Abstract
The pretreatment methods for enhancing biogas production from oat straw under study include hot maceration, steam explosion, and pressure shockwaves. The micropore area (9, 55, and 64 m2 g-1) inhibitor formations (0, 15, and 0 mL L-1) as well as the overall methane yields (67, 179, and 255 CH4 VS t-1) were robustly analyzed. It was confirmed that the operating conditions of the steam explosion must be precisely tailored to the substrate. Furthermore, it was beneficial to prepend the hot maceration before the steam explosion and the pressure shockwaves. The second alternative may give increased methane yields (246 in comparison to 273 CH4 VS t-1); however, the application of pressure shockwaves still faces limitations for deployment on a commercial scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
So far, there was only a negligible shift from purpose-grown phytomass (e.g., maize silage) to waste phytomass (e.g., straw) regarding biogas production. Based on its chemical composition, straw could also be a perfect source for biochemical processes. However, various costly pretreatment procedures (physical, chemical, and biological, as well as their various combinations) are necessary for its smooth utilization (Taherzadeh and Karimi 2008). The reason lies in the natural resistance of plant cell walls to microbial and enzymatic deconstruction, collectively known as “biomass recalcitrance” (Himmel 2008). Unfortunately, the recalcitrance lignocellulose ballast also inhibits the digestion of readily fermentable compounds through their inclusion (Wachendorf et al. 2009). There are low methane yields (Amon et al. 2007; Shiralipour and Smith 1984), low conversion efficiencies (Herrmann et al. 2007; Prochnow et al. 2005; Ress et al. 1998), and high retention times in the fermentor (Lemmer and Oechsner 2001; Noike et al. 1985). Several studies have shown a good correlation between the pore volume or population (accessible surface area for cellulase) and the enzymatic digestibility of lignocellulosic materials. The effect of this area may correlate with crystallinity, lignin protection, hemicellulose presentation, or all three factors (Taherzadeh and Karimi 2008).
It was hypothesized that focusing on the anaerobic fermentation of straw in order to reduce the negative agronomic and environmental aspects of the purpose-grown phytomass (Herrmann 2012) would be beneficial. The hypothesis was continued by an assumption that the micropore area (Brunauer et al. 1938) may serve as a supporting reference method for measuring the level of phytomass disintegration. The oat straw was the tested substrate, and hot maceration and steam explosion were the disintegration methods compared in a commercial scale. In addition, the promising technology of shockwaves generated by high-voltage discharges was taken into account, although its development for commercial use is not yet completed.
Materials and methods
Substrate
Medium-early oat variety with short straw called “Atego” with origin in varieties of Gramena and Auron was sown (450 seeds m-2) on 23 March 2011 in Dub (Czech Republic). The fertilization was carried out using the following soil properties to achieve the requirements (75 kg N, 60 kg P2O5, and 85 kg N ha-1) recommended by the breeder (SELGEN a.s., Czech Republic): loamy soil, pH 5.8, 109.4 mg P, and 168 mg K kg-1. The harvest was done at full maturity (3.7 t seeds and 2 t straw in fresh weight per hectare) on August 19. Raked straw was pelleted into 6-mm rolls (970.5 ± 23 kg m-3, 88.5 ± 0.2 % volatile solids (VS), 30 ± 2.1 % acidic-detergent fiber, 17.6 ± 0.4 % acidic-detergent lignin, 14.935 ± 0.092 MJ kg-1, labile pool 1 of carbon 28.1 ± 3.6 %, and labile pool 2 of carbon 23.5 ± 3, all n = 12; P < 0.05) using JGE 120 (PCC Ltd., Czech Republic) and stocked in plastic fabric bags.
Inoculate
Fresh inoculate was obtained from biogas station Nedvědice 1 (Miroslav Drs farm, Czech Republic). Its detailed analysis, origin, and method of production can be found in Maroušek (2013).
Hot maceration
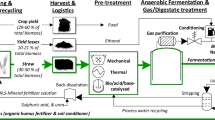
The hot maceration was performed by the M2 continuous phytomass macerator (Fig. 1, BiomassTechnology a.s., Czech Republic) based on the Krátký et al. (2012) laboratory prototype. The commercial-scale macerator operated at 300 kg VS h-1 (corresponding to 10 % VS). The temperature was in the range of 75 to 95 °C, and the retention time was between 20 and 200 s.
Steam explosion
he steam explosion process was performed by the TTP3 continuous high-pressure horizontal cylindrical reactor (Fig. 1, BiomassTechnology a.s., Czech Republic), ended with the expansion tourniquet (single 0.3-L explosion performed in 0.11–0.09 s) according to Maroušek et al. (2012). The entering substrate had approximately 15 % VS. The high-pressure reactor operated in the pressure range of 1.4–1.8 MPa, while the inner helix allowed gradual changes in the hydraulic retention between 2 and 20 min.
Shockwave pretreatment
The shockwave pretreatment was performed in prototype number 7 (Higa et al. 2012), which is a lockable, metallic-strengthened vessel (Fig. 1) with a volume of 1 L. The substrate was initially poured into a 200-mL plastic bottle. The bottle was inserted into the vessel filled with distilled water. Subsequently, the high-voltage generator circuit released several 3.5-kV discharges, resulting in 50- to 60-MPa pressure shockwaves (4.9 kJ, 1,500 m s-1).
Analytical methods
The elemental soil analysis was conducted externally (ÚKZÚZ S.p.A., Czech Republic). The VS was determined by OV400 oven (Memmert GmbH, Germany) and a LH 06/13 muffle furnace (Fisher Scientific Ltd., Czech Republic) according to the method developed by the U.S. Environmental Protection Agency. The amounts of acidic-detergent fiber and acidic-detergent lignin were determined using the Fibertec 1020(M6) fiber analyzer (FOSS Ltd., Denmark). The heat values were analyzed using auto-calculating bomb calorimeter (CA-4AJ, Shimadzu). The proportions of carbon pools were determined by the acid hydrolysis (H2SO4) approach according to Rovira and Vallejo (2002) modified by Shirato and Yokozawa (2006), using the automatic high-sensitivity N/C analyzer (NC-90A, Shimadzu). The pH and temperature were measured using the CyberScan 600 multi-meter (Chromservis Ltd., Czech Republic). The methane yields were qualitatively and quantitatively analyzed as described in Maroušek et al. (2012) and converted to 0 °C at 101,325 Pa. Analyses on formic and levulinic acid, hydroxymethylfurfural, and furfural acid were conducted using the 5890 Series II Gas Chromatograph (Hewlett Packard, USA) equipped with a flame ionization detector (300 °C) and a DBwax column (30 mm × 0.25 mm × 0.25 μm). The gas chromatograph settings were as follows: Helium was used as the carrier gas at a flow rate of 40 μL s-1, temperature was set to 50 °C, the flow rate of nitrogen was 30 mL min-1, the injector used was in split mode, and the injector port temperature was at 250 °C (all J&W Scientific Inc., Folsom, CA, USA). The gas chromatograph oven temperature was programmed in the following manner: the temperature was held steady at 50 °C for 2 min, increased at a rate of 10 °C min-1 for 20 min, and then held at 250 °C for 8 min. The micropore area was detected using the technique of helium gas adsorption using a TriStar 3000 surface area analyzer (Micromeritics Ltd., Tokyo, Japan) after 24 h of degassing at 200 °C and 1 h of degassing at 300 °C.
Results and discussion
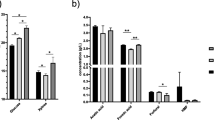
Analysis on the micropore area (m2 g-1) regarding the hot maceration pretreatment is shown in Fig. 2a. The data always analyzed 90 trials using polynomial function, while the lowest sum of squared absolute error (SSAE 2.6E − 19) reached and lowest root mean squared error (root mean square deviation (RMSE) 7.5E-17) were the main fitting criteria for all of the multi-parameter plots provided. The plot shows that there are no interacting effects between operating temperature and micropore area. In addition, it was observed that after a short delay in the macerator, the micropore area spiked, but subsequently, this speculated rise diminished. However, in comparison to manifestations of other pretreatment methods discussed below, the overall increase on the micropore area was relatively small (roughly from 2 to 9 m2 g-1). It is assumed that the plot solely reflects how the substrate loses its outer pellet form which may interfere with the analyses conducted. Figure 2b describes the change in micropore area regarding the steam explosion pretreatment. Approximation (SSAE 1.9E − 4, RMSE 1.7E − 3) of these data shows that both operating conditions (hydraulic retention time and operating pressure) greatly affect the micropore area. The hydraulic retention times in the high-pressure reactor shorter than approximately 7 min affect the increase on the micropore area less substantially, independent of the operating pressure. There likely exists a minimum amount of time necessary for the hot steam to penetrate deeper into the internal structures of lignocellulosic fibers to be more effectively exposed to the rapid pressure changes in the following expansion tourniquet. Similar manifestations may be seen in Fig. 2c, which describes the dynamics (SSAE 9.4E − 5, RMSE 3.7E − 7) of the pressure shockwaves. It appears that the first couple of shockwaves is absorbed by the substrate to lose its pellet form. The observations show that larger amounts of water in the pretreated substrate make the micropore expand easier. Once the water is almost incompressible, this phenomenon may be explained by better pressure shockwave transmissions into the inner structure of the substrate, resulting in deeper warping of the plant cells. This assumption is supported by the peaks which are being discreetly formed in the area where higher amounts of shockwaves meet the larger amounts of water (bottom right contours), respectively, in opposite conditions (smaller amounts of water and less pressure shockwaves, up left). Based on these observations, in the next trials, the hot maceration (100 s, 95 °C) was preceding the steam explosion (Fig. 2d, SSAE 5.7E − 9, RMSE 4.6E − 4) and the pressure shockwaves (Fig. 2e, SSAE 8.4E − 5, RMSE 3.7E − 7). Both figures show that the obstacles connected with the external form of the substrate were reduced. Admittedly, Richter et al. (2009) achieved methane yields of 397–426 CH4 VS t-1. The scope of their experiments is different as they used similar techniques on a grass silage which does not have recalcitrance-like properties as straw. In relation to the energy requirements (can be looked up in referred papers and manufacturer's manuals), this discovery could be of great economic significance. Further verification on how the increase on the micropore area correlates with the methane yields obtained was required. The hot maceration did not show any correlation (y = 1.34X + 73.13, R 2 = 0.062). The best non-linear approximation found (Fig. 3a) gave R 2 = 0.108. This result shows that the hot maceration does not significantly increase the micropore area and the micropore area achieved is not responsible for the methane yields obtained. It can thus be assumed that the increase in the methane yield was caused by other factors. According to Richter et al. (2009), it is likely the phenomenon of hydrothermal conditioning and mechanical dehydration of the organic matter. A significant correlation was not achieved even in case of steam explosion (y = 0.44X + 107, R 2 = 0.092). However, closer examination of the data approximated by non-linear function (Fig. 3b, R 2 = 0.782) allows us to speculate that the data may be divided into two groups which, under certain assumptions, may correlate linearly. According to Palmqvist and Hahn-Hägerdal (2000), this phenomena may be caused by a wide range of compounds which are inhibitory to microorganisms (mostly furan derivatives). Analyses of the main representatives of this group as well as other possible causes of the decrease of the methane yield are discussed later. Pretreatment by pressure shockwave showed positive correlations (y = 2.15X + 22.86, R 2 = 0.951) between the micropore area and the methane yields. More sensitive approximation of the data by non-linear function (Fig. 3c, R 2 = 0.983) shows that there may be other unidentified factors. In comparison to steam explosion, it does not show that these unidentified factors are dampening the methane production. Quite contrary, the data can be read as the generated pressure shockwaves are a gentle and effective method of increasing the micropore area and methane yields. According to Himmel (2008) and Taherzadeh and Karimi (2008), this phenomena may be explained as follows: The increased micropore area seems to correlate with accelerated enzymatic hydrolysis of the lignocellulose, which subsequently provided more fermentable compounds and higher methane yields. Increase on micropore area achieved by hot maceration followed by steam explosion did not show correlation with the methane yields subsequently obtained (Fig. 3d, y = -7.14X + 645.91, R 2 = 0.123). In comparison to self-standing steam explosion (Fig. 3b), it can be assumed that the hot maceration followed by steam explosion made the substrate more sensitive to the operating conditions of the high-pressure reactor, resulting in higher yields responsible for a steeper fall in excess of critical operating pressure. The methane yields achieved are exactly in the same range (250–300 CH4 VS t-1) as Dererie et al. (2011). However, their steam explosion was carried out in the presence of lime and dilute acid or followed by enzymatic hydrolysis. On the other hand, the hot maceration followed by pressure shockwaves showed a more significant correlation than the pressure shockwaves itself (y = 2.09X + 68.4, R 2 = 0.96). In addition, sensitive approximation by non-linear function (Fig. 3e, R 2 = 0.971) showed a small reduction of the methane yield regarding the highest micropore area achieved. According to Fan et al. (1980), this phenomena may be explained by the crystallinity of the remaining cellulose which is the next limiting factor. In order to confirm or refute the assumption about possible inhibitor formations on enzymatic hydrolysis (cellulases), analysis on the main inhibitors (formic and levulinic acid, hydroxymethylfurfural, and furfural) as defined by Palmqvist and Hahn-Hägerdal (2000) was performed. In the case of hot maceration, pressure shockwaves, and hot maceration followed by pressure shockwaves, the amounts of furan derivatives analyzed were under the limit of detection. However, pretreatment of the substrate by steam explosion formed certain quantities of these inhibitors, especially hydroxymethylfurfural (Fig. 4a, b). The data show that the inhibitors were formed mostly in the most severe conditions of the high-pressure reactor. In both cases, the dynamics of the operating pressure and the hydraulic retention are relatively similar in regard to the formation of such inhibitors. Following the previous results regarding the manifestations of the methane yield, it is assumed that the microorganism consortia in the anaerobic fermentation are able to adapt the presence of inhibitors to some extent. After exceeding a certain limit (10.7 ± 2.8 and 10.9 ± 2.5 mL L-1, both P < 0.05), their activity is strongly paralyzed. These observations give rise to the possibility of further work exploring if yields of methane may be further improved by developing specific detoxification methods, optimizing the process of anaerobic fermentation or choosing adapted microorganism.
Correlation of the micropore area and the methane yields achieved. a–e as in Fig. 2. The dashed line indicates the 95 % confidence intervals
Conclusion
The self-standing hot maceration does not significantly increase the micropore area or the methane yields. Pretreatment by steam explosion has potential to significantly increase the micropore area and the methane yields, but the operating conditions must be precisely tailored once inhibitor formations occur. This phenomenon is intensified if the steam explosion is following the hot maceration. The pressure shockwaves are capable of high increases of the micropore area as well as achieving high methane yields without the formations of inhibitors. Admittedly, this technology works so far only in volumes of liters per minute.
References
Amon T, Amon B, Kryvoruchko V, Zollitsch W, Mayer K, Gruber L (2007) Biogas production from maize and dairy cattle manure—influence of biomass composition on the methane yield. Agric Ecosyst Environ 118:173–182
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Dererie DY, Trobro S, Momeni MH, Hansson H, Blomqvist J, Passoth V, Schnürer A, Sandgren M, Ståhlberg J (2011) Improved bio-energy yields via sequential ethanol fermentation and biogas digestion of steam exploded oat straw. Bioresour Technol 102:4449–4455
Fan LT, Lee YH, David H (1980) Mechanism of the enzymatic hydrolysis of cellulose: effects of major structural features of cellulose on enzymatic hydrolysis. Biotechnol Bioeng 22:177–199
Herrmann A (2012) Biogas production from maize: current state, challenges and prospects. 2. Agronomic and environmental aspects. Bioenerg Res. doi:10.1007/s12155-012-9227-x
Herrmann C, Heiermann M, Idler C, Scholz V (2007) Parameters influencing substrate quality and biogas yield. In: Proceedings of the 15th European Biomass Conference and Exhibition, Berlin, Germany, Florence, Italy: ETA-Renewable Energies, pp 809–819
Higa O, Matsubara R, Higa K, Miyafuji Y, Gushi T, Omine Y, Naha K, Shimojima K, Fukuoka H, Maehara H, Tanaka S, Itoh S (2012) Mechanism of the shock wave generation and energy efficiency by underwater discharge. Int J Multiphys 6:89–98
Himmel ME (2008) Biomass recalcitrance: deconstructing the plant cell wall for bioenergy. Blackwell Publishing Ltd, Singapore
Krátký L, Jirout T, Nalezenec J (2012) Lab-scale technology for biogas production from lignocellulose wastes. Acta Polytech 52:54–59
Lemmer A, Oechsner H (2001) Co-fermentation of grass and forage maize. Landtechnik 56:412–413
Maroušek J (2013) Removal of hardly fermentable ballast from the maize silage to accelerate biogas production. Ind Crop Prod 44:253–257
Maroušek J, Kawamitsu Y, Ueno M, Kondo Y, Kolář L (2012) Methods for improving methane yield from rye straw. Appl Eng Agric 28:1–9
Noike T, Endo G, Chang JE, Yaguchi JI, Matsumoto JI (1985) Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol Bioeng 27:1482–1489
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Prochnow A, Heiermann M, Drenckhan A, Schelle H (2005) Seasonal pattern of biomethanisation of grass from landscape management. Agricultural Engineering International: the CIGR Ejournal, Manuscript EE 05 011, vol. VII, Manuscript EE 05 011. Available at: http://cigrjournal.org/index.php/Ejournal
Ress BB, Calvert PP, Pettigrew CA, Barlaz MA (1998) Testing anaerobic biodegrability of polymers in a laboratory-scale simulated landfill. Environ Sci Technol 32:821–827
Richter F, Graß R, Fricke T, Zerr W, Wachendorf M (2009) Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. II. Effects of hydrothermal conditioning and mechanical dehydration on anaerobic digestion of press fluids. Grass Forage Sci 64:132–143
Rovira P, Vallejo VR (2002) Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach. Geoderma 107:109–141
Shiralipour A, Smith PH (1984) Conversion of biomass into methane gas. Biomass 6:85–92
Shirato Y, Yokozawa M (2006) Acid hydrolysis to partition plant material into decomposable and resistant fractions for use in the Rothamsted carbon model. Soil Biol Biochem 38:812–816
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651
Wachendorf M, Richter F, Fricke T, Graß R, Neff R (2009) Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. I. Effects of hydrothermal conditioning and mechanical dehydration on mass flows of organic and mineral plant compounds, and nutrient balances. Grass Forage Sci 64:132–143
Acknowledgments
This work was supported by a grant from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Maroušek, J. Prospects in straw disintegration for biogas production. Environ Sci Pollut Res 20, 7268–7274 (2013). https://doi.org/10.1007/s11356-013-1736-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1736-4