Abstract
Effectiveness of steam explosion (SE) pretreatment for deconstructing the complex structural carbohydrates (SC) and lignin recalcitrance properties of rice straw (RS) for conjunctive improvement of biofuel yield and waste valorization was evaluated. This work exhibited successful pretreatment of RS at a different pressure (1.2, 1.5, and 1.8 MPa) and retention (3, 6, 9, and 12 min) for enhancement of SC contribution to biomethane production. Regression analysis demonstrated that SE pretreatment efficiency improved at high-temperature and short-retention time for biodegradation of RS. Maximum cumulative methane yield (EMY) achieved 254.8 mL/gvs at 1.2 MPa (3 min) of SE-treated RS with 62.7% of very significant improvement compared with untreated RS (156.6 mL/gvs). Furthermore, solid fraction of xylose, arabinose, cellobiose, glucose, and acid-soluble lignin in SE-treated RS of 1.2 MPa (3 min) were biodegraded by 27.4%, 46.4%, 100%, 48.8%, and 14.1%, respectively, after anaerobic digestion. Therefore, SE pretreatment was an encouraging approach for enhancing SC conversion to biomethane and waste resource to circular economy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is the prime diet of about 50 % of people worldwide as well as a major cultivated crop in many Asian countries. China is the world leading rice grower and produces around 300 million metric tons of rice crop residues each year, after harvesting the rice (Luo et al. 2019). Disposal of such a huge quantity of rice crop residues is a big issue because of high collection and transportation cost and low market value, and farmers are in a hurry to prepare their farm-field for the next crop cultivation. The commonly used effortless method for disposal a considerable proportion of rice crop residues is open field burning, which causes severe kinds of natural resource depletion and environmental issues (air pollution, haze, and global warming) (Medina et al. 2015). Hence, there is a need to develop an integrated and sustainable approach to mitigate the rice crop residues issues. Biofuel production from rice crop residues is regarded as a better option for value addition and beneficial re-use (Abraham et al. 2016; Marousek 2013).
Anaerobic digestion (AD) is a low-cost, environmentally friendly, and mature tool for efficiently treating solid organic waste of agricultural sectors. AD process transforms organic matter into biogas in an anaerobic environment and the presence of microorganisms (Chu et al. 2015). Two main products are achieved after the completion of AD process; one is a clean energy in terms of methane (CH4) and another is a nutrient-rich by-product as a fertilizer for crops (Ma et al. 2017). However, digestion efficiency of feedstock is significantly reduced by the recalcitrant properties of lignocellulose. Disruption of the recalcitrant lignin bonds with the SC components could improve the hydrolysis of biomass and AD efficiency. Barriers of lignocellulosic biomass can be removed by efficient pretreatment (Koyama et al. 2017b).
The variety of pretreatment mechanisms have been established to deconstruct recalcitrance properties of lignocellulosic biomass for improving anaerobic digestibility for biomethanation. Among the developed pretreatment methods, acid and alkaline pretreatment technologies were most commonly studied and widely used (Digman et al. 2010; Kumar and Sharma 2017). However, acid and alkaline pretreatments could not deploy without a large quantity of water as well as to treat the downstream process to avoid the chemical impacts on the environment. Chemical pretreatment also causes the corrosion of equipment, and chemicals are usually expensive too (Ruiz et al. 2017). SE pretreatment is an environment-friendly technique and could be used to open up complex construction of lignocellulosic biomass. Saturated steam quickly heats lignocellulosic biomass for a short time in SE pretreatment; then the pressure is swiftly released, which cause cleavage of fibers and tearing cross-linkages of cell walls of the biomass (Holtzapple et al. 1989; Zhang and Chen 2012). Overall, SE pretreatment causes the solubilization of hemicellulose and subsequent conversion into its structural units, while the lignin is relocated and partially transformed into fractional products in SE pretreatment and consequently cellulose undergoes to hydration steps (Aguilar et al. 2018; Ruiz et al. 2013; Siddhu et al. 2016a). However, SE pretreatment conditions greatly influence the open-up complex structure, biodegradation of lignocellulosic biomass, and biomethane yield. Inefficient SE pretreatment might convert the biomass components into inhibitors, which are not only waste of biomass, but also caused the loss of energy in terms of high input (Gikas 2014; Theuretzbacher et al. 2015). The statistical models might be also an effective and reliable approach for evaluating the SE pretreatment performance efficiency to improve anaerobic biodegradability of lignocellulosic feedstock and methane yield (Dandikas et al. 2018). So far, less information elucidated the efficiency of SE pretreatment for opening up the RS complex bonding of SC and lignin components and subsequent improvement of each component contribution in AD process for biomethanation.
Current study was intended with the following objectives: (1) to examine the SE pretreatment effects on methane production performance of RS; (2) to determine the each SC component contribution in biomethanation of SE-treated and untreated RS during AD; and (3) to compare the biodegradation of SC components of SE-treated and untreated RS after AD.
Materials and methods
RS and inoculant
RS was acquired from Jiangsu Province, People’s Republic of China. Then, chopped RS was dried at room temperature in laboratory, and large RS particles size was cut-down. After these steps, air-dried RS size in range of 1.5 to 2.0 cm was sieved out and wrapped airtightly and placed at 4 °C. The inoculant utilized in this experiment was effluent of Donghuashan biogas plant, Beijing, People’s Republic of China.
Pretreatment of RS with SE
Pretreatment of RS was carried out in an SE machine (Huada, Jiangsu, China). The highest operating temperature and pressure of SE was 250 °C and 20 MPa, respectively. Machine heating-up rate of temperature was 10° C/min, and maximum water flow rate was 30 mL/min to the steam generator. Each batch of 55 g of RS was soaked in 50 mL tap water to increase the moisture content (MC) to approximately 50% by using Eq. (1) and then fed into 5 L of the high-pressure chamber (Siddhu et al. 2016b). Each pressure (1.2, 1.5, and 1.8 MPa) was used to treat RS at the retention time of 3, 6, 9, and 12 min. All the samples were ejected out (cool down), as suddenly released the operating pressure (less than 1 s) of pretreatment. All the samples were collected (solid and liquid totally) from the collector after SE pretreatment. Then, the sample characteristics were measured for AD test. All SE-treated and untreated RS were put in 4 °C before AD (Li et al. 2015).
AD of SE-treated RS
The AD test was conducted in 1-L serum bottle to assess digestibility of untreated and SE-treated RS. Feed of each digester on the substrate to inoculant ratio on VS basis was controlled to be 0.50 (Li et al. 2014). After feeding the substrate and inoculant mixture, distilled water was used to fill up each digester up to 500 mL. Blank digesters were also prepared for correcting biogas yield calculation of RS and had the same amount of inoculant. After this step, nitrogen gas was purged into each digester for 3 min for anoxic condition. After fixing the rubber stopper and screw cap, each digester was set in an incubator for the mesophilic AD. After completion of the AD, effluent characteristics were determined (Zhao et al. 2017).
Analytical methods
Basic characteristics of RS and inoculant such as total solid (TS) and VS contents were measured by APHA protocol (APHA 2005). Elements, such as C, H, and N, of untreated RS and inoculant, were analyzed by a Vario El cube (Germany) detector, and O was determined as reported previously (Rincón et al. 2012). The daily pressures of biogas in all the digesters were determined as previously reported by our laboratory (Chen et al. 2016; Siddhu et al. 2016b). Biogas volume of each digester was determined by using Eq. (S1) (Shen et al. 2016). The equations form of S1, and details were presented in supplementary material.
Biogas composition was determined via 7890B gas chromatograph (Agilent, Technologies, USA) with thermal conductivity detector, while helium was used as carrier gas. GC’s detector and oven temperature during the biogas sample test were 220 and 60 °C, respectively. Volatile fatty acids (VFAs) in effluents after AD were determined via a flame ionization detector fitted in GC-7890A, and nitrogen gas (N2) was used as carrier gas. Total ammonia nitrogen (TAN) and total alkalinity (TA) in effluent were measured by HACH protocol, while the free ammonia nitrogen (FA) was calculated via following Eq. (2) (Chen et al. 2016).
where FA stands for free ammonia nitrogen (mg/L), pH is final pH of effluents, TAN indicates total ammonia nitrogen (mg/L), and Kb and Kw stand for dissociation constant for ammonia and water at 37 °C (1.855 × 10−5 and 2.355 × 10−14 mol/L, respectively).
RS SC and lignin biodegradation
SC and lignin components were determined in a solid fraction of untreated, SE-treated RS and after AD by using the protocols of the NREL of the US Department of Energy (Sluiter et al. 2008). RS samples of untreated, SE-treated and after AD were treated by 72% sulfuric acid for 1 h at 30 °C. Then 72% sulfuric acid treated samples were diluted up to 4% by adding water and later these samples were autoclaved at 121 °C for 1 h. Then, cooled down and undissolved solid from the liquid was separated by using a filtering crucible. High-performance liquid chromatography (SHIMADZU) separated with an Agilent Hi-Plex H column (30 × 0.77 cm, column temperature 65 °C), and a refractive index detector was used for determination of SC in liquid part. Five millimolar sulfuric acid was used for carrier solution (flow rate = 0.01 mL/s). Acid-soluble lignin (ASL) was determined at 320 nm wavelength by spectrophotometry method. After the AD, each component of RS (cellobiose, glucose, arabinose, xylose, xylitol, and ASL) biodegradation was calculated by Eq. (3).
where Mb stands for component biodegraded, Mfeeded refers to the component of the fed solid of RS in the digester, and Mleftover means the component in digested solid of RS.
Theoretical maximum methane potential yield and biodegradability
Theoretical maximum methane potential yield (MMY) of untreated RS was calculated by using Eqs. (S2) and (S3), and then biodegradability (Bd) was calculated by Eq. (S4) as reported previously (Buswell and Mueller 1952; Li et al. 2018). The equations form of S2, S3, and S4, and details were presented in supplementary material.
Kinetic analysis
A modified Gompertz model was applied for evaluating the kinetics of methane production of SE-treated RS by using Eq. (S5) (Siddhu et al. 2016b): The model equation and description was provided in supplementary material (S5).
Data analysis
Regression model and one-way analysis of variance were applied to analyse experimental data for determining a meaningful outcome. All statistical analysis was conducted at α level of 0.01 and 0.05, respectively. Data was analyzed by using software of OriginPro 9.1 (OriginLab, USA).
Results and discussion
Basic characteristics of RS and inoculant
Table 1 presents the basic characteristics of untreated RS and inoculant. TS and VS contents were calculated to be 94.6% and 80.5%, respectively, on the basis of the total mass of the sample. According to elemental analysis, the C/N ratio was found to be 34.0, which was slightly higher than the recommendation for an AD in previously reported studies (18 to 30) (Li et al. 2014). RS could be represented as an organic substrate in the following formula: C40.8H66.4O33.3N. MMY of RS was determined by Eq. (S2) to be 407.1 mL/gvs.
AD for SE pretreated RS
Table 1S revealed the daily methane yield (DMY) production of untreated and SE-treated RS. The maximum DMY of 1.2 MPa for 3, 6, 9, and 12 min of SE-treated RS was 12.0 ± 0.4, 13.5 ± 0.0, 12.1 ± 0.0, and 9.9 ± 0.0 mL/gvs, respectively, while that of untreated RS was only 8.5 ± 0.3 mL/gvs. In addition, the highest peaks of 1.2 MPa SE-treated RS for 3, 6, 9, and 12 min appeared on the fifth day, while the untreated appeared at the eighth day of digestion. The average DMY of SE-treated RS of 1.2 MPa for 3, 6, 9, and 12 min were 5.5 ± 0.4, 5.2 ± 0.3, 4.8 ± 0.4, and 4.2 ± 0.2 mL/gvs, respectively, while the untreated RS was observed to be 3.5 ± 0.3 mL/gvs. Average DMY of SE-treated RS of 1.2 MPa decreased, as increase of retention time and pressure for pretreatment, while average DMY of each SE-treated RS increased as evaluated with untreated.
Ninety percent of maximum cumulative methane yield (EMY) for all SE-treated RS groups (1.2, 1.5, and 1.8 MPa) was achieved within the first 39 days of digestion. Figure 1a exhibits that the EMYs at 1.2 MPa for 3, 6, 9, and 12 min of SE-treated RS were 254.8 ± 16.0, 246.2 ± 5.8, 234.0 ± 9.3, and 195.1 ± 5.6 mL/gvs, representing a significant improvement of 62.7%, 57.3%, 49.5%, and 24.6%, respectively, compared with the untreated RS (156.6 ± 3.5 mL/gvs). The EMY of SE-treated RS of 1.2 MPa (3 min) was 3.5%, 8.9%, and 30.6% higher, respectively, than retention time of 6, 9, and 12 min for pretreatment. A dwindling in methane yield was noticed per increase in retention time at constant pressure. It was considered that SE treatment at 1.2 MPa for 3 min was effective in altering RS recalcitrant properties compared with the longer retention time.
Figure 1b demonstrates that the EMY of SE-treated RS of 1.5 MPa for 3, 6, 9, and 12 min were 248.1 ± 0.2, 222.9 ± 4.6, 214.0 ± 4.7, and 196.1 ± 0.1 mL/gvs, which were significantly improved by 58.4%, 42.4%, 36.7%, and 25.3%, respectively, compared with untreated RS (156.6 ± 3.5 mL/gvs). EMY of SE-treated RS of 1.5 MPa for 3 min was 11.3%, 15.9%, and 26.5% higher, respectively, than the retention time of 6, 9, and 12 min pretreatment. A pronounced decrease of methane yield was detected with the increase of retention time at 1.5 MPa. It was considered that SE treatment at 1.5 MPa for 3 min was suitable to change the recalcitrant characteristics of RS for AD compared with the longer retention time, which caused increased energy input.
Figure 1c shows that the EMYs of SE-treated RS of 1.8 MPa for 3, 6, 9, and 12 min were 226.1 ± 17.3, 200.9 ± 18.2, 197.9 ± 2.2, and 196.6 ± 0.6, mL/gvs, which were significantly improved by 44.4%, 28.3%, 26.4%, and 25.6%, respectively, compared with the untreated RS (156.6 ± 3.5 mL/gvs). The EMYs of SE-treated RS of 1.8 MPa for 3 min were 12.6%, 14.3%, and 15.0% higher, respectively, than 6, 9, and 12 min of pretreatment. A noticeable decrease in methane yield at long retention for pretreatment at constant (1.8 MPa) pressure and increase input energy for pretreatment. It was considered that SE treatment of RS of 1.8 MPa (3 min) was deconstructed recalcitrant characteristics compared with the longer retention time.
Furthermore, DMY and EMYs of pretreated RS at three different pressures 1.2, 1.5, and 1.8 MPa and constant retention time of 3 min were compared and presented in supplementary material in Fig. 1S. The maximum DMY of 1.2, 1.5, and 1.8 MPa for 3 min of SE-treated RS was 12.0 ± 0.4, 12.1 ± 0.3, and 10.9 ± 0.5 mL/gvs, respectively, and appeared on the fifth day of digestion. EMY of SE-treated RS of 1.2 MPa was 2.7% and 12.7% higher than 1.5 and 1.8 MPa at 3 min. Therefore, looking at the methane yield, input energy, SE efficiently overcomes the recalcitrant properties; SE pretreatment at 1.2 MPa and 3 min might be the optimal condition for efficient anaerobic digestibility of RS.
Characteristics of effluent of untreated and SE-treated RS
Effluent parameters of untreated and SE-treated RS are shown in Table 2. The initial pH in AD reactors of untreated and 1.2 to 1.8 MPa SE-treated RS were ranged from 8.2 ± 0.0 to 8.3 ± 0.0. After the successful AD, the pH inside each reactor was also measured and found to be within a permissible range from 6.8 ± 0.0 to 8.2 ± 0.0 (Koyama et al. 2017a; Theuretzbacher et al. 2015).
After AD, effluent of untreated and SE-treated RS at 1.2, 1.5, and 1.8 MPa for 3 min was analyzed to assess the anaerobic process stability. Both the TA, VFA, and their relative proportions are important parameters for a successful AD. The TA (carbonate/bicarbonate) is an indicator to evaluate acid neutralization in the AD process. The final TA value for the untreated RS was the highest (2750 ± 50.0 mg CaCO3/L), but TA value decreased at higher pressure (1.2 MPa of 2600 ± 50.0, 1.5 MPa of 2275 ± 125.0, 1.8 MPa of 2250 ± 50.0 mg CaCO3/L) of SE at constant retention time (3 min). All the final values were found to be within the permissible range (1000 to 3000 mg CaCO3/L) (Theuretzbacher et al. 2015). VFAs in the effluent of SE-treated RS reactors were in the range of 18.6 to 57.5 mg/L, while inhibition starts over 350 mg/L concentration (Li et al. 2014).
Nitrogen is an essential nutrient for microbial growth of anaerobic function throughout the AD process. TAN values of all the digesters were analyzed and found to be within an acceptable range (less than 1700 mg/L) (Theuretzbacher et al. 2015), and no inhibition was occurred in any of the digesters. FA is a pH-dependent portion of the TAN, and FA is most toxic to methanogens. Each calculated value of FA by using an Eq. (3) was below the 50 mg/L and was considered to be sustainable for AD. (Ramos-Suárez et al. 2014). Therefore, no sign of FA-based inhibition was seen in the digesters. All of these results indicated that digesters were stable and conditions were appropriate for the AD after SE pretreatment.
Modified Gompertz model
Kinetics of methane production process of untreated and treated RS were investigated by modified Gompertz model, and parameters are presented in Table 3, While R2 varied from 0.993 to 0.994, which also proved that modified Gompertz model simulated the methane production process very well.
The model estimated simulated maximum cumulative methane yield (B0) of untreated RS (165.9 ± 4.1 mL/gvs) was slightly higher than the EMY (156.6 ± 3.5 mL/gvs). In addition, B0 of 1.2 MPa SE-treated RS for 3 min (272.0 ± 6.3 mL/gvs) was slightly more than the EMY achieved (254.8 ± 16.0 mL/gvs). While B0 and EMY of 1.5 and 1.8 MPa SE-treated RS for 3 min were almost identical. The maximum methane production rate (μm) of untreated and SE-treated RS ranged from 5.4 ± 0.2 to 9.5 ± 0.3 mL/gvs/day. The μm of untreated RS was the lowest (5.4 ± 0.2 mL/gvs/day) compared to 1.2, 1.5, and 1.8 MPa for 3 min SE-treated RS. On the other hand, the μm value at highest pressure (1.8 MPa) was the lowest among all the SE-treated RS at constant retention time (3 min). λ is the lag phase time and a higher λ value indicates a long start-up time, because of recalcitrant characteristics of RS. RS treated for 3 min at 1.2 MPa had the lowest λ of 1.7 ± 0.4 day, which was because of SE treatment impact for acceleration of hydrolysis, enabling a shorter AD initiating time. Furthermore, Bd is another indicator to evaluate and compare the performances of untreated and SE-treated RS of AD; “Bd was calculated from EMY obtained by experiment divided by the theoretical maximum methane potential.” At constant retention time of 3 min, 1.2 MPa pretreated RS achieved the highest Bd of 62.6%, compared to 1.5 MPa, 1.8 MPa, and untreated RS of 60.9%, 55.6%, and 38.5%, respectively. In general, SE-treated of 1.2 MPa for 3 min resulted in deconstructing complex structure of RS, and Bd significantly improved 62.6% compared with untreated.
Regression analysis for retention time and temperature of SE
Variation of retention time and temperature of SE causes influence of methane yield from RS. A regression analysis was developed to explain the consequence of temperature and retention time of SE pretreatment for RS on EMY (at constant pressure of 1.2 MPa of SE) and shown in Eq. (4).
where EMY1.2MPa stands for maximum cumulative methane yield at 1.2 MPa of SE-treated RS (mL/gvs), t represents the retention time of RS in steam chamber (min), and T means the temperature in the steam chamber (°C).
The coefficient of determination (R2) of the regression analysis was 0.963, which indicated that model was a reasonable fit. The long retention time for pretreatment of RS was impacted negative to EMY. The long retention time of SE on biomass causes the production of furfural and its derived compounds (Palmqvist and Hahn-Hägerdal 2000). While the temperature of SE treatment was implied as a positive contribution to breakdown the recalcitrance, interlinks of physical and chemical structure of RS and subsequent enhanced the hydrolysis for methane production. The temperature causes “cracking”: Moisture penetrates into the cell wall structure and swells the biomass. This destructive step caused shear stress on the RS tissues and might increase the biomass surface area (Sui and Chen 2014; Zhang et al. 2013).
SC and lignin components in solid fraction of untreated and SE-treated RS
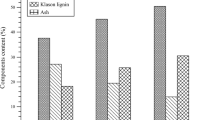
SC and lignin components of the solid fraction of untreated and SE-treated RS of 1.2 MPa (3, 6, 9, and 12 min) before AD are presented in Fig. 2. Cellobiose and glucose are mainly released from cellulose (Philippidis et al. 1992). The cellobiose component of SE-treated (1.2 MPa for 3 min) RS decreased as compared to untreated (0.8% vs 2.4%). The cellobiose component also decreased with the increase in retention time from 3 to 12 min of SE pretreatment of RS (0.8% to 0.5%). The glucose component of SE-treated (1.2 MPa for 3 min) RS was significantly reduced in comparison with the untreated RS (19.6% vs 34.2%). SE pretreatment might cause solubilization of sugar and destruction of microfibrils. Increasing the retention time of SE from 3 to 12 min could also cause solubilization of glucose as measured values indicated (19.6% to 16.7%) of RS (Sasaki et al. 2012).
Arabinose component of SE-treated (1.2 MPa for 3 min) RS decreased in comparison with untreated (2.9% vs 3.6%), and increase in retention time (3 to 12 min) of pretreatment also caused more decrease in arabinose (2.9 to 1.4%). The xylose component of the SE-treated RS for 3 min decreased in comparison to untreated (13.2% vs 16.4%). The xylose component also decreased (13.2% to 8.8%) with the increasing of SE retention time from 3 to 12 min. Xylitol was completely removed after the SE pretreatment, most likely because of its high solubility at high temperature. The ASL was slightly reduced to 10.1% in SE-treated RS as compared to untreated values of 11.0%. Long retention of SE pretreatment caused more hydrolysis of SC, resulting in more destruction of fibrils in RS. However, it not only increased the cost of the pretreatment process but also might lead to conversion of SC into inhibitors, which were toxic for AD process. Therefore, 1.2 MPa for 3 min of SE pretreatment condition for RS was preferred by deconstructing recalcitrance structure for the ultimate improvement in methane production.
Biodegradation of SC and lignin components in solid after AD
The impact of SE pretreatment on RS for enhancing biodegradation of SC and lignin was analyzed after the successful AD process and is presented in Fig. 3. AD is a very complex process involving consortia of bacteria and archaea. Lignin is highly recalcitrant by nature and is a primary barrier to microbial access of hemicellulose and cellulose during hydrolysis (Ahring et al. 2015). Biodegradation of ASL in untreated and SE-treated RS was 0.4% and 14.1% during the AD. In addition, each SC and lignin component (arabinose, xylose, glucose, cellobiose, and ASL) hydrolyzed differently, and the hydrolysis products formed also varied. It is not surprising that some variations were observed during AD. Hemicellulose plays the link role between cellulose and lignin. Other literatures have found that removal of the covalent bonding in xylose and arabinose with lignin could enhance the hydrolysis and biodegradation of hemicellulose (Barakat et al. 2013). Biodegradation of arabinose and xylose components of SE-treated RS (1.2 MPa, 3 min) was higher (46.4%, 27.4%) as compared to untreated (18.8%, 5.5%), respectively, after AD. Glucose and cellobiose components of untreated and SE-treated RS at 1.2 MPa (3 min) were biodegraded 16.1%, 48.8%, and 19.7%, 100%, respectively, after AD.
These collective results indicated that SE treatment of RS at 1.2 MPa for 3 min significantly altered the most recalcitrant lignin shield structure for SC and subsequently caused enhancement of hemicellulose–cellulose components hydrolysis. Glucose, cellobiose, arabinose, and xylose components of SE-treated RS were significantly biodegraded as compared to untreated after the AD process. Therefore, SE pretreatment of RS could deconstruct recalcitrance structure of RS, break fibrils down, enhance the hydrolysis, and was likely a key contributor to the higher biogas production.
Conclusions
Steam explosion pretreatment (SE) was applied at three different pressures (1.2, 1.5, and 1.8 MPa) for four different retention times to rice straw for improving structural carbohydrate (SC) contribution in methane production during AD. The EMY of SE-treated RS at 1.2, 1.5, and 1.8 MPa for 3 min was increased significantly by 62.7%, 58.4%, and 44.4%, respectively, over untreated. Bd of SE-treated RS of 1.2 MPa for 3 min achieved 62.6% as compared to 38.5% of untreated. Results confirmed that SE pretreatment significantly enhanced the anaerobic digestibility of SC components of RS for biomethanation and was a promising technique for advanced application.
Abbreviations
- AD:
-
Anaerobic digestion
- ASL:
-
Acid-soluble lignin
- Bd :
-
Biodegradability
- C/N:
-
Carbon to nitrogen ratio
- EMY:
-
Maximum cumulative methane yield obtained by experiment
- FA:
-
Free ammonia nitrogen
- MMY:
-
Theoretical maximum methane potential
- MPa:
-
Megapascal
- RS:
-
Rice straw
- SC:
-
Structural carbohydrates
- SE:
-
Steam explosion
- TA:
-
Total alkalinity
- TS:
-
Total solid
- VFA:
-
Volatile fatty acids
- VS:
-
Volatile solid
References
Abraham A, Mathew AK, Sindhu R, Pandey A, Binod P (2016) Potential of rice straw for bio-refining: an overview. Bioresour Technol 215:29–36
Aguilar DL, Rodriguez-Jasso RM, Zanuso E, de Rodriguez DJ, Amaya-Delgado L, Sanchez A, Ruiz HA (2018) Scale-up and evaluation of hydrothermal pretreatment in isothermal and non-isothermal regimen for bioethanol production using agave bagasse. Bioresour Technol 263:112–119
Ahring BK, Biswas R, Ahamed A, Teller PJ, Uellendahl H (2015) Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresour Technol 175:182–188
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, D.C
Barakat A, Gaillard C, Steyer J-P, Carrere H (2013) Anaerobic biodegradation of cellulose–xylan–lignin nanocomposites as model assemblies of lignocellulosic biomass. Waste Biomass Valoriz 5:293–304
Buswell AM, Mueller HF (1952) Mechanism of methane fermentation. Ind Eng Chem 44:550–552
Chen H, Wang W, Xue L, Chen C, Liu G, Zhang R (2016) Effects of ammonia on anaerobic digestion of food waste: process performance and microbial community. Energy Fuel 30:5749–5757
Chu X, Wu G, Wang J, Hu ZH (2015) Dry co-digestion of sewage sludge and rice straw under mesophilic and thermophilic anaerobic conditions. Environ Sci Pollut Res 22:20143–20153
Dandikas V, Heuwinkel H, Lichti F, Drewes JE, Koch K (2018) Predicting methane yield by linear regression models: a validation study for grassland biomass. Bioresour Technol 265:372–379
Digman MF, Shinners KJ, Casler MD, Dien BS, Hatfield RD, Jung HJ, Muck RE, Weimer PJ (2010) Optimizing on-farm pretreatment of perennial grasses for fuel ethanol production. Bioresour Technol 101:5305–5314
Gikas P (2014) Electrical energy production from biosolids: a comparative study between anaerobic digestion and ultra-high-temperature gasification. Environ Technol 35:2140–2146
Holtzapple MT, Humphrey AE, Taylor JD (1989) Energy requirements for the size reduction of poplar and aspen wood. Biotechnol Bioeng 33:207–210
Koyama M, Nakahashi N, Ishikawa K, Ban S, Toda T (2017a) Anaerobic co-digestion of alkali-pretreated submerged macrophytes and acidified food waste for reduction of neutralizing agents. Int Biodeterior Biodegrad 125:208–213
Koyama M, Watanabe K, Kurosawa N, Ishikawa K, Ban S, Toda T (2017b) Effect of alkaline pretreatment on mesophilic and thermophilic anaerobic digestion of a submerged macrophyte: inhibition and recovery against dissolved lignin during semi-continuous operation. Bioresour Technol 238:666–674
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4:7
Li J, Zhang R, Siddhu MAH, He Y, Wang W, Li Y, Chen C, Liu G (2015) Enhancing methane production of corn Stover through a novel way: sequent pretreatment of potassium hydroxide and steam explosion. Bioresour Technol 181:345–350
Li W, Siddhu MAH, Amin FR, He Y, Zhang R, Liu G, Chen C (2018) Methane production through anaerobic co-digestion of sheep dung and waste paper. Energy Convers Manag 156:279–287
Li Y, Zhang R, He Y, Zhang C, Liu X, Chen C, Liu G (2014) Anaerobic co-digestion of chicken manure and corn Stover in batch and continuously stirred tank reactor (CSTR). Bioresour Technol 156:342–347
Luo T, Huang H, Mei Z, Shen F, Ge Y, Hu G, Meng X (2019) Hydrothermal pretreatment of rice straw at relatively lower temperature to improve biogas production via anaerobic digestion. Chin. Chem. Lett. In Press, Corrected Proof
Ma Y, Yin Y, Liu Y (2017) New insights into co-digestion of activated sludge and food waste: biogas versus biofertilizer. Bioresour Technol 241:448–453
Marousek J (2013) Prospects in straw disintegration for biogas production. Environ Sci Pollut Res 20:7268–7274
Medina J, Monreal C, Barea JM, Arriagada C, Borie F, Cornejo P (2015) Crop residue stabilization and application to agricultural and degraded soils: a review. Waste Manag 42:41–54
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Philippidis GP, Spindler DD, Wyman CE (1992) Mathematical modeling of cellulose conversion to ethanol by the simultaneous saccharification and fermentation process. Appl Biochem Biotechnol 34-35:543–556
Ramos-Suárez JL, Martínez A, Carreras N (2014) Optimization of the digestion process of scenedesmus sp. and opuntia maxima for biogas production. Energy Convers Manag 88:1263–1270
Rincón B, Heaven S, Banks CJ, Zhang Y (2012) Anaerobic digestion of whole-crop winter wheat silage for renewable energy production. Energy Fuel 26:2357–2364
Ruiz HA, Rodríguez-Jasso RM, Fernandes BD, Vicente AA, Teixeira JA (2013) Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review. Renew Sust Energ Rev 21:35–51
Ruiz HA, Thomsen MT, Trajano HL (2017) Hydrothermal processing in biorefineries: production of bioethanol and high added-value compounds of second and third generation biomass. Springer International Publishing, 511 pp
Sasaki C, Sumimoto K, Asada C, Nakamura Y (2012) Direct hydrolysis of cellulose to glucose using ultra-high temperature and pressure steam explosion. Carbohydr Polym 89:298–301
Shen J, Zhao C, Liu G, Chen C (2016) Enhancing the performance on anaerobic digestion of vinegar residue by sodium hydroxide pretreatment. Waste Biomass Valoriz 8:1119–1126
Siddhu MAH, Li J, Zhang J, Huang Y, Wang W, Chen C, Liu G (2016a) Improve the anaerobic biodegradability by copretreatment of thermal alkali and steam explosion of lignocellulosic waste. Biomed Res Int 2016:2786598
Siddhu MAH, Li J, Zhang R, Liu J, Ji J, He Y, Chen C, Liu G (2016b) Potential of black liquor of potassium hydroxide to pretreat corn Stover for biomethane production. BioResources 11:4550–4563
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory, NREL/TP-510-42618
Sui W, Chen H (2014) Multi-stage energy analysis of steam explosion process. Chem Eng Sci 116:254–262
Theuretzbacher F, Lizasoain J, Lefever C, Saylor MK, Enguidanos R, Weran N, Gronauer A, Bauer A (2015) Steam explosion pretreatment of wheat straw to improve methane yields: investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour Technol 179:299–305
Zhang Y, Chen H (2012) Multiscale modeling of biomass pretreatment for optimization of steam explosion conditions. Chem Eng Sci 75:177–182
Zhang Y, Wang L, Chen H (2013) Formation kinetics of potential fermentation inhibitors in a steam explosion process of corn straw. Appl Biochem Biotechnol 169:359–367
Zhao C, Cui X, Liu Y, Zhang R, He Y, Wang W, Chen C, Liu G (2017) Maximization of the methane production from durian shell during anaerobic digestion. Bioresour Technol 238:433–438
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFD0800801) and China Scholarship Council.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 259 kb)
Rights and permissions
About this article
Cite this article
Siddhu, M.A.H., Li, W., He, Y. et al. Steam explosion pretreatment of rice straw to improve structural carbohydrates anaerobic digestibility for biomethanation. Environ Sci Pollut Res 26, 22189–22196 (2019). https://doi.org/10.1007/s11356-019-05382-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05382-w