAOB in the CANON granular system enhanced by exogenous N2H4 has been reported (Xiao et al. 2015) to be approximately 85.75% Candidatus Scalindua and 14.25% Ca. Brocadia anammoxidans. The effects of N2H4 addition on the metabolism of AnAOB of different candidate genera (e.g., Ca. Scalindua), especially with regard to putative N2O production pathway(s), merit further investigation.
Potential biochemical mechanism of N2O reduction
In the present study, exogenous N2H4 was completely degraded during aeration phase I (0–1 h, Fig. 3). NH4+ abundance rapidly decreased during this period, whereas the NO2− concentration increased in the initial 0.5 h, after which it decreased to a low concentration. This result was in good agreement with batch tests A, B, C, and D, which suggested that some of the added N2H4 was utilized by AnAOB to accelerate anammox activity during aeration.
The peak average N2O production rate was reached during the initial 0.5 h of aeration (Fig. 3). This finding basically coincided with a previous report, in which the peak average N2O production rate was reached in the initial 40 min (approximately 0.67 h) of the aeration period, and the HO pathway was the primary mechanism of N2O production (Rathnayake et al. 2013). N2O production by HO is favored at high NH4+ concentrations and NH4+ removal rates (Wunderlin et al. 2013a, b). Sudden fluctuations in the DO concentration, NH4+ concentration, and pH during reactor start-up generally facilitate HO (Rathnayake et al. 2013). N2O production by ND has been reported to be favored by a high NO2− concentration and a low DO concentration (Wunderlin et al. 2013a; Peng et al. 2015), as well as by elevating the NH4+ and DO concentrations in CANON reactors (Harris et al. 2015). Although NO2− accumulated in the initial 0.5 h, the concentration of NO2− was quite low, and it decreased rapidly in the latter part of aeration phase I. Decreasing the NH4+ concentration and maintaining low concentrations of NO2− and DO in the initial 1 h of operation inhibited the formation of N2O by the ND pathway. Hence, the HO pathway, in which N2O is formed from intermediates (e.g., NOH or N2O2H2) (Hollocher et al. 1981) of HAO via NH2OH oxidation (Poughon et al. 2001), is more likely to dominate N2O production in the first hour of SBR operation in the presence of exogenous N2H4, whereas N2O produced via ND is relatively unimportant. Batch tests A, B, C, and D suggested that N2H4 could inhibit AOB activity to reduce total N2O production in the CANON process under oxygen-limited conditions. As expected, the N2O concentration and production rate were quite low (0.8 ppm and 0.15 mgN/L, respectively) even during a single cycle of SBR operation. Consequently, in addition to accelerating anammox activity, exogenous N2H4 competed with NH2OH for HAO in AOB to inhibit the formation of intermediates and facilitate N2O reduction by the HO pathway within the first hour of SBR operation.
During aeration phase II (1–2.5 h, Fig. 3), without N2H4, the NH4+ concentration slowed decreased, but NO2− accumulated rapidly. Increasing the NO2− concentration and decreasing the NH4+ concentration with a low DO level in the reactor could facilitate NO2− reduction to increase the rate of N2O production by the ND pathway (Harris et al. 2015; Peng et al. 2015). In addition, N2O production by HO was gradually inhibited. Enhancement of the ND pathway and weakening of the HO pathway could slightly elevate N2O production during aeration phase II. Therefore, after N2H4 degradation, the contribution of the ND pathway to N2O production was comparable with that of the HO pathway in the latter aeration phase. This finding is similar to that reported for a PN granular reactor (Rathnayake et al. 2013).
Finally, in anaerobic phase III (2.5–4 h, Fig. 3), declining N2O production due to low HD activity during the entire anaerobic phase of SBR operation showed that the ND pathway associated with anammox activity did not form N2O, because exogenous N2H4 added during the aeration phase was completely degraded.
Conclusion
Exogenous N2H4 minimized N2O production from the CANON SBR and retaining high nitrogen removal efficiency. N2O production rate during aeration was mitigated because AOB activity inhibited by N2H4, but N2O production rate under anaerobic conditions was increased by accelerated NH2OH production due to anammox activity enhanced by N2H4. AOB was the main producer of N2O during the enhanced CANON process. A potential biochemical mechanism of N2O reduction in the CANON process enhanced by N2H4 could be explained as follows: added N2H4 completely degraded in the initial aeration period of the SBR to facilitate anammox activity, and meanwhile, compete with NH2OH for HAO in AOB to inhibit intermediates forming of HO pathway to significantly reduce N2O production.
References
Ali M, Rathnayake RM, Zhang L, Ishii S, Kindaichi T, Satoh H, Toyoda S, Yoshida N, Okabe S (2016) Source identification of nitrous oxide emission pathways from a single-stage nitritation-anammox granular reactor. Water Res 102:147–157. https://doi.org/10.1016/j.watres.2016.06.034
APHA (1998) Standard methods for examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC. (No DOI Found)
Blum JM, Jensen MM, Smets BF (2018) Nitrous oxide production in intermittently aerated partial nitritation-anammox reactor: oxic N2O production dominates and relates with ammonia removal rate. Chem Eng J 335:458–466. https://doi.org/10.1016/j.cej.2017.10.146
Castro-Barros CM, Daelman MRJ, Mampaey KE, van Loosdrecht MCM, Volcke EIP (2015) Effect of aeration regime on N2O emission from partial nitritation-anammox in a full-scale granular sludge reactor. Water Res 68:793–803. https://doi.org/10.1016/j.watres.2014.10.056
Chandran K, Smets BF (2008) Biokinetic characterization of the acceleration phase in autotrophic ammonia oxidation. Water Environ Res 80:732–739. https://doi.org/10.2175/106143008X296442
Choi J, Jung S, Ahn YH (2013) Increased hydrazine during partial nitritation process in upflow air-lift reactor fed with supernatant of anaerobic digester effluent. Korean J Chem Eng 30:1235–1240. https://doi.org/10.1007/s11814-013-0041-8
De Clippeleir H, Vlaeminck SE, De Wilde F, Daeninck K, Mosquera M, Boeckx P, Verstraete W, Boon N (2013) One-stage partial nitritation/anammox at 15°Con pretreated sewage:feasibility demonstration at lab-scale. Appl Microbiol Biotechnol 97:10199–10210. https://doi.org/10.1007/s00253-013-4744-x
Domingo-Félez C, Mutlu AG, Jensen MM, Smets BF (2014) Aeration strategies to mitigate nitrous oxide emissions from single-stage nitritation/anammox reactors. Environ Sci Technol 48:8679–8687. https://doi.org/10.1021/es501819n
Frear DS, Burrell RC (1955) Spectrophotometric method for determining hydroxylamine reductase activity in higher plants. Anal Chem 27:1664–1665. https://doi.org/10.1021/ac60106a054
George M, Nagaraja KS, Balasubramanian N (2008) Spectrophotometric determination of hydrazine. Talanta 75:27–31. https://doi.org/10.1016/j.talanta.2007.09.002
Harris E, Joss A, Emmenegger L, Kipf M, Wolf B, Mohn J, Wunderlin P (2015) Isotopic evidence for nitrous oxide production pathways in a partial nitritation-anammox reactor. Water Res 83:258–270. https://doi.org/10.1016/j.watres.2015.06.040
Hollocher TC, Tate ME, Nicholas DJD (1981) Oxidation of ammonia by Nitrosomonas europaea: definite 18O-tracer evidence that hydroxylamine formation involves a monooxygenase. J Biol Chem 256:10834–10836 (No DOI Found) http://www.jbc.org/content/256/21/10834.full.pdf
Hu Z, Lotti T, De Kreuk M, Kleerebezem R, van Loosdrecht M, Kruit J, Mike MSM, Kartal B (2013) Nitrogen removal by a nitritation-anammox bioreactor at low temperature. Appl Environ Microbiol 79:2807–2812. https://doi.org/10.1128/AEM.03987-12
IPCC (2011) Climate Change 2001: The scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge and New York. https://doi.org/10.1256/004316502320517344
Joss A, Salzgeber D, Eugster J, König R, Rottermann K, Burger S, Fabijan P, Leumann S, Mohn J, Siegrist H (2009) Full-scale nitrogen removal fromdigester liquid with partial nitritation and anammox in one SBR. Environ Sci Technol 43:5301–5306. https://doi.org/10.1021/es900107w
Joss A, Derlon N, Cyprien C, Burger S, Szivak I, Traber J, Siegrist H, Morgenroth E (2011) Combined nitritation-anammox: advances in understanding process stability. Environ Sci Technol 45:9735–9742. https://doi.org/10.1021/es202181v
Kampschreur MJ, van de Star WR, Wielders HA, Mulder JW, Jetten MS, van Loosdrecht MC (2008) Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res 42:812–826. https://doi.org/10.1016/j.waters.2007.08.022
Kampschreur MJ, Poldermans R, Kleerebezem R, van der Star WR, Haarhuis R, Abma WR, Jetten MS, van Loosdrecht MC (2009a) Emission of nitrous oxide and nitric oxide from a full-scale single-stage nitritation-anammox reactor. Water Sci Technol 60:3211–3217. https://doi.org/10.2166/wst.2009.608
Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, van Loosdrecht MCM (2009b) Nitrous oxide emission during wastewater treatment. Water Res 43:4093–4103. https://doi.org/10.1016/j.watres.2009.03.001
Kartal B, Maalcke WJ, de Almeida NM, Cirpus I, Gloerich J, Geerts W, Op den Camp HJM, Harhangi HR, Janssen-Megens EM, Francoijs KJ, Stunnenberg HG, Keltjens JT, Jetten MSM, Strous M (2011) Molecular mechanism of anaerobic ammonium oxidation. Nature 479:127–130. https://doi.org/10.1038/nature10453
Li K, Fang F, Wang H, Wang C, Chen Y, Guo J, Wang X, Jiang F (2017) Pathways of N removal and N2O emission from a one-stage autotrophic N removal process under anaerobic conditions. Sci Rep-UK 7:42072 https://www.nature.com/ articles/srep42072
Ma J, Yao H, Yu H, Zuo L, Li H, Ma J, Xu Y, Pei J, Li X (2018) Hydrazine addition enhances the nitrogen removal capacity in an anaerobic ammonium oxidation system through accelerating ammonium and nitrite degradation and reducing nitrate production. Chem Eng J 335:401–408. https://doi.org/10.1016/j.cej.2017.10.132
Okabe S, Oshiki M, Takahashi Y, Satoh H (2011) NO emission from a partial nitrification–anammox process and identification of a key biological process of no emission from anammox granules. Water Res 45:6461–6470. https://doi.org/10.1016/j.watres.2011.09.040
Oshiki M, Ali M, Shinyako-Hata K, Satoh H, Okabe S (2016) Hydroxylamine-dependent anaerobic ammonium oxidation (anammox) by “Candidatus Brocadia sinica”. Environ Microbiol 18:3133–3143. https://doi.org/10.1111/1462-29200.13355
Peng L, N J YL, Yuan Z (2015) The combined effect of dissolved oxygen and nitrite on N2O production by ammonia oxidizing bacteria in an enriched nitrifying sludge. Water Res 73:29–36. https://doi.org/10.1016/j.watres.2015.01.021
Peng L, Liu Y, Ni BJ (2016) Nitrous oxide production in completely autotrophic nitrogen removal biofilm process: a simulation study. Chem Eng J 287:217–224. https://doi.org/10.1016/j.cej.2015.11.026
Pocquet M, Wu Z, Queinnec I, Spérandio M (2016) A two pathway model for N2O emissions by ammonium oxidizing bacteria supported by the NO/N2O variationl. Water Res 88:948–959. https://doi.org/10.1016/j.watres.2015.11.029
Poughon LC, Dussap G, Gros JB (2001) Energy model and metabolic flux analysis for autotrophic nitrifiers. Biotechnol Bioeng 72:416–433. https://doi.org/10.1002/1097-0290(20000220)72:4<416::AID-BIT1004>3.3.CO;2-4
Rassamee V, Sattayatewa C, Pagilla K, Chandran K (2011) Effect of oxic and anoxic conditions on nitrous oxide emissions from nitrification and denitrification processes. Biotechnol Bioeng 108:2036–2045. https://doi.org/10.1002/bit.23147
Rathnayake RMLD, Song Y, Tumendelger A, Oshiki M, Ishii S, Satoh H, Toyoda S, Yoshida N, Okabe S (2013) Source identification of nitrous oxide on autotrophic partial nitrification in a granular sludge reactor. Water Res 47:7078–7086. https://doi.org/10.1016/j.watres.2013.07.055
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. https://doi.org/10.1126/science.1176985
Ritchie GAF, Nicholas DJ (1972) Identification of sources of nitrous-oxide produced by oxidative and reductive processes in nitrosomonaseuropaea. Biochem J 126:1181–1191. https://doi.org/10.1042/bj1261181
Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3:1–24. https://doi.org/10.3389/fmicb.2012.00372
Shapleigh JP, Payne WJ (1985) Differentiation of c,d1 cytochrome and copper nitrite reductase production in denitrifiers. FEMS Microbiol Lett 26:275–279. https://doi.org/10.1111/j.1574-6968.1985.tb01610.x
Sliekers AO, Derwort N, Gomez JL, Strous M, Kuenen JG, Jetten MS (2002) Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res 36:2475–2482. https://doi.org/10.1016/S0043-1354(01)00476-6
van der Star WR, van de Graaf MJ, Kartal B, Picioreanu C, Jetten MSM, van Loosdrecht MCM (2008) Response of anaerobic ammonium-oxidizing bacteria to hydroxylamine. Appl Environ Microbiol 74:4417–4426. https://doi.org/10.1128/AEM.00042-08
Vázquez-Padín JR, Pozo MJ, Jarpa M, Figueroa M, Franco A, Mosquera-Corral A, Campos JL, Méndez R (2009) Treatment of anaerobic sludge digester effluents by the CANON process in an air pulsing SBR. J Hazard Mater 166:336–341. https://doi.org/10.1016/j.jhazmat.2008.11.055
Wang X, Fang F, Chen Y, Guo J, Li K, Wang H (2017) N2O micro-profiles in biofilm from a one-stage autotrophic nitrogen removal system by microelectrode. Chemosphere 175:482–489. https://doi.org/10.1016/j.chemosphere.2017.02.026
Watt GW, Chrisp JD (1952) A spectrophotometric method for the determination of hydrazine. Anal Chem 24:2006–2008. https://doi.org/10.1021/ac60072a044
Weissenbacher N, De Clippeleir H, Boeckx P, Hell M, Chandran K, Murthy S, Wett B (2011) Control of N2O-emissions from sidestream treatment. Proc Water Environ Fed 18:236–248. https://doi.org/10.2175/193864711802639255
Wunderlin P, Mohn J, Joss A, Emmenegger Land Siegrist H (2012) Mechanisms of N2O production in biological wastwater treatment under nitrifying and denitrifying conditions. Water Res 46:1027–1037. https://doi.org/10.1016/j.watres.2011.11.080
Wunderlin P, Siegrist H, Joss A (2013a) Online N2O measurement: the next standard for controlling biological ammonia oxidation? Environ Sci Technol 47:9567–9768. https://doi.org/10.1021/es402971p
Wunderlin P, Lehmann MF, Siegrist H, Tuzson B, Joss A, Emmenegger L, Mohn J (2013b) Isotope signatures of N2O in a mixed microbial population system: constraints on N2O producing pathways in wastewater treatment. Environ Sci Technol 47:1339–1348. https://doi.org/10.1021/es303174x
Xiao P, Cai Q, Zhang D, Yao Z, Lu P (2014) Characteristics of nitrogen removal and nitrous oxide production in canon process. J Chem Technol Biot 89:552–558. https://doi.org/10.1002/jctb.4153
Xiao P, Lu P, Zhang D, Han X, Yang Q (2015) Effect of trace hydrazine addition on the functional bacterial community of a sequencing batch reactor performing completely autotrophic nitrogen removal over nitrite. Bioresour Technol 175:216–223. https://doi.org/10.1016/j.biortech.2014.10.084
Yang J, Trela J, Plaza E, Tjus K (2013) N2O emissions from a one stage partialnitrification/anammox process in moving bed biofilm reactors. Water Sci Technol 68:144–152. https://doi.org/10.2166/wst.2013.232
Yao Z, Cai Q, Zhang D, Xiao P, Lu P (2013) The enhancement of completely autotrophic nitrogen removal over nitrite (CANON) by N2H4 addition. Bioresour Technol 146:591–596. https://doi.org/10.1016/j.biortech.2013.07.121
Yao Z, Lu P, Zhang D, Wan X, Li Y, Peng S (2015) Stoichiometry and kinetics of the anaerobic ammonium oxidation (anammox) with trace hydrazine addition. Bioresour Technol 198:70–76. https://doi.org/10.1016/j.biortech.2015.08.098
Yao Z, Zhang D, Xiao P, Peng S, Lu P, Wan X, He Q (2016) Long-term addition of micro-amounts of hydrazine enhances nitrogen removal and reduces NO and NO3− production in a sbr performing anammox. J Chem Technol Biotechnol 91:514–521. https://doi.org/10.1002/jctb.4606
Funding
This research was financially supported by National Natural Science Foundation of China (41502328, 51708077), Science and Technology Funds of Chongqing Education Commission and (KJ1600905), and Chongqing Water Environment Basic Investigation and Technology Application Development (Huan Ke Zi NO. (04) 2018).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengying Xiao and Shuo Ai contributed to the work equally and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Xiao, P., Ai, S., Zhou, J. et al. N2O profiles in the enhanced CANON process via long-term N2H4 addition: minimized N2O production and the influence of exogenous N2H4 on N2O sources. Environ Sci Pollut Res 27, 37188–37198 (2020). https://doi.org/10.1007/s11356-019-06508-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06508-w