Abstract
This study is the first attempt to evaluate the limno-ecological status of freshwater ecosystems in Aras River system (Turkey) using diatom metrics and multivariate analyses according to the European Union Water Framework Directive requirements. Samples were collected using the standard protocols from 17 sampling stations in August and October of 2014, and June of 2015. Deterioration of water quality in the Aras River catchment was remarked from the downstream of Karakoyunlu, Sarısu, and Bozkuş creeks, which showed higher values of trophic index Turkey (TIT), trophic index (TI), and eutrophication and/or pollution index-diatom (EPI-D). These sites were also characterized by pollution tolerant taxa e.g., Cocconeis placentula, Gomphonema parvulum, Navicula cryptocephala, Navicula trivialis, and Tryblionella angustata. Sites S14–S17 had high ecological status according to TIT, supported by the score of EPI-D which indicated a good ecological condition for S14. The occurrence of pollution-sensitive species (e.g., Hannaea arcus, Cymbella affinis, Didymosphenia geminata, Meridion circulare, and Encyonema silesiacum) and the low nutrients like total phosphorus, orthophosphate and nitrate, conductivity, and BOD5 supported the high ecological status of S14–S17. The relationships between diatom assemblages and environmental variables were also illustrated by multivariate analyses. Spearman correlation analysis revealed that TIT had positive important correlations with total phosphorus (p < 0.01, r = 0.768). Trophic index and EPI-D had also significant correlations with environmental variables, but they had lower correlation coefficient than that of TIT. It seems that TIT could be a suitable diatom metric for assessing the ecological status of sampling stations in Aras River system and Mediterranean region. Our findings showed also that ecological statuses of surface waters should be evaluated due to the indices developed in their ecoregions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities through worldwide threaten the quality of surface waters, which is one of the major environmental problems (Hering et al. 2010; Birk et al. 2012). Various environmental stressors such as climate changes, eutrophication, acidification, discharging of hazardous and other effluents, runoff of fertilizers from land uses, hydromorphological degradation, catchment land use, habitat destruction, riparian habitat alteration, flow modification, and invasive alien species have strong impact on the ecological quality of water bodies (Hering et al. 2010; Birk et al. 2012). Reliable information about water quality is required to achieve a good ecological condition based on biological quality tools to ensure the biotic integrity of ecosystems. The European Union Water Framework Directive (WFD) (Directive 2000) is mainly inspired by the ecological approaches of US Clean Water Act published in 1972 and then developed in 1977 and 1980s relevant to water quality standards. In order to assess the ecological quality of surface waters, five aquatic bioindicator organisms (diatoms as phytobenthos, phytoplankton, benthic invertebrates, macrophytes, and fish) with the requirements of the WFD are used (Birk et al. 2012; Kelly et al. 2014; Toudjani et al. 2017). This attempt has provided important information to achieve an environmental sustainability according to bioindicator organisms’ responses to environmental stress.
Different biological communities have been used to assess and monitor the quality of freshwaters. Among them, epilithic diatoms have been widely used as efficient indicators of water bodies for the assessment ecological status of surface waters (Kelly et al. 1998; Potapova and Charles 2003; Kelly et al. 2008; Delgado and Pardo 2014; Lobo et al. 2015, 2016; Rimet 2012).
Diatom indices were developed by the numerical evaluation of the species responses to environmental variables using different statistical approaches. To assess water ecological quality, several diatom metrics have been developed Europe like specific pollution index (SPI) (Cemagref 1982), trophic index (TI) (Rott et al. 1999), eutrophication and/or pollution index-diatom (EPI-D) (Dell’Uomo 2004), and trophic diatom index (TDI) (Kelly et al. 2008). They highlighted that the ecological preferences of diatom species can be changed with temporal and spatial variation in different ecoregions. In Turkey, Çelekli et al. (2019) proposed the use of the trophic index Turkey (TIT) for evaluating water quality using diatoms. In this study, the overall hypothesis was that direct use of foreign diatom index scores obtained from different ecoregions could lead to a wrong interpretation of the water quality. Thereby, the objectives of this research as the first attempt were to use diatom indices developed from different ecoregions for assessing the limno-ecological status of various sampling stations of Aras River system (Turkey).
Materials and methods
Study area and field sampling
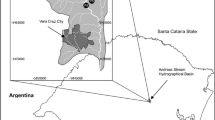
The Kura–Aras River as a transboundary basin located in the North-Eastern Turkey. It is one of the largest running water systems of the Caucasus. Aras River rises from Bingöl Mountains in the south of Erzurum. It flows along the Turkish–Armenian border, and connects Turkey to Nakhchivan exclave. Also, the Aras River forms some part of boundaries of Turkey with Iran, Armenia, and Azerbaijan. The river joins the Kura stream in the north region, and outlets into the Caspian Sea. The catchment is dominated by the continental climate, and the reported average temperature was about 17 °C (DGWM 2016). Aquatic ecosystems in the Aras River system are fed by snowfall since the winters are dominated by terrestrial climatic conditions. The total surface area of the catchment is approximately 27,548 km2 and is mainly constituted by five provinces (Erzurum, Kars, Igdır, Ardahan, and Ağrı). Mean precipitation values varied between 400 mm in Iğdır province and 750 mm in Ardahan province during the present study. Of the provinces in the border of basin, Iğdır located relatively at low altitude and so agriculture land use and livestock farming are the income source.
A total of 17 sampling stations in the Aras River catchment (a transboundary river) of Turkey (Fig. 1) were sampled in August and October of 2014 and June of 2015. Data were collected from stations S1–S11 during three seasons. Then, stations S12–S14 and S15–S17 were added to the study program in October of 2014 and in June of 2015, respectively.
Geographic situation of Aras River Basin and locations of sampling stations. S1, S17—Çot Creek; S2, S6, S9—Aras River; S3—Eğritaş Creek; S4—Karakoyunlu Creek; S5—Sarısu Creek; S7—Karasu Stream; S8—Ilıgöze Creek; S10—Handere Creek; S11—Bozkuş Creek; S12—S12 Creek; S13—Karahan Creek; S14—Digor Creek; S15—Zeveta Creek; and S16—Posof Stream
Before the epilithic diatom sampling in situ, pH, water temperature, electrical conductivity (EC), dissolved oxygen (DO), and salinity were measured using an YSI Professional Plus oxygen–temperature meter. A geographical positioning system was used to determine geographical data. Water samples were then taken and preserved in a storage container with ice packs until the transfer to laboratory. At least five stones were randomly collected in the riffle sections of running water systems for the epilithic diatom samples. Subsequently, the upper surface of substrata was scraped and scrubbed with a hard bristled toothbrush in 100 ml of distilled water by following standard methods of European Committee for Standardization (2004, 2014) and fixed with a Lugol–glycerol solution.
Laboratory analysis
Chemical variables (e.g., total phosphorus (TP), orthophosphate (P-PO4), total nitrogen (TN), nitrate nitrogen (N-NO3), ammonium nitrogen (N-NH4), and biochemical oxygen demand (BOD5)) were analyzed using standard methods (APHA 2012).
The diatom cleaning process with hot hydrogen peroxide and preparation of permanent slides was applied according to the European Committee for Standardization (2004). During the examination of permanent slides, at least 500 diatom valves were enumerated at a magnification of × 1000 under a light microscope (Olympus BX53 model) equipped with a DP73 model digital camera and an Olympus cellSens imaging software. The taxonomic guides provide in Krammer and Lange-Bertalot 1991a, b, 1999a, b, Lange-Bertalot (2001), and Krammer (2000, 2002) were used to identify diatom species.
Determination of ecological status
TIT (Çelekli et al. 2019) was calculated using the following equation (Eq. 1).
where bi, i-diatom taxon trophic weight; ei, i-diatom taxon indicator scores; and ci, the percentage of i-diatom species (Çelekli et al. 2019).
Ecological quality ratios (EQR) were calculated based on TIT scores. An EQR value close to 0 indicates bad ecological status while a value close to 1 shows high ecological status. The ecological status boundary classes were determined according to the WFD using the box-plot analysis for each typology.
EPI-D (Dell’Uomo 2004) was calculated using the following equation (Eq. 2).
where aj abundance of the species j; rj reliability of the species j with inversely proportional to its ecological “range”; 5 is an optimum indicator; 3 is a good indicator; 1 is a sufficient indicator only; ij weighted integrated sensitivity index of the species j; the values attributed go from 0 (for environment of excellent quality) to 4 (degraded water body).
Trophic index (TI) (Rott et al. 1999) was also used and calculated by the following equation:
where TWi is i-taxon trophic weight, Gi is i-taxon indicator value, and Hi is i-taxon’s valve number (Rott et al. 1999).
Statistical analyses
Physico-chemical variables of the sampling stations were compared using multiple range test of Duncan (SPSS version 15.0, SPSS Inc., Chicago, IL, USA). The Spearman correlation was performed to determine the relationships between environmental variables and diatom indices. Detrended correspondence analysis was applied to the dataset of diatom assemblages to determine the gradient length. The result of this analysis revealed the gradient lengths are larger than 3.0, which justified the performing of unimodal ordination techniques (ter Braak and Šmilauer 2002). Thus, canonical correspondence analysis (CCA) was performed to assess the relationship between environmental factors as predictor variables and diatom assemblages as response variables in the 17 sampling stations of the Aras River catchment. The predictor variables were logarithmically transformed except pH to decrease skewness (ter Braak and Šmilauer 2002). Partial CCA was used to determine the most important explanatory variable(s) that drive the distribution of diatom composition. CANOCO software was used for the ordination analyses. The Monte Carlo Permutation test was performed to test which environmental factors played significance roles on the distribution of diatom assemblages. Thus, the ordination consisted of 40 diatom species, 8 environmental variables, and 17 sampling stations as supplementary variables. In order to predict optima and tolerance values of diatom taxa for environmental variables, weighted averaging regression was applied using the Calibrate program (Juggins and ter Braak 1992). Diatom species occurred more than one with an abundance larger than 1% were used in the statistical analyses (Leps and Smilauer 2003).
Results
Environmental variables of sites
Descriptive analyses of main environmental variables are summarized in Table 1. The Aras River system had mainly alkaline water and the mean pH values ranged from 7.4 in Digor Creek to 8.7 in Karasu Stream. Stations S12–S17 had smaller pH levels than those of S1–S14. The highest mean water temperature (22.0 °C) was measured at Karakoyunlu Creek located at 843 m, whereas the lowest mean value (12.8 °C) in Karahan Creek (S12) at 2059 m.
The highest mean EC (1166 μS cm−1) value was found in Sarısu Creek followed by Karakoyunlu Creek with 825 μS cm−1 while stations S12–S17 had low EC values ranged from 25 μS cm−1 in Zeveta Creek to 136 μS cm−1 in Posof Stream (see more in Table 1). Nutrient content of sampling stations fluctuated during the present study. Karakoyunlu Creek (S4), Bozkuş Creek (S11), and Aras River (S2) had high nutrients values than the others (p < 0.05). Low values of TP, P-PO4, TN, and salinity were mainly measured in stations S15–S17 and in Aras River (S9) (p < 0.05).
Diatom composition–environment relationships
A total of 65 diatom taxa were found in the Aras River catchment during the current study. Cocconeis placentula, Cymbella affinis, Gomphonema parvulum, Ulnaria ulna, Diatoma vulgaris, and Navicula cryptocephala were commonly found in the river system. Forty diatom species with more than 1% relative abundance at least one sample were retained for the multivariate statistical analyses (Table 2).
The first two axes of CCA explained 10.6% of the cumulative variance in the diatom assemblages with 0.951 of the species–environment correlation. The diatom species–environmental relationship was highly significant (F = 1.948, p = 0.002) according to results of the Monte Carlo Permutation test. Partial CCA indicated that the most effective explanatory environmental factors e.g., EC, TP, altitude, temperature, TOC, and BOD5 that affected the distribution of diatom species in the Aras River system.
The ordination of diatom assemblages in the CCA diagram was given in Fig. 2. High values of TOC, TSS, BOD5, and EC are associated with Sarısu Creek (S5). Water temperature and TP are closely related with Karakoyunlu creek (S4) and Aras River (S2). S12–S17 stations located far away from the arrows of TOC, TSS, BOD5, and conductivity, but S15–S17 closely related to high altitude, characterized by low nutrient contents, conductivity, temperature, and BOD5. These stations were also associated with species such as Hannaea arcus, Cymbella affinis, Didymosphenia geminata, Meridion circulare, Encyonema silesiacum, and Navicula radiosa.
CCA plot of diatom assemblage (circle)-environmental (arrow) relationships in the sampling sites (up triangular). S1, S17—Çot Creek; S2, S6, S9—Aras River; S3—Eğritaş Creek; S4—Karakoyunlu Creek; S5—Sarısu Creek; S7—Karasu Stream; S8—Ilıgöze Creek; S10—Handere Creek; S11—Bozkuş Creek; S12—S12 Creek; S13–Karahan Creek; S14—Digor Creek; S15—Zeveta Creek; and S16—Posof Stream. TSS total suspended solids, BOD5 biological oxygen demand, TOC total organic carbon, and TP total phosphorus
The pollutant tolerant species (e.g., Gomphonema parvulum, Navicula cryptocephala, and Navicula trivialis) were commonly found in the studied stations except S12–S17, and they mainly showed relationships with high conductivity, water temperature, nutrients, TOC, and BOD5 (Fig. 2).
According to the weighted average regression results, C. placentula (354 μS cm−1 EC optima and 0.384 mg L−1 TP optima), N. cryptocephala (555 μS cm−1 EC optima and 0.470 mg L−1 TP optima), Navicula trivialis (573 μS cm−1 EC optima and 0.859 mg L−1 TP optima), and Tryblionella angustata (945 μS cm−1 EC optima and 0.999 mg L−1 TP optima) preferred high TP and EC. On the other hand, a few diatom species associated with relatively low TP optima e.g. H. arcus (0.065 g L−1 TP), D. geminata (0.115 mg L−1 TP), M. circulare (0.028 mg L−1 TP), and E. silesiacum (0.083 mg L−1 TP).
Ecological status
The bioassessment results of the sampling stations in the Aras River system based on diatom indices are given in Table 3. All diatom indices indicated that the lowest value was recorded in Zeveta Creek (S15). Sarısu Creek (S5) had the high scores of TI and EPI-D whereas high TIT scores were found in Karakoyunlu Creek (S4) that is followed by Sarısu Creek. TIT indicated had high ecological status for S14–S17, which also supported by EPI-D except S14 since it had a good status. Only S15 and S16 showed a high ecological status while bad ecological status was noted for Sarısu Creek according to the TI.
Significant correlations (*p < 0.05 and **p < 0.01) between diatom indices and environmental variables are given in Table 4. TIT indicated positively significant correlations with TP (p < 0.01, r = 0.768), P-PO4 (p < 0.01, r = 0.744), BOD5 (p < 0.01, r = 0.568), and EC (p < 0.01, r = 0.552). Also, TI and EPI-D had significant correlations with environmental variables (for more see Table 4).
Discussion
In the Aras River system, Karakoyunlu Creek (S4), Bozkuş Creek (S11), and Aras River (S2) had higher nutrient values than those of the other sites (p < 0.05). The high EC value was measured in Sarısu Creek (S5) and Karakoyunlu Creek (S4) in this system (p < 0.05). EC as a good monitor can reflect the total content of the ions in the watercourses. In the present study, EC values in urban down sites of the Aras catchment averaged 1166 μS/cm in S5, followed by S4 with 825 μS/cm when the lowest value (25 μS/cm) was observed in S15 and S17 that was located at the up side of this catchment (p < 0.001). Limno-ecological studies also showed similar EC results among stations in USA (Potapova and Charles 2003), Luxembourg (Rimet et al. 2004), China (Chen et al. 2016), and in the western Mediterranean river catchment of Turkey (Toudjani et al. 2017).
The deterioration of the water quality of the mentioned stations could be the consequences of a number of anthropogenic pressures including sewage discharges, livestock, runoff from fertilized agriculture soils, and partial interventions in the riverbed (DGWM 2016). Besides, nutrient and pollutant deposition along the watersheds of S4 lead to the increase in nutrient concentrations.
In the Aras River system, sampling stations especially S15–S17 had the lowest nutrient content. This could be due to the stations’ areas located at high altitudes and the absence of settlements and agricultural land use. The lowest TP value (0.02 mg/L) was measured in Çot Creek (S17), followed by Posof Creek (S16 with 0.04 mg/L) and Zeveta Creek (S15 with 0.04 mg/L). Furthermore, the lowest values of EC, TOC, BOD5, other nutrients, and salinity were also measured from these stations located at high altitude. Besides, hydromorphological features of mentioned stations especially S15–S17 composed of pebbles, stones, and rocks and have clear waters. These findings were also supported by the water quality of these ecosystems during the monitoring study.
TIT scores varied from 0.87 in Zeveta creek (S15) to 2.86 in Karakoyunlu (S4) during the study time. The lowest scores of TI and EPI-D were recorded in Zeveta Creek, but the highest was found at Sarısu Creek (S5) when compared to TIT. TIT and EPI-D indicated high ecological status for S14–S17 while good status was found at S14. In contrast, only S15 and S16 had a high status according to the results of TI, when Sarısu creek indicated bad ecological status. With regard to the ecological status of sampling stations, TIT and EPI-D showed concordant results compared to results of TI. This could be due to the optima and tolerance ranges of diatom species for environmental conditions that can vary from one ecoregion to another. This shows the needing of indices to be developed for different ecoregions (Gomá et al. 2004; Lobo et al. 2004). Previous studies (e.g. Kelly et al. 1998; Rott et al. 1999; Rott et al. 2003; Dell’Uomo 2004) also indicated that developed indices based on diatoms in certain regions of Europe could not be effective when applied in other parts of Europe.
The environmental variables especially EC, TP, altitude, temperature, TOC, and BOD5 in Aras River system play a significant role in the distribution of diatom assemblages with 0.951 the species–environment correlation (F = 1.948, p = 0.002, Fig. 2). Ecological status of the stations related to environmental variables was clearly indicated by the CCA ordination. With regard to the ordination, Sarısu Creek (S5) was associated with high nutrients, TOC, TSS, BOD5, and conductivity. Water temperature and TP were related with Karakoyunlu creek (S4) and Aras River (S6). These stations were characterized by the occurrence of pollutant tolerant taxa such as G. parvulum, N. cryptocephala, N. trivialis, and T. angustata. The occurrence of these species in the aforementioned stations could be the consequence of the combination of several pressures especially agriculture activities known as a great source of organic and inorganic pollutants, domestic wastes, and effluent release from their catchments (DGWM 2016). In accordance with our findings, several previous studies indicated the characterization of polluted and/or eutrophic sites by these species (Lobo et al. 2004; Potapova et al. 2004; Bere and Tundisi 2011; Delgado et al. 2012; Wang et al. 2014).
Gomphonema parvulum as an environmental tolerant species (Delgado et al. 2012) exhibits a wide biogeographical distribution on the earth including the Eastern Highlands of Zimbabwe (Bere 2016), a karstic limno-crene spring of Turkey (Çelekli and Külköylüoğlu 2007), the subtropical temperate Brazilian rivers (Lobo et al. 2010), Mountain Rivers of the Segre basin of Catalonia (Goma et al. 2005), the western Mediterranean river basin of Turkey (Toudjani et al. 2017), the water bodies of Austria (Rott et al. 1999), the running waters of UK (Kelly et al. 2008), the Richmond River Catchment of Australia (Oeding and Taffs 2017), and the watercourses of Italy (Dell’Uomo 2004).
Navicula cryptocephala and N. trivialis have been indicated a pollution (Rott et al. 1999; Wang et al. 2014; Kelly et al. 2008), and also nutrient tolerant species in freshwater ecosystems in Austria (Rott et al. 1999), Brazilian (Lobo et al. 2010), Argentinia (Cochero et al. 2015), Beijing, China (Chen et al. 2016), the Bloukrans River system in the Eastern Cape province of South Africa (Dalu et al. 2017), and Australia (Oeding and Taffs 2017). Similarly, T. angustata has been cited as a pollution tolerant species and was found in the nutrient-rich streams in the Anatolia (Çelekli et al. 2019) and in Argentinia (Cochero et al. 2015).
High ecological status was found in Posof Stream and Digor, Zeveta, and Çot creeks, which were associated with relatively low nutrient content and characterized by the presence of pollution sensitive diatom species e.g., H. arcus, C. affinis, D. geminata, M. circulare, and E. silesiacum. They have been known as low trophic indicator species and were found at high ecological status (Rott et al. 1999; Kelly et al. 2008; Çelekli et al. 2019). Hannaea arcus had high abundance in the S15–S17 sites having high flow and high dissolved oxygen in agreement with previous study (Goma et al. 2005). Cymbella affinis considered as a dominant species in less polluted ecosystems (Gómez and Licursi 2001) as a pollution-sensitive diatom taxon (Delgado et al. 2012) and as a low trophic indicator taxon (Wang et al. 2014). Didymosphenia geminata has been described as a representative species of relatively less polluted sites (Rott et al. 1999; Beltrami et al. 2008). It is also a typical species of oligotrophic rivers (Krammer and Lange-Bertalot 1997), and it is included in the Red List of Diatoms (Lange-Bertalot and Steindorf 1996). Meridion circulare was associated with relatively less polluted sites in Austria (Binder 2001), USA (Potapova et al. 2004), and in the headwater streams of Luxembourg (Rimet et al. 2004). Encyonema silesiacum, Fragilaria arcus (Hannaea arcus), and M. circulare as dominant taxa are considered as indicator species of low nutrient, and they were also found in the upstream stretches of mountain rivers of the Segre basin (Goma et al. 2005).
Ecological preferences and occurrences of aforementioned species confirmed the different ecological status of the sampling stations and the suitable applicability of the TIT index in ecoregion approach. Recently, Toudjani et al. (2017) proposed that TIT is more competitive than EPI-D developed in Italy (Dell’Uomo 2004) when assessing the ecological status of freshwater ecosystems in the Western Anatolia basin (Turkey). Results of Spearman correlation analysis confirmed that all diatom indices had significant correlations to environmental variables. According to values of Spearman correlation coefficient, TIT had better results in the assessment of the ecological quality of freshwater ecosystems in Aras River system than those of TI and EPI-D. This could be due to the effects of ecoregion environmental factors on the trophic weight of diatom taxa. These indices were developed for a particular region, integrated with different trophic weight and indicator values of diatom taxa may not be fit as competent for the assessment of biointegrity of water bodies in other regions. Regional variation in each ecoregion, especially in geology, anthropogenic activities, climate, and land use can significantly change predictor factors, constraints, and diatom assemblages (Stevenson 1997; Soininen 2007; Çelekli et al. 2019).
Conclusion
Deterioration of water quality in the Aras River was mainly found in the downstream of this catchment (e.g., in S4, S5, and S11) where higher values of TIT, TI, and EPI-D were recorded. These sites were also characterized by the pollution tolerant taxa. On the other hand, S14–S17 had high ecological status according to results of TIT and TI except S14 which showed a good status based on TI. The occurrences of pollution-sensitive species (e.g., H. arcus, C. affinis, D. geminata, M. circulare, and E. silesiacum) supported the high ecological status of mentioned stations confirmed by the low nutrients, conductivity, and BOD5, which were also supported by the multivariate analyses. TI and EPI-D had significant positive correlations with TP, conductivity, BOD5, TN, P-PO4, and N-NO3, but were not more competitive than TIT based on values of Spearman correlation coefficient. From that point, TIT as a diatom metric could be a useful tool for assessing the ecological status of running waters in the Aras River system.
References
APHA (2012) American Public Health Association. American Water Works Association, Water Environment Federation Standard Methods for the Examination of Water and Wastewater. 22nd Edition, 1496 p
Beltrami ME, Cappelletti C, Ciutti F (2008) Didymosphenia geminata (Lyngbye) M. Schmidt (Bacillariophyta) in the Danube basin: new data from the Drava river (northern Italy). Plant Biosyst 142:126–129
Bere T (2016) Challenges of diatom-based biological monitoring and assessment of streams in developing countries. Environ Sci Pollut Res 23(6):5477–5486
Bere T, Tundisi JG (2011) Diatom-based water quality assessment in streams influence by urban pollution: effects of natural and two selected artificial substrates, São Carlos-sp, Brazil. Braz J Aquat Sci Technol 15(1):54–63
Binder N (2001) Wege zur Anwendung numerischer Methoden für die Indikationsbewertung von Algenarten für das Fließgewässermonitoring. Institut für Botanik, Arbeitsgruppe Hydrobotanik, Innsbruck, Austria
Birk S, Bonne W, Borja A, Brucet S, Courrat A, Poikane S, Soliminie A, Van de Bund W, Zampoukas N, Hering D (2012) Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecol Indic 18:31–41
Çelekli A, Külköylüoğlu O (2007) On the relationship between ecology and phytoplankton composition in a arstic spring (Çepni Bolu). Ecol Indic 7:497–503
Çelekli A, Toudjani AA, Gümüş EY, Kayhan S, Lekesiz HÖ, Çetin T (2019) Determination of trophic weight and indicator values of diatoms in Turkish running waters for water quality assessment. Turk J Bot 43:90–101. https://doi.org/10.3906/bot-1704-40
Cemagref (1982) Etude de Mthodes Biologiques Quantitatives d’Appreciation de la Qualit des Eaux. Rapport Q.E. Lyon- A.F.B. Rhȏne-Mediterrannee-Corse
Chen X, Zhou W, Pickett STA, Li W, Han L, Ren Y (2016) Diatoms are better indicators of urban stream conditions: a case study in Beijing, China. Ecol Indic 60:265–274
Cochero J, Licursi M, Gómez N (2015) Changes in the epipelic diatom assemblage in nutrient rich streams due to the variations of simultaneous stressors. Limnologica 51:15–23
Dalu T, Wasserman RJ, Magoro ML, Mwedzi T, Froneman PW, Weyl OLF (2017) Variation partitioning of benthic diatom community matrices: effects of multiple variables on benthic diatom communities in an Austral temperate river system. Sci Total Environ 601–602:73–82
Delgado C, Pardo I (2014) Comparison of benthic diatoms from Mediterranean and Atlantic Spanish streams: community changes in relation to environmental factors. Aquat Bot 120:304–314
Delgado C, Pardo I, García L (2012) Diatom communities as indicators of ecological status in Mediterranean temporary streams (Balearic Islands, Spain). Ecol Indic 15:131–139
Dell’uomo A (2004) L’indice diatomico di eutrofizzazione/polluzione (EPI-D) nel monitoraggio delle azque correnti, line guida. Dipartimento di Botanica ed Ecologia, Universita di Camerino
Directorate General for Water Management (DGWM) (2016) Project on the establishment of an ecological assessment system for water quality in Turkey: monitoring report of Aras River Basin. Ankara, Turkey, p 488
Directive (2000) Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. O J E C 327:1–72
European Committee for Standardization (2004) Water quality-guidance standard for the identification, enumeration and interpretation of benthic diatoms from running waters. European standard EN, 13946, 12 pp Brussels
European Committee for Standardization (2014) Water quality-guidance for the routine sampling and preparation of benthic diatoms from rivers and lakes. European standard EN 13946, p 17. Brussels
Gomá J, Ortiz R, Cambra J, Ector L (2004) Water quality evaluation in Catalonian Mediterranean rivers using epilithic diatoms as bioindicators. Vie Milieu 54:81–90
Goma J, Rimet F, Cambra J, Hoffmann L, Ector L (2005) Diatom communities and water quality assessment in mountain rivers of the upper Segre basin (La Cerdanya, Oriental Pyrenees). Hydrobiologia 551:209–225
Gómez N, Licursi M (2001) The Pampean Diatome Index (PDI) for assessment of rivers and streams in Argentina. Aquat Ecol 35:173–181
Hering D, Borja A, Carstensen J, Carvalho L, Elliott M, Feld CK, Heiskanen A-S, Johnson RK, Moe J, Pont D, Solheim AL, van de Bund W (2010) The European Water Framework Directive at the age of 10: a critical review of the achievements with recommendations for the future. Sci Total Environ 408:4007–4019
Juggins S, ter Braak CFJ (1992) Calibrate-program for species-environment calibration by (weighted averaging) partial least squares regression. Environmental Change Res Cent University College London
Kelly M, Cazaubon A, Coring E, Dell’Uomo A, Ector L, Goldsmith B, Guasch H, Hurlimann J, Jarlman A, Kawecka B, Kwadrans J, Laugaste R, Lindstrom E, Leitao M, Marvan P, Padisak J, Pipp E, Prygiel J, Rott E, Sabater S, Van Dam H, Vizinet J (1998) Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10:215–224
Kelly M, Juggins S, Guthrie R, Pritchard S, Jamieson J, Rippey B, Hirst H, Yallop M (2008) Assessment of ecological status in U.K. rivers using diatoms. Freshw Biol 53:403–422
Kelly M, Urbanic G, Acs E, Bennion H, Bertrin V, Burgess A, Denys M, Gottschalk S, Kahlert M, Karjalainen SM, Kennedy B, Kosi G, Marchetto A, Morin S, Picinska-Fałtynowicz J, Poikane S, Rosebery J, Schoenfelder I, Schoenfelder J, Varbiro G (2014) Comparing aspirations: intercalibration of ecological status 2 concepts across European lakes for littoral diatoms. Hydrobiologia 734:125–141
Krammer K (2000) The genus Pinnularia, Lange-Bertalot H. (ed.), diatoms of Europe—diatoms of the European inland waters and comparable habitats. Gantner Verlag, Ruggel 1:1–703
Krammer K (2002) The genus Cymbella, Lange-Bertalot H. (ed.), diatoms of Europe—diatoms of the European inland waters and comparable habitats. Gantner Verlag, Ruggel 3:1–584
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae. 3 Teil: Centrales. Fragilariaceae. Eunotiaceae. In Ettl. H. J. Gerloff. H. Heynig, D. Mollenhauer (Hrsgb.). Süß-wasser- flora von Mitteleuropa. Band 2. Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. 4 Teil: Achnanthaceae. Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Ettl. H.G. Gartner. J. Gerloff. H. Heynig and D. Mollenhauer (eds) Süßwasserflora von Mitteleuropa. Band 2. Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1997) Bacillariophyceae. 1. Naviculaceae. Süßwasserflora von Mitteleuropa 2/1. Fischer, Jena
Krammer K, Lange-Bertalot H (1999a) Bacillariophyceae. 1 Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa. Band 2. Akademischer Verlag. Heidelberg, Berlin
Krammer K, Lange-Bertalot H (1999b) Bacillariophyceae. 2 Teil: Bacillariaceae. Epithemiaceae. Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süß-wasser- flora von Mitteleuropa. Band 2. Akademischer Verlag, Heidelberg. Berlin
Lange-Bertalot H (2001) Navicula sensu stricto 10 genera separated from Navicula sensu lato Frustulia, Lange-Bertalot H. (ed.), diatoms of Europe—diatoms of the European inland waters and comparable habitats. Gantner Verlag, Ruggel 2:1–526
Lange-Bertalot H, Steindorf A (1996) Rote Liste der limnischen Kieselalgen (Bacillariophyceae) Deutschlands. Schr Vegetationsk 28:633–677
Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Lobo EA, Callegaro VLM, Hermany G, Gómez N, Ector L (2004) Review of the use of microalgae in South America for monitoring rivers, with special reference to diatoms. Vie Milieu 54:105–114
Lobo EA, Wetzel CE, Ector L, Katoh K, Blanco S, Mayama S (2010) Response of epilithic diatom communities to environmental gradients in subtropical temperate Brazilian rivers. Limnetica 29(2):323–340
Lobo EA, Schuch M, Heinrich CD, Costa AB, Düpont A, Wetzel CE, Ector L (2015) Development of the Trophic Water Quality Index (TWQI) for subtropical temperate Brazilian lotic systems. Environ Monit Assess 187:354–366
Lobo EA, Heinrich CD, Schuch M, Wetzel CE, Ector L (2016) Diatoms as bioindicators in rivers. In: NECCHI Jr (ed) River Algae. Springer International Publishing, p 245–271
Oeding S, Taffs KH (2017) Developing a regional diatom index for assessment and monitoring of freshwater streams in sub-tropical Australia. Ecol Indic 80:135–146
Potapova MG, Charles DF (2003) Distribution of benthic diatoms in US rivers in relation to conductivity and ionic composition. Freshw Biol 48:1311–1328
Potapova M, Charles DF, Ponade KC, Winter DM (2004) Quantifying species indicator values for trophic diatom indices: comparison of approaches. Hydrobiologia 517:25–41
Rimet F (2012) Recent views on river pollution and diatoms. Hydrobiologia 683:1–24
Rimet F, Ector L, Cauchie HM, Hoffmann L (2004) Regional distribution of diatom assemblages in the headwater streams of Luxembourg. Hydrobiologia 520:105–117
Rott E, Pipp E, Pfister P, Van Dam H, Ortler K, Binder N, Pall K (1999) Indikationslisten für Aufwuchsalgen in österreichischen Fliessgewässern. Teil 2: Trophieindikation (sowie geochemische Präferenzen, taxonomische und toxikologische Anmerkungen). Wasserwirtschaftskataster, Bundesministerium f. Land-u. Forstwirtschaft, Wien, p 248
Rott E, Pipp E, Pfister P (2003) Diatom methods developed for river quality assessment in Austria and a cross-check against numerical trophic indication methods used in Europe. Algol Stud 110:91–115
Soininen J (2007) Environmental and spatial control of freshwater diatoms—a review. Diatom Res 22:473–490
Stevenson RJ (1997) Scale-dependent determinants and consequences of benthic algal heterogeneity. J N Am Benthol Soc 16:248–262
ter Braak CJF, Šmilauer P (2002) CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (version 4.5). Microcomputer Power Press, Ithaca
Toudjani AA, Çelekli A, Gümüş EY, Kayhan S, Lekesiz HÖ, Çetin T (2017) A new diatom index to assess ecological quality of running waters: a case study of water bodies in western Anatolia. Ann Limnol Int J Limnol 53:333–343
Wang X, Zheng B, Liu L, Li L (2014) Use of diatoms in river health assessment. Annu Res Rev Biol 4(24):4054–4074
Acknowledgments
We thank the General Directorate of Water Management of the Ministry of Forestry and Water Affairs (Republic of Turkey), which supported this research; the DOKAY-ÇED Co., and the Scientific Research Projects Executive Council of Gaziantep University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çelekli, A., Kayhan, S., Lekesiz, Ö. et al. Limno-ecological assessment of Aras River surface waters in Turkey: application of diatom indices. Environ Sci Pollut Res 26, 8028–8038 (2019). https://doi.org/10.1007/s11356-019-04295-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04295-y