Abstract
Nitrous oxide (N2O) accumulation in biological nitrogen removal has drawn much attention in recent years; however, nitric oxide (NO) accumulation in denitrification was rarely studied. In this study, NO and N2O accumulation during nitrite denitrification in a lab-scale sequencing batch reactor (SBR) were investigated. Results showed that low pH (< 7) and high influent loading (> 360:90) (COD:NO2−-N) caused serious NO and N2O accumulation. The maximal NO accumulation of 4.96 mg L−1 was observed at influent loading of 720:180 and the maximal N2O accumulation of 46.29 mg L−1 was found at pH of 6. The NO accumulation was far higher than the values reported in previous studies. In addition, the high NO accumulation could completely inhibit the activities of reductases involved in denitrification. High NO and N2O accumulation were mainly caused by significant free nitrous acid (FNA) and NO inhibition at low pH and high influent loading. There were significant differences on NO and N2O accumulation at different carbon to nitrogen (COD/N). Low COD/N (≤ 4) could mitigate NO accumulation, but led to high N2O accumulation. It is speculated that NO accumulation is related to the rapid denitrification with accumulated electron in anaerobic stage at high COD/N. N2O accumulation is attributed to intense electron competition at low COD/N. High dissolved oxygen (DO) of 4.04 mg L−1 was detected during NO detoxification in this experiment, which is speculated to be partly caused by NO dismutation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological nitrogen removal (BNR) is an effective and economic technology in wastewater treatment. Heterotrophic denitrification, as one of important process in BNR, has been widely investigated in the past few decades. The complete heterotrophic denitrification is the sequential reduction of nitrate (NO3−-N), nitrite (NO2−-N), nitric oxide (NO), nitrous oxide (N2O), and finally to nitrogen gas (N2) (Brotto et al. 2015). Sequential actions of several enzymes, such as nitrate reductase (Nar), nitrite reductase (Nir), nitric oxide reductase (Nor), and nitrous oxide reductase (Nos), are involved in this process (Pan et al. 2012). However, recent research showed that NO and N2O were detected under certain conditions (Lu and Chandran 2010; Zhang et al. 2015). It is well known that NO and N2O have adverse effects on the environment and living organisms. N2O, as a potent greenhouse gas, can cause highly undesirable greenhouse effect even at a low emission rate. Meanwhile, N2O is suggested as the dominant ozone depleting substance in the twenty-first century (Pan et al. 2012). NO is a potent cytotoxin, exerting severe biological impacts, such as being toxic to the metabolism of microorganism, including many groups of prokaryotes and eukaryotes (Wang et al. 2016b). Moreover, literature showed that NO also contributed to destruction of the ozone layer and to precursors of photochemical smog (Lu and Chandran 2010). Therefore, in order to mitigate the NO and N2O production during heterotrophic denitrification, it is imperative to investigate the mechanism of NO and N2O accumulation in this process.
Many studies reported the factors affecting N2O production in denitrification. Pan et al. (2012) investigated the effect of pH on N2O accumulation during denitrification. Results showed that substantial N2O accumulation was observed at low pH levels (6.0–6.5). Kim and Bae (2000) noticed that 25–40% of the NO3−-N was converted to N2O at influent concentration of 1500 mg L−1, while no N2O was accumulated when influent concentration was 750 mg L−1 (Kim and Bae 2000). Riya et al. (2015) also found that high nitrogen loading could lead to high N2O emission. Wu et al. (2009) studied the effects of carbon to nitrogen (COD/N) on N2O emission, showing that both high and low COD/N could cause high N2O emission. Alinsafi et al. (2008) found that NO2−-N accumulation could inhibit Nos, leading to N2O production. However, Zhou et al. (2008) believed that free nitrous acid (FNA), rather than NO2−-N, might be the true inhibitor on N2O reduction.
Compared with N2O, NO has received limited attention in recent researches. Adouani et al. (2015) investigated the impact of the temperature (5–20 °C) on NO emissions during heterotrophic denitrification, showing that NO emission increased when the temperature decreased. Wang et al. (2016a) studied the characterization of NO emission from a full-scale biological aerated filter, suggesting that influent ammonia loading, NO2−-N concentration, and dissolved oxygen (DO) had significant effects on NO emission. Ribera-Guardia and Pijuan (2017) found that NO linearly correlated with the ammonia oxidation rate. In addition, results showed that NO is chemically produced when pH is decreased with HCl (Ribera-Guardia and Pijuan 2017). Schulthess et al. (1995) reported that NO accumulation occurred after a pulse of the nitrite, which caused a slight inhibition on reductases involved in denitrification. Ettwig et al. (2010) found that NO also was produced in nitrite-based anaerobic methane oxidation (n-DAMO), but the NO was decomposed into N2 and O2 instead of reducing to N2O.
According to previous researches, most of the them were focused on NO production during ammonia oxidation. However, the factors and mechanisms responsible for NO accumulation as well as its relationship with N2O during nitrite denitrification was not characterize well. In addition, high NO accumulation was observed in our pre-experiment, which has a significant on denitrification. Therefore, in order to better understand the process of nitrite denitrification, further research is needed regarding the NO and N2O accumulation in this process.
In the present study, a serial of tests were performed to investigate the NO and N2O accumulation during nitrite denitrification. The main objectives were to (1) characterize NO and N2O accumulation under different conditions; (2) clarify the mechanism of NO and N2O accumulation; and (3) demonstrate the relationship between high NO accumulation and nitrite denitrification.
Materials and methods
Reactor setup and operation
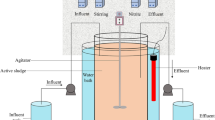
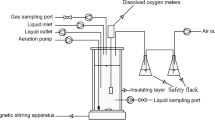
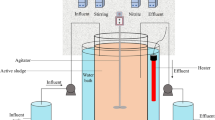
A glucose utilizing denitrifying culture was enriched in an 8 L sequencing batch reactor (SBR), which was inoculated with activated sludge from the oxidation ditch of a domestic wastewater treatment plants in Xi’an, China. The SBR was operated at 30 ± 1 °C with a cycle time of 8 h, consisting of 5-min feeding, 60-min anaerobic reaction, 390-min anoxic reaction, 20-min setting, and 5-min decanting. The pH of the mixed liquor in reactor was 7.0 ± 0.1 after feeding. Three liters of synthetic wastewater (the composition is shown in the “Synthetic wastewater” section) was pumped into the reactor in each feeding period, resulting in a hydraulic retention time (HRT) of 21 h. Two hundred fifty milliliters of mixed liquor was wasted per day to achieve the solids retention time (SRT) of approximately 30 days. The SBR operation was automatically controlled by a programmable logic controller (PLC). The mixed liquor suspended solids (MLSS) and mixed liquor volatile suspended solids (MLVSS) concentrations were about 5000 and 3500 mg L−1, respectively.
The SBR was operated about 6 months to achieve steady state, with > 99% removal of NO2−-N and chemical oxygen demand (COD). After that, the batch tests were performed. Before the tests, the active sludge was washed three times with tap water to remove residual COD, NO2−-N, and other substances, and then, sparged with N2 for 5 min to ensure the anaerobic condition. Each test was performed in triplicates. After each test, the SBR was operated normally at least for 24 h before another test was performed.
Synthetic wastewater
The synthetic wastewater used as influent in SBR consisted of (per liter) 0.37 g C6H12O6, 0.44 g NaNO2, 0.016 g KH2PO4 g, 0.041 g CaCl2, and 1 mL trace element solution. The trace element solution contained (per liter) 0.15 g H3BO3, 0.03 g CuSO4·5H2O, 0.18 g KI, 0.12 g MnCl2·4H2O, 0.06 g Na2MoO4·2H2O, 0.12 g ZnSO4·7H2O, 0.15 g CoCl2·6H2O, and 10 g C10H14N2Na2O8·2H2O.
Experimental strategy
Test 1: Effect of COD/N on NO and N2O accumulation
To investigate the effect of COD/N on NO and N2O accumulation, the tests were conducted at three different COD/N: 1, 4, and 6. The initial COD concentrations were set to 90, 360, and 540 mg L−1, respectively. The initial pH of all tests was controlled at 6.9 ± 0.1. After 60 min of anaerobic reaction, the NO2−-N of 90 mg L−1 was added to reactor. The tests were operated until NaNO2 was exhausted when COD/N were 4 and 6. As to COD/N of 1, the test lasted for 540 min.
Test 2: Effect of initial pH on NO and N2O accumulation
Five tests were performed on initial pH of 6, 7, 8, 9, and 10 to investigate the effect of pH on NO and N2O accumulation. At the beginning of each test, the C6H12O6 and other nutrient substances were added to the reactor, resulting in the initial COD of 360 mg L−1. After 60 min of anaerobic reaction, the NaNO2 was added to achieve an initial NO2−-N concentration of 90 mg L−1. Each test was operated until NaNO2 was exhausted.
Test 3: Effect of influent loading on NO and N2O accumulation
Test 3 were carried out to study the effect of influent loading on NO and N2O accumulation. The same initial pH for all tests is used in test 2. The initial COD concentrations were set to 90, 180, 360, and 720 mg L−1. The NO2−-N concentrations of 22.5, 45, 90, and 180 mg L−1 were injected into the reactor after 60 min of anaerobic reaction to achieve same initial COD/N of 4. Each test was operated until NaNO2 was exhausted.
Analysis and measurements
Ammonium nitrogen (NH4+-N), NO2−-N, NO3−-N, COD, MLSS, and MLVSS were measured following Standard Method 5220 (APHA 2005). Total nitrogen (TN) was defined as the sum of NH4+-N, NO2−-N, and NO3−-N concentrations. DO and pH were monitored by DO meter (HACH HQ30d, USA) and pH meter (PHS-3C, China) respectively. Dissolved NO and N2O in the tests were continuously monitored using calibrated real-time online NO and N2O microsensors (NO-500, Unisense A/S, Aarhus, Denmark; N2O-500, Unisense A/S, Aarhus, Denmark) with a response time of 10 s. The absolute abundances of Nir, Nor, Nos, and NO dismutase (Nod) were quantified by flurogenic quantitative polymerase chain reaction. All statistical analyses were performed using SPSS 19.0 and Origin 8.0.
Results
Effect of COD/N on NO and N2O accumulation
NO and N2O accumulation under different COD/N are shown in Fig. 1. The peak of NO accumulation increased with the increase of COD/N. However, N2O accumulation showed an opposite trend with NO. It suggests that the mechanism of NO accumulation under different COD/N may be different with that of N2O.
As shown in Fig. 1a, NO accumulation increased quickly as soon as NaNO2 was added in the reactor at COD/N of 1, which decreased gradually after reaching the peak of 0.14 mg L−1. NO accumulation lasted for about 110 min. Interestingly, two different stages were observed in N2O accumulation. In the first stage, N2O increased rapidly for about 96 min, and then decreased in a short time. After that, N2O increased again, but the accumulation rate (0.03 mg L−1 min−1) was lower than the first one (0.041 mg L−1 min−1). N2O accumulation decreased gradually after the peak was achieved. The decrease rate of N2O was closely equal to emission rate, which indicated no N2O reduction occurred during this period.
At COD/N of 4, NO accumulation lasted for about only 2 min, which was much shorter than that COD/N of 1. Two different stages of N2O accumulation also were observed at COD/N of 4. In the first stage, N2O accumulation increased quickly at beginning of anoxic stage, and then decreased to a low level in a few minutes. In the second stage, N2O accumulation showed a similar variation, but the N2O accumulation rate and reduction rate were lower than that in the first stage. Furthermore, a higher peak of N2O accumulation was found in the second stage.
When COD/N was 6, the variation of NO accumulation was similar to COD/N of 1, but its duration is relatively short. N2O accumulation and its duration were much smaller than that COD/N of 1 and 4. Zhao et al. (2013) also found that a low COD/N could stimulate more N2O accumulation, which was similar with present test.
Effect of initial pH on NO and N2O accumulation
NO and N2O accumulation at different initial pH are shown in Fig. 2. The peak of NO and N2O accumulation decreased with the increase of initial pH, and the maximal NO and N2O accumulation were 3.58 and 46.29 mg L−1, respectively. At initial pH of 6, NO and N2O concentration increased immediately as soon as NaNO2 was added in the reactor. However, N2O did not increase when NO increased to the peak, which maintained at a constant level until NO reduced to a very low level. After that, N2O increased again, and then reduced to N2 after the peak was achieved. Unexpectedly, high DO concentration (4.04 mg L−1) was observed during NO accumulation in this test. When the initial pH was higher than 6, NO decreased quickly after an increase. It was different with that initial pH of 6, and the peaks of NO were also significantly lower than pH of 6. As shown in Fig. 2, the peak of N2O accumulation at different initial pH showed a similar trend with NO; the maximal N2O accumulation was achieved at initial pH of 6. However, when the initial pH was higher than 7, little N2O was detected, and the duration of N2O accumulation also is shorten simultaneously.
Effect of influent loading on NO and N2O accumulation
As shown in Fig. 3, the maximal NO accumulation of 4.96 mg L−1 was observed with the influent loading of 720:180. There was a slight decrease when influent loading decreased from 180:45 to 90:22.5. The duration of NO accumulation increased with the increase of influent loading except for 360:90. Interestingly, Fig. 3b showed that NO increased rapidly as soon as NaNO2 was added in the reactor and then decreased quickly after reaching the peak. After a few minutes decrease, the NO decrease rate slowed down gradually, which was different with other influent loadings. N2O accumulation increased with the increase of influent loading, and the maximum of N2O accumulation was 20.43 mg L−1. The duration of N2O accumulation increased when influent loading increased from 90:22.5 to 360:90. However, the duration of N2O accumulation decreased when influent loading was 720:180, and the variation of N2O accumulation was similar to that at pH of 6 (Fig. 1).
Discussion
Mechanism of NO and N2O accumulation under different COD/N
Results showed that NO increased rapidly at the beginning of anoxic stage, which may be related to the quantity of electron accumulation during the anaerobic stage. Pan et al. (2013b) found that COD could be converted to reduced mediator (Mred) in the absence of electron acceptors. When electron acceptor (such as NO2−-N) was added in the system, Mred would be quickly oxidized to oxidized mediator (Mox) (\({\mathrm{NO}}_2^{-}+1/2\mathrm{Mred}+{\mathrm{H}}^{+}\to \mathrm{NO}+1/2\mathrm{Mox}+{\mathrm{H}}_2\mathrm{O}\)). The total concentration of electron carriers (Mtot) was defined as the sum of Mred and Mox. Therefore, it can be speculated that the number of Mred is approximately equal to Mtot during anaerobic stage, namely most of the electron mediators are reduced form. The accumulated Mred made the conversion rate of reductases in optimal state. When the NO2−-N was added in the reactor, the accumulated Mred could react with NO2−-N immediately due to the high activity of Nir, which resulted in rapid NO accumulation at beginning of anoxic stage.
In addition, result showed that high COD/N could cause a serious effect on NO accumulation. It is known that NO2−-N is first converted to NO, then to N2O, and finally to N2 during nitrite denitrification. Therefore, NO accumulation was determined by nitrite reduction rate. As shown in Table 1, NO reduction rate was slower than the nitrite reduction rate under all COD/N at beginning of anoxic stage, which resulted in NO accumulation. Furthermore, nitrite reduction rate decreased with the decrease of COD/N due to the lack of sufficient electron donor, leading to low NO accumulation occurred at low COD/N. Similar result was found during nitrate to nitrite (Li et al. 2013). However, the duration of NO accumulation showed a different trend. The duration of NO accumulation under different COD/N may be caused by following reasons: when COD/N was 1, the serious shortage of carbon source may result in a slower NO reduction rate during the stage of NO decrease. Subsequently, a long duration of NO accumulation was observed at COD/N of 1. In addition, previous report showed that high NO accumulation was harmful to microbial activities (Schulthess et al. 1995). When NO accumulation reached to a relatively high level (but the microbial activities was not inhibited completely), microorganisms would reduce NO to N2O immediately to mitigate the NO toxicity. Therefore, fast NO reduction was found at high NO accumulation. However, in present experiment, the shortest duration of NO accumulation occurred when the COD/N was 4. The possible reason is that the parent reactor was operated at COD/N of 4 during normal operation; microorganisms had adapted this condition for a long time, which may lead to a faster NO reduction rate than other COD/N.
As shown in Fig. 1, the peak of N2O accumulation decreased with the increase of COD/N, which was similar with the result reported by Yan et al. (2017). Pan et al. (2013a) reported that electron competition among four reductases would occur during denitrification, and low COD/N could potentially intensify the electron competition through their possible effect of slowing down the electron supply rate. It is known that Nos has lower affinity for electron than other reductases, resulting in more serious N2O accumulation at low COD/N. Thus, it indicates that the different N2O accumulations may be caused by different electron competition among denitrification reductases.
Results showed that there were two different N2O accumulation stages at COD/N of 1 and 4. Figure 1 a and b showed that COD was not stored completely during anaerobic stage. Thus, two different reduction stages were observed in this test: denitrification in the presence of external carbon source (exogenous denitrification) and denitrification without an external carbon source (endogenous denitrification). As shown in Fig. 1 a and b, COD concentration did not decrease after the first N2O accumulation was reduced completely. It indicates that the external carbon source had been consumed exhaustively during this stage. Meanwhile, the pH increased rapidly until N2O accumulation increased again. It is widely known that exogenous denitrification rate is faster than endogenous denitrification. Therefore, a faster pH variation was observed in exogenous denitrification. According to the variation of COD and pH, it can be inferred that the two different N2O accumulation stages at COD/N of 1 and 4 may be mainly attributed to the two stages of denitrification. In addition, literature showed that more N2O accumulation occurred with intracellular polymer as electron donor compared with external carbon source. Therefore, a higher N2O accumulation was observed in the second stage (Wu et al. 2014).
Mechanism of NO and N2O accumulation under different pH
Ribera-Guardia and Pijuan (2017) suggested that there was a close correlation between NO accumulation and pH. However, they found that NO was produced by chemodenitrification, but not by biodenitrification. In their experiment, when HCl was added in the reactor, NO increased rapidly to a peak and then decreased sharply. Thus, they believed that it could be caused by the deprotonation of HNO2 (2HNO2 ↔ NO + NO2 + H2O). Compared with previous study, there were some different results in the present tests. As shown in Fig. 2, high NO accumulation was achieved at initial pH of 6, which lasted for about 200 min. It was different with the result that reported by Ribera-Guardia and Pijuan (2017). Therefore, it indicates that NO accumulation may be produced biologically in the present test. In addition, literature showed that nitrite could inhibit the Nor activity, leading to NO accumulation (Schulthess et al. 1995). In test 1, the same initial NO2−-N concentration was supplied, but the different NO accumulations were achieved. It indicates that NO accumulation is not caused by NO2−-N concentration in the present test. However, Fig. S1 showed that there was a good correlation between NO and FNA at different initial pH; the peak of NO accumulation increased with the increase of FNA. Therefore, it can be speculated that NO accumulation is mainly caused by high FNA. In addition, literature showed that pH could affect the conversion rates of enzymes involved in NO production (Nir) and reduction (Nor), leading to NO accumulation (Blum et al. 2018).
Figure 2 a showed that high NO accumulation caused a significant inhibition on reductases during denitrification. At the beginning of the anoxic stage, Nor and Nos were inhibited due to the low pH and high FNA, resulting in rapid increase of NO and N2O. When NO concentration reached to the maximum of 3.57 mg L−1, there were no obvious change in NO2−-N and N2O until NO reduced to a low level. It is possible that Nir and Nor were inhibited completely by NO. The decrease of NO during this period may be attributed to emission and detoxification by another way. It is known that dissolved NO in the liquid phase could emit to atmosphere through air stripping and diffusion. Therefore, when NO was accumulated in the reactor, a part of NO would emit to atmosphere, resulting in the decrease of NO. However, result showed that the NO decline rate was higher than that of emission rate, indicating that the decline of NO was not only contributed to emission but also related to biodegradation. Ettwig et al. (2010) found that NO could be decomposed into N2 and O2 by Nod during n-DAMO (2NO = N2 + O2), which may result in the decrease of NO. Results showed that DO was detected during NO accumulation, and the more NO accumulated, the higher the DO detected. Therefore, it is possible that NO dismutation occurs in this experiment. However, literature showed that NO could cause a positive inference with some models of optical DO probes (Klaus et al. 2017), including the HACH DO used in this experiment. Therefore, the interference caused by NO may be an important reason for the increase of DO. In order to determine if DO was produced during NO reduction, an abiotic test was performed. In this test, NO was bubbled into the reactor with no other substances were added; DO and NO were recorded simultaneously until no DO was detected. The correlation equation between NO and DO was obtained by regression analysis. Then, the real DO could be calculated by the correlation equation. As shown in Fig. S2a, the detected DO was higher than interfering DO, which indicates that DO was produced in this test. Similar result was observed at influent of 720:180 (Fig. S2b). In addition, in order to further determine the possibility of NO dismutation, the absolute abundances of functional genes in this system were identified (Table 2). Results showed that high Nod abundance of (5.94 ± 0.74) × 105 copies·g−1 was detected, which was higher than that of Nor. This confirmed that NO dismutation could occur in this experiment. According to the results and literatures, it can be inferred that NO dismutation may be another important way to cause the decrease of NO in the present study. When NO decreased to the low level, the inhibitions of Nir and Nor were mitigated gradually, but Nos was still inhibited by FNA. Therefore, the end product was mainly N2O rather than N2. After a period of operation, Nos activity was recovered gradually with the decrease of FNA; the N2O was transformed into N2, and it was stabilized at low level during the rest period of the cycle.
Results showed that pH had a significant effect on N2O accumulation. There are three possible reasons for this phenomenon. Firstly, pH may have direct effect on denitrifiers and activities of four reductases involved in denitrification. It has been previously suggested that a slower turnover of Nos at low pH as compared with Nar, Nir, and Nor, resulting in N2O as the end product of denitrification (Bergaust et al. 2010). Liu et al. (2014) also reported that the Nos activity declined dramatically when pH was lower than 6.1, which was similar to the present test. In addition, as shown in Table 3, N2O reduction rate increased with the increase of initial pH. Therefore, it can be speculated that the high N2O accumulation is attributed to low Nos activity and turnover at low pH. Secondly, denitrification involves carbon oxidation to supply electrons and nitrogen oxides reduction to consume electrons. When electron production rate cannot meet the electron consumption, electron competition among different reductases would occur. Literature showed that low pH had adverse impact on carbon oxidation rate, which would intensify electron competition (Pan et al. 2012). Therefore, it could be inferred that high N2O accumulation at low pH was possibly related to the reduced carbon oxidation rate. Thirdly, it has been proven that FNA could bind to the active sites of copper-contained enzymes, resulting in N2O accumulation, and the FNA inhibition concentration was 0.0007–0.001 mg L−1 (Zhou et al. 2008). However, in this experiment, the minimal FNA inhibition concentrations at pH of 6 and 7 (Table 3) were lower than the inhibition threshold reported in previous study. It may be caused by the different culture systems and microbial structures. According to present study and previous reports, it can be concluded that FNA played an important role in N2O accumulation during denitrification.
Mechanism of NO and N2O accumulation under different influent loadings
Based on Michaelis-Menten kinetics, a higher substrate concentration could result in a faster reaction rate. Subsequently, the faster reaction rate might lead to more NO accumulation when NO2−-N was used as electron acceptor. In addition, Castro-Barros et al. (2016) reported that nitrite could improve the expression of Nir, which stimulated the NO production. Therefore, the more NO was accumulated with the increase of influent loading. As shown in Table 4, nitrite reduction rate increased with the increase of influent loading at the beginning of anoxic stage except for at 720:180. The peak of NO accumulation followed with the trend of nitrite reduction rate. However, when influent loading was 720:180, the nitrite reduction rate decreased remarkably, but NO accumulation with influent loading of 720:180 was much higher than other influent loadings. As discussed in the “Mechanism of NO and N2O accumulation under different pH” section, high FNA was harmful to the activities of reductases involved in denitrification, resulting in an inhibition on denitrification. Compared with Nir, Nor was easier to be inhibited when suffering high FNA concentration. In this experiment, FNA concentration increased with the increase of nitrite in the influent. The NO reduction would be inhibited significantly when FNA concentration was 0.12 mg L−1, but nitrite reduction was not inhibited obviously. Therefore, it can be inferred that the NO accumulation with influent loading of 720:180 may be mainly attributed to FNA inhibition, when NO concentration reached to a high level, which would cause a more serious inhibition on Nor, resulting in a higher NO accumulation. In order to mitigate NO toxicity, microorganisms would convert NO to other substances. The mechanism of NO detoxification at influent loading of 720:180 was similar with that of at pH of 6 (Mechanism of NO and N2O accumulation under different pH).
Figure 3 b showed that two different NO decrease rates were observed. The possible reasons are as follows. The activities of Nir, Nor, and Nos were inhibited mostly when NO reaches to the peak. In order to mitigate the NO inhibition, most of the NO might be converted to other substances in a short time through another pathway, namely NO dismutation (as discussed in “Mechanism of NO and N2O accumulation under different pH” section). NO decreased rapidly during this stage. The inhibition on denitrification was relieved, and the activities of Nir and Nor recovered gradually when NO decreased to a low level. After that, NO2−-N and NO were reduced by normal denitrification, which led to NO decrease rate slowed down.
Results showed that high influent loading was more likely to lead to N2O accumulation, which was similar with Kim and Bae (Kim and Bae 2000). They found that 25–40% of the nitrate in the influent was converted to N2O at a nitrate concentration of 1500 mg L−1. However, no N2O was produced when the nitrate concentration decreased to 750 mg L−1. In this experiment, two main reasons were responsible for the different N2O accumulations. FNA concentration increased with the increase of nitrite in the influent, resulting in higher N2O accumulation at high influent loading. In addition, NO inhibition might be another possible reason for N2O accumulation. It is known that NO is a toxic substance, which can inhibit the reductases in denitrification. Ni and Yuan found that NO inhibit constants for the reductions of nitrite, NO, and N2O were 0.5, 0.3, 0.075, respectively (Ni and Yuan 2013). It suggests that Nos is most susceptible to NO inhibition, which may be inhibited by a relative low NO concentration compared with other reductases. Therefore, it can be speculated that the higher the NO concentration, the stronger the Nos inhibited, and the more N2O accumulation was observed in this test (Table 4).
Implications to the operation of nitrite denitrification process
Wastewater treatment plants are always subjected to large fluctuations in water quality, which would result in NO and N2O accumulation. It is known that NO and N2O have adverse effect on environment, human, and microorganism. Therefore, in order to reduce NO and N2O production during nitrite denitrification, it is significant to investigate the mechanism of NO and N2O production under different conditions.
According to the results of this study, there were mainly three aspects that should be cautious to avoid high NO and N2O production in nitrite denitrification. Firstly, results suggest that low COD/N could cause high N2O accumulation, but it is conducive to mitigate NO accumulation. Thus, an appropriate COD/N should be controlled to reduce NO and N2O accumulation simultaneously. Based on the above results, the COD/N should be controlled in 4–6. Secondly, results showed that NO and N2O accumulation was much high at low pH conditions (Fig. 2). Literature showed that strong alkalizer carbon source, such as CH3COONa, could potentially reduce the risk of NO and N2O accumulation due to the high increase of pH in denitrification (Li et al. 2014). In addition, the alkaline matter (e.g., calcium hydroxide) can be added for pH adjustment. Thirdly, the high influent loading could cause a significant NO and N2O accumulation (Fig. 3). Therefore, it is important to reduce influent loading as much as possible in the practical operation. The addition of regulating pool would be an optimal choice.
Conclusion
The NO and N2O accumulation during nitrite denitrification were investigated in this study. The main conclusions are as follows:
- (1)
High NO accumulation of 4.96 mg L−1 was detected in this experiment, which was far higher than the values reported in previous studies. The nitrite denitrification could be inhibited completely by the high NO accumulation.
- (2)
NO accumulation increased with the increase of COD/N due to the rapid denitrification with accumulated electron in anaerobic stage at high COD/N. However, N2O accumulation showed an opposite trend with NO, which is related to the intense electron competition at low COD/N.
- (3)
Low pH and high influent loading could result in serious NO and N2O accumulation. The maximal NO and N2O accumulation of 3.58 and 46.29 mg L−1 were observed at pH of 6. When influent loading was 720:180, the maximums of NO and N2O accumulation were 4.96 and 20.43 mg L−1, respectively. High NO and N2O accumulation are mainly attribute to the significant FNA and NO inhibition at low pH and high influent loading.
- (4)
High DO of 4.04 mg L−1 was detected during NO accumulation, which is presumed to be partly caused by NO dismutation.
References
Adouani N, Limousy L, Lendormi T, Sire O (2015) N2O and NO emissions during wastewater denitrification step: influence of temperature on the biological process. Comptes Rendus Chimie 18:15–22. https://doi.org/10.1016/j.crci.2014.11.005
Alinsafi A, Adouani N, Béline F, Lendormi T, Limousy L, Sire O (2008) Nitrite effect on nitrous oxide emission from denitrifying activated sludge. Process Biochem 43:683–689. https://doi.org/10.1016/j.procbio.2008.02.008
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington
Bergaust L, Mao Y, Bakken LR, Frostegard A (2010) Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrous oxide reductase in Paracoccus denitrificans. Appl Environ Microbiol 76:6387–6396. https://doi.org/10.1128/AEM.00608-10
Blum J-M, Su Q, Ma Y, Valverde-Pérez B, Domingo-Félez C, Jensen MM, Smets BF (2018) The pH dependency of N-converting enzymatic processes, pathways and microbes: effect on net N2O production. Environ Microbiol 20:1623–1640. https://doi.org/10.1111/1462-2920.14063
Brotto AC, Kligerman DC, Andrade SA, Ribeiro RP, Oliveira JL, Chandran K, de Mello WZ (2015) Factors controlling nitrous oxide emissions from a full-scale activated sludge system in the tropics. Environ Sci Pollut Res Int 22:11840–11849. https://doi.org/10.1007/s11356-015-4467-x
Castro-Barros CM, Rodríguez-Caballero A, Volcke EIP, Pijuan M (2016) Effect of nitrite on the N2O and NO production on the nitrification of low-strength ammonium wastewater. Chem Eng J 287:269–276. https://doi.org/10.1016/j.cej.2015.10.121
Ettwig KF et al (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548
Kim EW, Bae JH (2000) Alkalinity requirements and the possibility of simultaneous heterotrophic denitrification during sulfur-utilizing autotrophic denitrification. Water Sci Technol 42:233–238. https://doi.org/10.1038/nature08883
Klaus S, Sadowski M, Jimenez J, Wett B, Chandran K, Murthy S, Bott CB (2017) Nitric oxide production interferes with aqueous dissolved oxygen sensors. Environ Eng Sci 34:687–691. https://doi.org/10.1089/ees.2016.0634
Li C, Wang T, Zheng N, Zhang J, Ngo HH, Guo W, Liang S (2013) Influence of organic shock loads on the production of N2O in denitrifying phosphorus removal process. Bioresour Technol 141:160–166. https://doi.org/10.1016/j.biortech.2013.03.117
Li W, Zheng P, Guo J, Ji J, Zhang M, Zhang Z, Zhan E, Abbas G (2014) Characteristics of self-alkalization in high-rate denitrifying automatic circulation (DAC) reactor fed with methanol and sodium acetate. Bioresour Technol 154:44–50. https://doi.org/10.1016/j.biortech.2013.11.097
Liu B, Frostegard A, Bakken LR (2014) Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. MBio 5:e01383–e01314. https://doi.org/10.1128/mBio.01383-14
Lu H, Chandran K (2010) Factors promoting emissions of nitrous oxide and nitric oxide from denitrifying sequencing batch reactors operated with methanol and ethanol as electron donors. Biotechnol Bioeng 106:390–398. https://doi.org/10.1002/bit.22704
Ni B-J, Yuan Z (2013) A model-based assessment of nitric oxide and nitrous oxide production in membrane-aerated autotrophic nitrogen removal biofilm systems. J Membr Sci 428:163–171. https://doi.org/10.1016/j.memsci.2012.10.049
Pan Y, Ye L, Ni B-J, Yuan Z (2012) Effect of pH on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Water Res 46:4832–4840. https://doi.org/10.1016/j.watres.2012.06.003
Pan Y, Ni BJ, Bond PL, Ye L, Yuan Z (2013a) Electron competition among nitrogen oxides reduction during methanol-utilizing denitrification in wastewater treatment. Water Res 47:3273–3281. https://doi.org/10.1016/j.watres.2013.02.054
Pan Y, Ni BJ, Yuan Z (2013b) Modeling electron competition among nitrogen oxides reduction and N2O accumulation in denitrification. Environ Sci Technol 47:11083–11091. https://doi.org/10.1021/es402348n
Ribera-Guardia A, Pijuan M (2017) Distinctive NO and N2O emission patterns in ammonia oxidizing bacteria: effect of ammonia oxidation rate, DO and pH. Chem Eng J 321:358–365. https://doi.org/10.1016/j.cej.2017.03.122
Riya S, Zhou S, Kobara Y, Sagehashi M, Terada A, Hosomi M (2015) Influence of nitrogen loading and plant nitrogen assimilation on nitrogen leaching and N2O emission in forage rice paddy fields fertilized with liquid cattle waste. Environ Sci Pollut Res Int 22:5762–5771. https://doi.org/10.1007/s11356-014-3811-x
Schulthess RV, Kühni M, Gujer W (1995) Release of nitric and nitrous oxides from denitrifying activated sludge. Water Res 29:215–226. https://doi.org/10.1016/0043-1354(94)E0108-I
Wang Y, Fang H, Zhou D, Han H, Chen J (2016a) Characterization of nitrous oxide and nitric oxide emissions from a full-scale biological aerated filter for secondary nitrification. Chem Eng J 299:304–313. https://doi.org/10.1016/j.cej.2016.04.050
Wang Y, Lin X, Zhou D, Ye L, Han H, Song C (2016b) Nitric oxide and nitrous oxide emissions from a full-scale activated sludge anaerobic/anoxic/oxic process. Chem Eng J 289:330–340. https://doi.org/10.1016/j.cej.2015.12.074
Wu G, Zhai X, Li B, Jiang C, Guan Y (2014) Endogenous nitrous oxide emission for denitrifiers acclimated with different organic carbons. Procedia Environ Sci 21:26–32. https://doi.org/10.1016/j.proenv.2014.09.004
Wu J, Zhang J, Jia W, Xie H, Gu RR, Li C, Gao B (2009) Impact of COD/N ratio on nitrous oxide emission from microcosm wetlands and their performance in removing nitrogen from wastewater. Bioresour Technol 100:2910–2917. https://doi.org/10.1016/j.biortech.2009.01.056
Yan X, Zheng J, Han Y, Liu J, Sun J (2017) Effect of influent C/N ratio on N2O emissions from anaerobic/anoxic/oxic biological nitrogen removal processes. Environ Sci Pollut Res 24:23714–23724. https://doi.org/10.1007/s11356-017-0019-x
Zhang S, Liu F, Xiao R, Li Y, Zhou J, Wu J (2015) Emissions of NO and N2O in wetland microcosms for swine wastewater treatment. Environ Sci Pollut Res Int 22:19933–19939. https://doi.org/10.1007/s11356-015-5210-3
Zhao W, Wang Y, Liu S, Pan M, Yang J, Chen S (2013) Denitrification activities and N2O production under salt stress with varying COD/N ratios and terminal electron acceptors. Chem Eng J 215-216:252–260. https://doi.org/10.1016/j.cej.2012.10.084
Zhou Y, Pijuan M, Zeng RJ, Yuan Z (2008) Free nitrous acid inhibition on nitrous oxide reduction by a denitrifying-enhanced biological phosphorus removal sludge. Environ Sci Technol 42:8260–8265. https://doi.org/10.1021/es800650j
Funding
This work was financially supported by the National Natural Science Foundation of China (no. 51778057) and the Key Research and Development Program of Ningxia Hui Autonomous Region (no. 2019BFG02031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• High NO accumulation during nitrite denitrification can inhibit reductases completely
• The effects of COD/N on peaks of NO and N2O accumulation were different.
• Low pH and high influent loading have serious effect on NO and N2O reduction.
• High DO was detected during NO detoxification, it is partly caused by NO dismutation.
Electronic supplementary material
ESM 1
(DOCX 280 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Zhao, J. & Huang, T. High NO and N2O accumulation during nitrite denitrification in lab-scale sequencing batch reactor: influencing factors and mechanism. Environ Sci Pollut Res 26, 34377–34387 (2019). https://doi.org/10.1007/s11356-019-06391-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06391-5