Abstract
Stream biomonitoring tools are largely lacking for many developing countries, resulting in adoption of tools developed from other countries/regions. In many instances, however, the applicability of adopted tools to the new system has not been explicitly evaluated. The objective of this study was to test the applicability of foreign diatom-based water quality assessment indices to streams in Zimbabwe, with the view to highlight challenges being faced in diatom-based biological monitoring in this developing country. The study evaluated the relationship between measured water quality variables and diatom index scores and observed some degree of concordance between water quality variables and diatom index scores emphasising the importance of diatom indices in characterisation and monitoring of stream ecological conditions in developing countries. However, ecological requirements of some diatom species need to be clarified and incorporated in a diatom-based water quality assessment protocol unique to these regions. Resources should be channelled towards tackling challenges associated with diatom-based biological monitoring, principally taxonomic studies, training of skilled labour and acquiring and maintaining the necessary infrastructure. Meanwhile, simpler coarse taxonomy-based rapid bioassessment protocol, which is less time and resource consuming and requires less specialised manpower, can be developed for the country.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
River health monitoring and assessment is increasingly becoming important because of the anthropogenic activities that have significantly affected the water quality of aquatic systems. Diatoms have been used extensively in river health assessment programmes, especially in developed countries (Slàdeček 1986; Watanabe et al. 1986; Leclerq and Maquet 1987; Descy and Coste 1991; Kelly and Whitton 1995; Prygiel et al. 1996; Rott et al. 1997, 1999; Lobo et al. 2004; Lavoie et al. 2008). They respond rapidly to degradation of water quality, and the integrity of these communities provides a direct, holistic and integrated measure of river ecosystem health. They have gained momentum in their usage as alternatives to chemical analyses because the latter techniques provide, at best, a fragmented overview of the state of river systems as sporadic or periodic sampling cannot reflect fluxes of effluent discharge typical of real-world experiences. Diatom-based monitoring tools are particularly essential for the management of rivers in developing countries as they are fast and cost-effective approaches for assessing the effects of environmental stressors (McCormick and Cairns 1994; Taylor et al. 2007a; Harding et al. 2015; Bere and Tundisi 2010). Several diatom-based river health assessment indices have been developed, most of which are general pollution indices, especially indicative of eutrophication and organic pollution. These indices are thought to have universal applicability across geographic areas and environments because of the cosmopolitan nature of most diatom species (McCormick and Cairns 1994; Harding et al. 2015). Thus, due to lack of information on ecological preferences and tolerances of diatoms in developing countries, indices developed in developed countries are often borrowed.
There is, however, evidence that diatom indices developed in one geographic area or environment are less successful when applied in other areas (Pipp 2002). This is due to the floristic differences among regions (Taylor et al. 2007a) and the environmental differences that modify species responses to water quality characteristics (Potapova and Charles 2007). Strict testing of these borrowed indices is required to ensure that diatom index scores give a realistic reflection of the specific type of environmental pollution being tested.
Attempts to use diatoms as indicators of water quality changes are fairly recent and have relatively few precedents in Zimbabwe. Phiri et al. (2007) studied periphytic diatoms attached to the leaves of the submerged macrophyte Vallisneria aethiopica in the shallow waters of the Sanyati Basin in Lake Kariba, Zimbabwe. They concluded that diatoms may potentially be useful in assessing ecological conditions or the impact of human activities within the shallow marginal waters of the lake. Recent studies have also demonstrated the robust and quantifiable nature of the relationship between diatoms and environmental variables indicating the potential use of diatoms as ecological indicators in the country (Bere et al. 2013; Bere et al. 2014; Mangadze et al. 2015). Besides this study, diatom communities and their ecological requirements have largely been unexplored in the study region, hampering the use of diatoms as ecological indicators.
The objective of this study was to test the applicability of foreign diatom-based water quality assessment indices to streams in Zimbabwe, with the view to highlight challenges being faced in diatom-based biological monitoring in developing countries. In Zimbabwe, the potential for using diatoms as bioassessment tools has not been fully realised and benthic diatoms have not been widely used in state water quality assessment programs. The applicability of the diatom indices developed in other regions and calculated by the OMNIDIA version 5.3 software was tested in two ecological settings: (1) streams draining urban areas (Chinhoyi, Zimbabwe) and (2) relatively pristine streams draining the protected Nyanga National Park in the Eastern Highlands of Zimbabwe.
Materials and methods
Study area
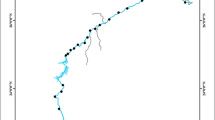
The study was carried out in two ecological settings: (1) streams draining urban areas (Chinhoyi, Zimbabwe) and (2) relatively pristine streams draining the protected Nyanga National Park in the Eastern Highlands of Zimbabwe (Fig. 1). In the first ecological setting, the study streams passed through Chinhoyi city. The area has average annual temperature of around 24.5 °C, with a mean monthly maximum of 29.9 °C recorded in October and November and a mean monthly minimum of 18.9 °C recorded in July. In 2012, the population of Chinhoyi was estimated at 79 368 inhabitants by the Zimbabwe National Statistics Agency (ZNSA). The expansion of the city does not meet the technical standards that go with it in terms of sewage treatment, collection of garbage, urban drainage and so on. Streams in the study area, therefore, receive untreated or semi-treated effluent from various domestic and industrial sources as well as other diffuse sources as they pass through the city. This disorderly growth of the city, typical of most developing countries, results in stream health deterioration especially due to organic pollution and eutrophication. A total of eight sites were established along streams in Chinhoyi. The criterion for selecting sampling sites was to enable evaluation of the impact of breakdown in municipal service delivery (especially sewage treatment) on water quality and the associated diatom communities in the study streams. Site 1 was located downstream on the Manyame River, while site 6 was located upstream on the same river, in a relatively less-polluted area. Large flow volumes in the Manyame River (the upper reaches of which drain less polluted commercial farms) are expected to have a dilution effect on pollutants at sites 1 and 6. Sites 2, 5, and 7 were located just after sewage effluent discharge points. Sites 3, 4, and 8 were located in the town centre where uncollected garbage and effluent from broken sewage pipes finds its way into the stream. All of the sites were sampled four times on the following dates: 04 March 2012, 16 May 2012, 30 July 2012 and 27 August 2012.
In the second ecological setting, headwaters of the study streams fall within the protected Nyanga National Park (Fig. 1). As the rivers leave the park, they flow into low-lying relatively pristine sparsely populated areas characterised by plantations and rural communities with highly preserved riparian buffer zones. Mean annual temperature of lower lying areas range from 17.5 to 20 °C as compared to 15 °C for higher altitude areas. A total of 21 sites were established in the study area (Fig. 1). Diatom and water quality sampling were recorded once off at 21 sites between May and August 2007. Along the Nyangombe River, sites N1 to N6 were located in protected areas while sites N6 to N9 were located in unprotected areas. Along the Pungwe River, sites P1 to P5 were located in protected areas while sites P6 and P7 were located in unprotected areas. All the five sites sampled along the Kairezi River were located in unprotected areas except K3, which was located in communal area management program for indigenous resources (CAMPFIRE), a semi-protected area.
In all the ecological settings, sampling was done during the stable flow periods to avoid variable effects of rainy season such as great variations in water level and velocity, floods and inundations, which are known to affect diatom development, especially growth rate and relative abundance of different species (Round 1991). More detailed descriptions of the study areas can be found in Bere and Tundisi (2011), Bere et al. (2013) and Bere et al. (2014).
Environmental variables
In Chinhoyi, temperature, pH and electrical conductivity were measured with ERMA meters (ERMA Inc. Japan). Dissolved oxygen (DO) was measured with a Bante 820 meter (Bante, China). Turbidity, nitrate (NO3 −) and phosphate (PO4 3−) were measured with Hach DR/2010. In the Eastern Highlands, temperature, pH, electrical conductivity, dissolved oxygen, turbidity and nitrate (NO3 −) were measured using Horiba U-23 and W-23XD Water Quality meter (Horiba Ltd., Japan).
Biological elements
At all the sites, epilithic diatom samples were sampled by brushing stones with a toothbrush. Dead wood was used as a substrate in the absence of boulders at two sites as suggested by Kelly and Whitton (1995). Prior to sampling of epilithic surfaces, all substrata were gently shaken in stream water to remove any loosely attached sediments and non-epilithic diatoms. At least five pebble- to cobble-sized stones were randomly collected at each sampling site and brushed, and the resulting diatom suspensions were pooled to form a single sample, which was then put in a labelled plastic bottle. In the laboratory, sub-samples of the diatom suspensions were cleaned of organic material using wet combustion with concentrated sulphuric acid and mounted in Naphrax (Northern Biological supplies Ltd., UK, RI = 1.74), following Biggs and Kilroy (2000). Three replicate slides were prepared for each sample. Around 400 valves per sample (based on counting efficiency determination method by Pappas and Stoermer (1996)) were identified and counted using a phase contrast light microscope (×1000; Leica Microsystems, Wetzlar GmbH, Type 020-519.503 LB30T, Germany). The diatoms were identified to species level based on the following studies: Metzeltin et al. (2005), Bicudo and Menezes (2006), Taylor et al. (2007c) and Metzeltin and Lange-Bertalot (1998, 2007).
Indices and data analysis
The diatom species counts were entered into the diatom database and index calculation tool OMNIDIA version 5.3 (Lecointe et al. 1993). Seventeen indices were calculated and tested (Table 1). For full data analysis, results and discussion of benthic diatom communities in relation to environmental variables in the study area, refer to Bere et al. (2013) and Bere et al. (2014). Pearson’s correlation (performed using Palaeontological Statistics (PAST) software version 2.16; Hammer et al. 2009) was used to determine the relationship between the calculated index scores and measured physical and chemical water quality data.
Results
Environmental variables
The values of physical and chemical variables measured in these studies are shown in Table 2. Pollution levels were generally high in the sites draining Chinhoyi urban area. Temperature and pH were comparable among sites, though the later tended to be generally low at sites 2 and 7 that were affected by sewage effluent compared to the rest of the sites. Conductivity was relatively lower at sites 1 and 6 where large flow volumes of ‘clean water’ had a dilution effects on pollutants compared to the rest of the sites. Turbidity, nitrite and phosphate levels were relatively higher at sites 2 and 7 that were affected by sewage effluent compared to the rest of the sites, while DO was relatively lower at sites 2 and 7 compared to the rest of the sites. On the other hand, most of the sites in the Eastern Highlands of Zimbabwe were pristine, especially those within the Nyanga National Park.
Indices
Diatom community structure and composition in the two ecological settings generally tended to reflect pollution gradients. In the Eastern Highlands of Zimbabwe, a total of 119 diatom species belonging to 38 genera were recorded in 21 diatom samples collected. Diatom communities characterising these samples included low to medium pollution tolerant taxa such as Cocconeis placentula, Cymbella javanica, Cymbella perpusilla, Cymbella tumida, Cymbella kappii, Cymatopleura solea, Hantzschia amphioxys, Navicula theronii, Eunotia fallax, Navicula rhynchocephala and Placoneis dicephala. In streams draining Chinhoyi urban area, a total of 101 diatom species belonging to 35 genera were recorded in 39 samples. All the sites were subject to some form of pollution; hence, species distribution was strongly biased towards those that are cosmopolitan and tolerant of elevated or slightly elevated levels of pollution. Diatom species characterising these sites include species such as Aulacoseira muzzanensis, Cyclotella ocellata, Gomphonema parvulum, Gomphonema gracile, Gomphonema pseudoaugur, Navicula gregalis, and Nitzschia palea. The distribution of most frequently occurring diatom taxa in Chinhoyi and the Eastern Highlands of Zimbabwe is shown in Appendix 1. Detailed, results and discussion of benthic diatom communities in relation to environmental variables in these two study areas can be found in Bere et al. (2013) and Bere et al. (2014).
A total of 25 and 43 % of the diatom species were not entered into OMNIDIA for calculation of indices in Chinhoyi and the Eastern Highlands, respectively. Significant correlations (p < 0.05) were generally observed between most of the index scores and water quality variables, especially in highly polluted urban areas (Table 3). The correlations were significantly low (p < 0.05) for Eastern Highlands compared to Chinhoyi urban sites. Indices such as the saprobity index (SLA), Leclercq and Maquet’s index (LMI) by Leclerq and Maquet (1987, see Table 1), Generic diatom index (GDI) and Artoise-Picardie diatom index (APDI) were not significantly correlated with any of the measured environmental variables in the Eastern Highlands.
Discussion
The two case studies provide abundant evidence that epilithic diatom communities are good reflectors of human-induced degradation of water quality in tropical streams as evidenced by observed correlations between some diatom index scores and some environmental variables in the study regions. This indicates the importance of foreign diatom indices in assessing anthropogenic changes in water quality in the study regions as recorded elsewhere (Prygiel and Coste 1993; Kwandrans et al. 1998; Taylor et al. 2007a, b). Although concerns have been raised as to the feasibility of transferring data concerning the ecological tolerance limits of diatoms among regions (Round 1991), most of the dominant diatom species encountered in these studies (detailed in Bere et al. (2013) and Bere et al. (2014)) are cosmopolitan species well-documented in international literature (e.g. Krammer and Lange-Bertalot 1986–1991). For that reason, most foreign diatom indices may be used in the study regions, especially in eutrophic, organically enriched waters, as they are based on the ecology of widely distributed or cosmopolitan taxa. Thus, diatom-based biotic indices constitute appropriate technical tools for environmental monitoring programs and water quality assessment in tropical streams. These techniques are particularly essential for stream monitoring and assessment in developing countries as they are fast and cost-effective approaches for assessing the effects of environmental stressors (McCormick and Cairns 1994; Taylor et al. 2007a; Harding et al. 2015; Bere and Tundisi 2010).
In highly polluted Chinhoyi urban area, the correlation coefficients were better than the correlations demonstrated in Europe and South Africa. Diatom-based biomonitoring techniques were born in the wake of the Industrial Revolution and urbanisation to measure the effects of environmental stressors, especially eutrophication and organic pollution, on aquatic systems. Thus, most of the indices are general pollution indices, especially indicative of eutrophication and organic pollution, hence their better reflection of water quality in eutrophication/organic pollution-prone streams draining urban industrialised areas.
On the other hand, in relatively pristine waters of the Eastern Highlands of Zimbabwe, correlations between index scores and water quality variables were general week and indices such as the SLA, LMI, Watanabe index (WAT), GDI and APDI were not significantly correlated to all the water quality variables (Table 3). This was not the case in studies carried out in eutrophic waters in Chinhoyi as well as from other regions (Kwandrans et al. 1998; Taylor et al. 2007a, b; Bere and Tundisi 2011). This highlights the limitations of the current diatom-based biotic indices in assessing water quality in relatively pristine environments.
In addition, cosmopolitan genera such as Navicula, Nitzschia, and Gomphonema with a wide range of tolerances to various water quality variables were among the dominant species in these studies (Bere and Tundisi 2011; Bere et al. 2013, 2014). Environmental optima and tolerance ranges of these ‘cosmopolitan’ species may vary in tropical regions (Lobo et al. 2004) affecting the correlation of temperate-based diatom index scores with water quality parameters. Thus, their use in calculation of indices may not always yield consistent results (Gómez and Licursi 2001). In addition, the hypothesis of cosmopolitanism of microorganisms (Finlay et al. 2002), one of the reasons given for universal applicability of diatom indices across geographic areas, is now a subject of much debate. For example, recent evidence suggest that certain species, originally believed to have a cosmopolitan distribution such as N. palea and G. parvulum, exhibit biogeographical or phylogeographical pattern of distribution, probably not supporting the hypothesis that only the environment selects (Trobajo et al. 2009; Boo et al. 2010; Kermarrec et al. 2013; Abarca et al. 2014). Separate taxa exist within these species complexes among geographic regions (Kermarrec et al. 2013; Abarca et al. 2014), which may not all share the same environmental optima and tolerances, hence discrepancies in the correlations between the calculated index scores and water quality variables. These taxa are currently being lumped in the calculation of most of the diatom indices. Round (2004) discovered that lumping of several similar looking taxa into one ‘morphospecies’ diminishes discriminative ability of diatom indices. Better predictive capacity of diatom-based assessment models has been shown with finer taxonomic resolutions than that with coarser ones (Rimet and Bouchez 2012). Thus, detailed taxonomic and ecological studies are required to fine tune diatom indices for water quality assessment.
However, Rimet and Bouchez (2012) showed that taxonomic resolution has little influence on diatom assemblage structure description, with little ecological information being lost when resolution is decreased from species to order level. This has also been observed with other biotic indicators such as freshwater benthic macroinvertebrates (Bowman and Bailey 1997; Metzeling et al. 2006). Though information content increases with taxonomic resolution, taxonomic identifications have been shown to become less certain at finer resolutions (Jones 2008) and data noise has also been shown to increase with increasing taxonomic resolution (Bowman and Bailey 1997). Identification to species level in this study has been difficult. The taxonomic challenges faced in this study can partially mask the diatom assemblage environmental relationships (Rimet and Bouchez 2012), hence affecting the sensitivity of indices. For this reason, ecological assessments using diatoms are less common than those using macroinvertebrates in Zimbabwe, with the majority of diatom indices being based on species or sub-species levels (Rimet 2012) and requiring highly qualified staff and resources currently lacking in the country. One way of circumventing taxonomic resolution challenges faced in this study is to resort to coarse taxonomy, which is also fair easy, less time and resource consuming and require less specialised manpower, with care being taken to ensure that not much ecological information is lost when resolution is decreased as this may compromise model inference. Indeed, several studies have shown good results with coarse diatom taxonomy in rapid bioassessment of ecological quality in freshwater systems (e.g. Growns 1999; Hill et al. 2001; Wunsam et al. 2002; Raunio and Soininen 2007). The recent demonstration of existence of a phylogenetic signal for ecological traits at lower taxonomic resolution (Keck et al. 2015) is promising for the development of simpler coarse taxonomy-based rapid bioassessment protocols.
Several common and abundant taxa (25 and 43 % in Chinhoyi and the Eastern Highlands, respectively), were not taken into account during index calculation, and this may lead to erroneous results. There were also a large number of unidentified taxa, especially in the Eastern Highlands, that require further taxonomic work. The ecological preferences of these diatoms species have yet to be determined, with some probably being endemic to these regions, rendering them useless in the calculation of index scores using OMNIDIA. Incorporation of these taxa in index calculation may give a better picture of the investigated water. In addition, some of the indices, such as SLA and WAT, have been developed three or four decades ago and have never been updated in keeping with highly dynamic diatom taxonomy.
Conclusion
Foreign indices are generally applicable to the study regions, especially in urban settings, because many widely distributed diatom species have similar environmental tolerances to those recorded for these species elsewhere, but there are several issues to be considered. There is need for taxonomic clarification (major challenge of the present study) and research on ecological requirements of some diatom species in the study region. When these undescribed taxa are abundant, as in the case of the Eastern Highlands of Zimbabwe, water quality may be misinterpreted. Meanwhile, a simpler coarse taxonomy-based rapid bioassessment protocol, which is less time and resource consuming and require less specialised manpower, can be developed for the country.
References
Abarca N, Jahn R, Zimmermann J, Enke N (2014) Does the cosmopolitan diatom Gomphonema parvulum (Kützing) Kützing have a biogeography? PLoS ONE. doi:10.1371/journal.pone.0086885
Bere T, Tundisi JG (2010) Biological monitoring of lotic ecosystems: the role of diatoms. Braz J Biol 70:493–502
Bere T, Tundisi JG (2011) Influence of ionic strength and conductivity on benthic diatom communities in a tropical river (Monjolinho) São Carlos-SP, Brazil. Hydrobiologia 671:261–276
Bere T, Phiri C, Kadye WT, Utete B (2013) Benthic diatom assemblages in mountain streams: community structure in relation to environmental and human pressures. Afr J Ecol 51:625–634
Bere T, Mangadze T, Mwedzi T (2014) The application and testing of diatom-based indices of water quality assessment in the Chinhoyi Town, Zimbabwe. Water SA 40:503–512
Bicudo CEM, Menezes M (2006) Gêneros de água de águascontinentais do Brazil: chave para identificação e descrições. Rima Editora, São Carlos-SP Brazil
Biggs BJF, Kilroy C (2000) Stream periphyton monitoring manual. NIWA, Christchurch, New Zealand
Boo SM, Kim HS, Shin W, Boo GH, Gho SM et al (2010) Complex phylogeographic patterns in the freshwater alga Synura provides new insights into ubiquity vs endemism in microbial eukaryotes. Mol Ecol 19:4328–4338
Bowman MF, Bailey RC (1997) Does taxonomic resolution affect the multivariate description of the structure of freshwater benthic macroinvertebrate communities? Can J Fish Aqua Scie 54:1802–1807
BUWAL (2002) Methodenzur Untersuchung und Beurteilung der Fließgewässer: Kieselalgen Stufe F (flächendeckend) Entwurf, Bern
CEMAGREF (1982) Etude des methods biologiquesd’appréciation quantitative de la qualité Deseaux. Rapport Q. E. Lyon, Agence de l’eauRhône-Méditerranée-Corse-Cemagref, Lyon
Coste M, Ayphassorho H (1991) É tude de la qualitédêseaux du Bassin Artois-Picardie à l’aide des communautés de diatoméesbenthiques (application des índicesdiatomiques). Rapport Cemagref. Bordeaux - Agence de l’Eau Artois-Picardie, Douai
Dell’Uomo A (1996) Assessment of water quality of an Apennine river as a pilot study. In BA Whitton, E Rott (EDs), Use of Algae for Monitoring Rivers II. Institutfür Botanik, Universität Innsbruck, pp 65–73
Descy JP (1979) A new approach to water quality estimation using diatoms. Nova Hedwigia 64:305–323
Descy JP, Coste M (1991) A test of methods for assessing water quality based on diatoms. Verhand der Internat Ver für Limnol 24:2112–2116
Finlay BL, Monoghan EB, Maberly SC (2002) Hypothesis: the rate and scale of dispersal of freshwater diatom species is a function of their global abundance. Protist 153:261–273
Gómez N, Licursi M (2001) The pampean diatom index (Idp) for assessment of rivers and streams in Argentina. Aquat Ecol 35:173–181
Growns I (1999) Is genus or species identification of periphytic diatoms required to determine the impacts of river regulation? J Appl Phycol 11:273–283
Hammer O, Harper DAT, Ryan PD (2009) PAST—PAlaeontological STatistics, version 1.90. http://folk.uio.no/ohammer/past Accessed 18 November 2013
Harding WR, Archibald CGM, Taylor JC (2015) The relevance of diatoms for water quality assessment in South Africa: a position paper. Water SA 3:41–46
Hill BH, Stevenson RJ, Pan YD, Herlihy AT, Kaufmann PR, Johnson CB (2001) Comparison of correlations between environmental characteristics and stream diatom assemblages characterized at genus and species levels. J N Am Benthol Soc 20:299–310
Jones FC (2008) Taxonomic sufficiency: the influence of taxonomic resolution on freshwater bioassessments using benthic macroinvertebrates. Environ Rev 16:45–69
Keck F, Rimet F, Franc A, Bouchez A (2015) Phylogenetic signal in diatom ecology: perspectives for aquatic ecosystems biomonitoring. Ecol Appl
Kelly MG, Whitton BA (1995) The trophic diatom index: a new index for monitoring eutrophication in rivers. J Appl Phycol 7:433–444
Kermarrec L, Bouchez A, Remit F, Humbert J-F (2013) First evidence of exsistence of semi-cryptic species and phylogeographic structures in Gomphonema parvulum (Kützing) Kützing complex. Protist 64:686–705
Krammer K, Lange-Bertalot H, Süßwasserflora von Mitteleuropa (1986–1991) Band 2. Bacillariophyceae. Teil 1–4. Gustav Fischer Verlag, Stuttgart. Germany
Kwandrans J, Eloranta P, Kawecka B, Kryzsysztof W (1998) Use of benthic diatom communities to evaluate water quality in rivers of southern Poland. J Appl Phycol 10:193–201
Lavoie S, Campeau S, Darchambeau F, Cabana F, Dillon PJ (2008) Are diatoms good integrators of temporal variability in stream water quality? Freshw Biol 53:827–841
Leclerq L, Maquet B (1987) Deux nouveaux índices chimiqueet diatomique de qualitéd’eau courante. Application au Samson et à sesaffluents (bassin de la Meuse belge). Comparaison avec d’autresíndiceschimiques, biocénotiqueset diatomiques. Institut Royal des Sciences Naturelles de Belgique, document de travail 28
Lecointe C, Coste M, Prygiel J (1993) “Omnidia”: software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia 269(270):509–513
Lenoir A, Coste M (1996) Development of a practical diatom index of overall water quality applicable to the French National Water Board network. In BAWhitton & E Rott (EDs.), Use of Algae for Monitoring Rivers II. Institut für Botanik. Universität Innsbruck, pp. 29–43
Lobo EA, Callegaro VL, Hermany G, Bes D, Wetzel CE, Oliveira MA (2004) Use of epilithic diatoms as bioindicator from lotic systems in southern Brazil, with special emphasis on eutrophication. Acta Limnol Bras 16:25–40
Mangadze T, Bere T, Mwedzi T (2015) Epilithic diatom flora in contrasting landuse settings in tropical streams, Manyame Catchment, Zimbabwe. Hydrobiologia 755:163–173
McCormick PV, Cairns JJ (1994) Algae as indicators of environmental change. J Appl Phycol 6:509–526
Metzeling L, Perriss S, Robinson D (2006) Can the detection of salinity and habitat simplification gradients using rapid bioassessment of benthic macroinvertebrtes be improved through finer taxonomic resolution or alternative indices? Hydrobiologia 572:235–252
Metzeltin D, Lange-Bertalot H (1998) Tropical diatoms of South America I. Iconographia diatomologica 5:1–695
Metzeltin D, Lange-Bertalot H (2007) Tropical diatoms of South America II. Iconographia diatomologica 18:1–877
Metzeltin D, Lange-Bertalot H, García-Rodríguez F (2005) Diatoms of Uruguay. Iconographia Diatomologica: 1–736
Pappas JL, Stoermer EFF (1996) Quantitative method for determining a representative algal sample count. J Phycol 32:693–696
Phiri C, Day J, Chimbari M, Dhlomo E (2007) Epiphytic diatoms associated with a submerged macrophyte, Vallisneria aethiopica, in the shallow marginal areas of Sanyati Basin (Lake Kariba): a preliminary assessment of their use as biomonitoring tools. Aquat Ecol 41:169–181
Pipp E (2002) A regional diatom-based trophic state indication system for running water sites in upper Austria and its overregional applicability. Verhand der Internat Ver für Limnol 27:3376–3380
Potapova MG, Charles DF (2007) Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol Indic 7:48–70
Prygiel J, Coste M (1993) The assessment of water quality in the Artois-Picardie water basin (France) by the use of diatom indices. Hydrobiologia 269(279):343–349
Prygiel J, Lévéque L, Iserentant R (1996) Unnouve lindice diatomiquepratique pour l’évaluation de La qualité des eaux en réseau de surveillance. Rev Sci Eau 1:97–113
Raunio J, Soininen J (2007) A practical and sensitive approach to large river periphyton monitoring: comparative performance of methods and taxonomic levels. Boreal Environ Res 12:55–63
Rimet F (2012) Recent views on river pollution and diatoms. Hydrobiologia 683:1–24
Rimet F, Bouchez A (2012) Biomonitoring river diatoms: implications of taxonomic resolution. Ecol Indic 15:92–99
Rott E, Hofmann G, Pall K, Pfister P, Pipp E (1997) Indikationslistenfür Aufwuchsalgen. Teil 1. Saprobielle Indikation. Bundesministeriumfür Land- und Forstwirtschaft. Wien: 1–73.
Rott E, Pipp E, Pfister P, van Dam H, Ortler H, Binder N, Pall K (1999) Indikationslisten für Aufwuchsalgen in ö sterreichischen Fliessgewässern. Teil 2: Trophieindikation (sowiegeochemische Präferenzen; taxonomische und toxikologische Anmerkungen). Wasserwirtsch aftskatasterherasgegebenvom Bundesministeriumf. Land-u. Forstwirtschaft, Wien, ISBN 3–85 174-25-4, 248
Round FE (1991) Diatoms in river water-monitoring studies. J Appl Phycol 3:129–145
Round FE (2004) pH scaling and diatom distribution. Diatom Res 20:9–12
Schiefele S, Schreiner C (1991) Use of diatoms for monitoring nutrient enrichment acidification and impact salts in Germany and Austria. In B. A., Whitton, E. Rott, & G. Friedrich. (EDs), Use of Algae for Monitoring Rivers. InstitütfürBotanik, Univ. Innsbruk
Slàdeček V (1986) Diatoms as indicators of organic pollution. Acta Hydrochim Hydrobiol 14:555–566
Taylor JC, Archibald CGM, Harding WR (2007a) An illustrated guide to some common diatom species from South Africa. Water research commission report no. TT 282/07. Water Research Commission, Pretoria, p 210
Taylor JC, Janse Van Vuuren MC, Pieterse AJH (2007b) The application and testing of diatom-based indices in the Vaal and Wilge Rivers, South Africa. Water SA 33:51–59
Taylor CJ, Prygiel AV, de La Rey PA, Van Rensburg S (2007c) Can diatom-based pollution indices be used for biological monitoring in SA? A case study of the crocodile west and Marico water management area. Hydrobiologia 592:455–464
Trobajo R, Clavero E, Chepurnov VA, Sabbe K, Mann DG, Ishihar S, Cox EJ (2009) Morphological, genetic and mating diversity within the widespread bioindicator Nitzschia palea (Bacillariophyceae). Phycologia 48:443–459
Watanabe T, Asai K, Houki A (1986) Numerical estimation of organic pollution of flowing waters by using the epilithic diatom assemblage—diatom assemblage index (DIApo). Sci Total Environ 55:209–218
Wunsam S, Cattaneo A, Bourassa N (2002) Comparing diatom species, genera and size in biomonitoring: a case study from streams in the Laurentians (Quebec, Canada). Freshw Biol 47:325–340
Acknowledgments
This study was made possible by the provision of funds from British Ecological Society (BES), International Foundation for Science (IFS) and Third World Academy of Science in conjunction with Conselho Nacional de Desenvolvimento Científico e Tecnológico (TWAS/CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Hein
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Bere, T. Challenges of diatom-based biological monitoring and assessment of streams in developing countries. Environ Sci Pollut Res 23, 5477–5486 (2016). https://doi.org/10.1007/s11356-015-5790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5790-y