Abstract
Fruit shell residue from Xanthoceras sorbifolia was investigated as a potential biosorbent to remove crude oil from aqueous solution. The shell powder and its carbonized material were compared while assessing various factors that influenced oil removal capacity. The structure and sorption mechanism were characterized using scanning electron microscopy and Fourier-transform infrared spectroscopy. The oil removal capacity of the raw material (75.1 mg g−1) was better than the carbonized material (49.5 mg g−1). The oil removal capacity increased with greater saponin content, indicating that hydrophobic and lipophilic surface characteristics of the saponins improved adsorption by the raw X. sorbifolia shell. An orthogonal experimental design was used to optimize the adsorption. Using 4 g L−1 of raw X. sorbifolia shell (particle size of < 0.15 mm), the highest crude oil removal efficiency was obtained using an initial oil concentration of 400 mg L−1, adsorption temperature of 30 °C, adsorption time of 10 min at a shaking speed of 150 rpm. The adsorption of crude oil onto X. sorbifolia shell was best described using a pseudo-second-order kinetic model. Raw X. sorbifolia shell material was more efficient than the carbonized material at crude oil removal from aqueous solution. This was attributable to the functional groups of saponins in raw X. sorbifolia shell. This study highlights that some agricultural and forest residues could be a promising source of low-cost biosorbents for oil contaminants from water—without requiring additional processing such as carbonization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A global increase in fuel demand has resulted in increased oil exploration, extraction, and refining, which in turn has led to increased oil pollution (Bandura et al. 2015). Major environmental contamination may occur due to accidental losses associated with extraction or transportation, or even intentional discharge by illegal refineries (Vollaard 2017). Crude oil is a complex mixture that often contains persistent organic pollutants that are hazards to human and environmental health (Jacquin et al. 2017; Osin et al. 2017). Although oil can be efficiently removed using physical or chemical methods, treatment is usually expensive and difficult to maintain—even with advanced technologies (Hassanshahian et al. 2014; Cheng et al. 2017). Adsorption is seen as an attractive treatment process for oily wastewater because it is environmentally benign and offers excellent removal efficiencies. However, an effective and economical biosorbent is required (Yao et al. 2017).
Unlike synthetic sorbents, natural sorbents derived from animal, plant residues, or minerals are a logical choice because of their large quantity and availability (Zadaka-Amir et al. 2013; Wu et al. 2017). Natural oil sorbents contain micro- and macroporous structures that permit oil attachment and inter- and intrafiber structures that allow entrapment (Abdullah et al. 2010). To date, many natural and modified biomass sorbents are available and include walnut shell, cotton, bagasse, biochars, etc. (Srinivasan and Viraraghavan 2008; Singh et al. 2013; Yang et al. 2017), and several were reported as sorbents for remediating oil-containing water (Urum et al. 2006; Wang et al. 2013; Zhu et al. 2017). Bagasse has even been modified by grafting to improve oil-removal efficiency by improving hydrophobic and lipophilic properties (Said et al. 2009). Shi et al. (2017) modified silica aerogels using polyacrylonitrile and the new surface properties enhanced the oil adsorption capacity. In other studies, organic (loose natural wool fibers and recycled wool-based nonwoven) and inorganic (sepiolite) materials were compared using actual oily wastewater—contaminated with motor oils rather than synthetic mixtures. They found that the natural wool-based fibers had a higher oil sorption capacity, and that oil removal efficiency was affected by contact time and oil concentration (Rajakovic et al. 2007).
Xanthoceras sorborifolia is an economically valuable hardy (drought, cold, and salt tolerant) Chinese tree. The seed kernel is used as high-quality feedstock for biodiesel production in China (Li et al. 2010). The seed oil is a rich source of saponins and is an excellent health-care product that can effectively treat hyperlipaemia, arteriosclerosis, and coronary heart disease and improve microcirculation (Zhang et al. 2010) (Fig. 1). However, large amounts of seed shell residue are generated that have no industrial or commercial use. The seed coat can remove methylene blue from aqueous solutions and is a potential sorbent for cationic dye removal from wastewaters (Yao et al. 2009). Ionic liquids have been used to enhance the pore structure of the seed-shell residues, which increased oil sorption capacity (Li et al. 2013). Activated carbon derived from Xanthoceras sorbifolia can adsorb Cd (II) and Hg (II) from wastewater, presumably due to the highly porous structure and surface functional groups (Zhang et al. 2016). Previous research in our group has indicated acid functional groups (such as carboxyl and hydroxyl groups) in the shell that have sorbent potential (Liu et al. 2014). In the present study, X. sorbifolia shell was assessed as a biosorbent for crude oil removal from aqueous solution. In addition, carbonized material was compared to the raw material to establish the effects on oil removal efficiency.
Materials and methods
Materials
X. sorbifolia was collected from Chifeng, Inner Mongolia, China. Its coat was manually peeled, washed with distilled water, dried, finely crushed (0.1 to 1 mm), and stored for analysis. To prepare a material with a high specific surface area, the carbonization process was based on methodology established by Zhang et al. (2009) and Hao et al. (2013). The material was placed in porcelain crucible in a programmed high-temperature box-type electric furnace, at a heating rate of 15 °C min−1 and a residence time of 2 h at 750 °C. The compositional characteristics of the raw and carbonized material are compared in Table 1. The apparent bulk densities (g cm−3) were determined measuring the mass of volume-packed material (Sun et al. 2017). Here, material was placed in graduated cylinder, hand-shaken for maximum compaction, and the mass determined for a specific volume. The moisture and ash contents were determined according to the method of Sewu et al. (2017). Samples (2500 mg) were put in the pre-weighed and pre-dried crucibles, then dried at 105 °C until a constant weight and the mass loss was attributed to the moisture content. The dried samples were combusted at 750 °C for 6 h until a constant weight, and the residual mass was attributed to the ash content. Elemental analysis for total carbon and nitrogen in the shells were determined using an Elemental Analyzer (Flash EA 1112, Thermo Flasher) and expressed per dry mass. The two sorbents were further characterized by pressing material into KBr pellets and analyzing by Fourier-transform infrared (FTIR) spectroscopy (Nicolet 6700 FTIR spectrometer, Thermo Fisher). Samples were fixed on aluminum stubs and sputter-coated with gold and viewed by scanning electron microscopy (SEM) using Quanta™ 250 SEM (FEI). The crude oil used in this study was obtained from Liaohe heavy-oil pipeline. The crude oil had a viscosity of 0.006 Pa⋅s and a density of 0.8337 g cm−3 at 25 ± 1 °C.
Oil sorption tests
A range-finding experiment was used to compare the oil removal efficiencies of raw and carbonized X. sorbifolia shells to commercial and traditional biosorbents (activated carbon, hazelnut shells, aspen bark, and peanut shells). Biosorbents (1.0 g) with the particle size of 0.18–0.30 mm were placed in an oil-water emulsion with an initial crude oil content of 520 mg L−1 for 0.5 h at a shaker speed of 110 rpm. Results are listed in Table 2. To determine the effect of saponins on oil removal efficiency, saponins extracted from the raw shells of X. sorbifolia were added with 4 g L−1 (< 0.18 mm) into the raw and carbonized shells in 520 mg L−1 oily water. The results supported our assumption that oil removal efficiency increased with increasing saponin content (Fig. 2).
To optimize parameter combinations for removal capacity and efficiently determine the most precise information, a five-level six-factor orthogonal experiment L25 (Table S1) was designed to evaluate the influence of several factors on the oil removal efficiency of raw and carbonized X. sorbifolia shell. The factors were initial oil concentration, oscillation rate, contact time, temperature, adsorbent concentration, and particle size. Each factor was studied at five levels, and each test was conducted in triplicate. Oil sorption capacities were determined according to a modified method of Singh et al. (2013) and Ribeiro et al. (2000). Crude oil (ranging from 150 to 600 μL) was placed in 500-mL flasks and emulsified in 250 mL of tap water. The sorbent materials had a particle size range of 0.11–0.38 mm and were assessed across a concentration range of 4.0–6.0 g L−1 in the crude oil emulsion. Flasks were agitated by vibration at 70–150 cycles min−1 for 5–120 min in a horizontal rotator. The experiments were conducted across a temperature range of 25–45 °C to account for the impact of temperature on viscosity. The results for orthogonal design conditions are shown in Table S2. All results from each design are expressed as the mean of three tests. The experimental data were analyzed using univariate analysis of variance (ANOVA) and Duncan’s multiple range tests to account for differences among averaged performance.
Analytical methods for determination of oil concentration
Oil content was determined using an extractive–gravimetric method using tetrachloromethane as the solvent. First, the sorbent was removed by filtration through a stainless-steel mesh. The steel mesh was rinsed then with tetrachloromethane to remove any trace of adsorbed oil. The solvent was added to the filtered aqueous solution in a separation funnel shaken for 2 min. The organic solvent phase was transferred to a 150-mL round-bottom flask and concentrated by drying under an inert atmosphere (nitrogen), and the mass of the remaining oil mass (g) was determined. Each wastewater sample was extracted twice (Rajakovic et al. 2007; Singh et al. 2013). The oil removal efficiency (q, %) was calculated using the following equation:
where Ci and Cf are the initial and final oil masses in grams.
Kinetics study of oil sorption capacity
According to the optimum conditions selected in the pre-test, 1-g shell material with a particle size between 0.177 and 0.250 mm was placed into a series of conical flasks containing approximately 0.13 g of crude oil in 250 mL of water and shaken at 110 rpm at 25 °C in a time-course experiment. The adsorption kinetics of biosorbents were modeled using pseudo-first order, pseudo-second order, and intra-particle diffusion models by linear methods (Hameed et al. 2008; Yao et al. 2009; Yousef et al. 2011; Wang et al. 2015; Sewu et al. 2017).
The pseudo-first order kinetic model initially proposed by Lagergren is widely used for adsorption in liquid/solid system and may be expressed as the following:
where qe (mg g−1) and qt (mg g−1) are the amount of adsorbed oil at equilibrium and at any time t (min), respectively, while the constant k1 (min−1) represents the adsorption rate in the pseudo-first order reaction. The constants (listed in Table 3) were calculated by plotting log (qe- qt) versus time, as shown in Fig. 5a.
The pseudo-second-order kinetic model used the following linear formula as proposed by Ho and Mckay (1999):
where k2 (g mg−1 min−1) is the adsorption rate in the pseudo-second-order reaction. qe and k2 (Table 3) were determined by plotting t/qt versus time, as shown in Fig. 5b.
The intra-particle diffusion model proposed by Weber and Morris (1963) was used to predict the rate-limiting step in the adsorption process by examining the relationship between qt and t 0.5 in the following formula:
kd (mg g−1 min−0.5) is the rate constant for the intra-particle diffusion kinetic model, while I (mg g−1) is a constant associated with the boundary layer thickness. In this model, the adsorption process was controlled by intra-particle diffusion if the plots of qt vs. t 0.5 could be fitted to a straight line.
Results and discussion
Oil removal capacity of Xanthoceras sorbifolia shells
Preliminary experiments (Table 2) indicated that the raw X. sorbifolia shells had greater oil removal efficiency (88.1%) than the carbonized form (77.5%) and was second only to a commercial activated carbon (92.0% removal efficiency). Activated carbon is the most popular and widely used adsorbent in wastewater treatments, because it has a relatively low cost and a high removal rate due to its high surface area (Altmann et al. 2014; Skouteris et al. 2015; El-Naas et al. 2017). However, it requires more expensive chemicals and complex processing for removing pollutants such as crude oil (Ahmad et al. 2005; Gunatilake and Bandara 2017). Therefore, raw and carbonized X. sorbifolia shells were selected for further analyses and mechanistic studies regarding the adsorption of crude oil. Given their relatively high adsorption efficiencies, low cost, and environmental benefits, the results from this study will serve as benchmark for the development of other practical low-cost biosorbents.
The lower crude oil removal efficiency of the carbonized shell is most likely due to the removal of the amphipathic compounds that adsorb oil and water, in which water inevitably interferes the contact between oil and sorbent. Moreover, the raw X. sorbifolia shells contain large amount of saponins, which decomposed when the shell was carbonized. Saponins are a type of biosurfactant and decrease the interfacial tensions of oil molecules on its surface and increase the bioavailability of hydrophobic compounds (Pacwa-Płociniczak et al. 2011). This increases the amount of oil, rather than water, drawn onto the shell and leads to a higher oil adsorption capacity (Paria and Khilar 2004; Wei et al. 2005). Emulsion and foam properties due to saponins in the raw shell may also enhance oil removal during treatment (Urum and Pekdemir 2004; Pekdemir et al. 2005). The oil removal efficiencies (Fig. 2) increased when saponins were added to the raw or carbonized material (up to 100 mg g−1). The increase was more pronounced for the carbonized material (from 4.8 to 49.5 mg g−1) than for the raw material (from 53.3 to 75.1 mg g−1), as would be expected for material no longer containing saponins. In conclusion, raw X. sorbifolia shell appears a better natural biosorbent for oil removal from water than its carbonized form, due to the chemical reaction or chemisorption of the hydrophobic oils with lipophilic compounds such as saponins.
Determination of the optimal adsorption conditions
A total of 75 trials (25 tests, each in triplicate) were conducted using an orthogonal array L25, with six factors and five levels. Based on the Taguchi method, the percentage of crude oil removed from water was selected as the dependent variable. The results are listed in Table S2. The highest Xbar-R value represents the best level for each factor, which indicates the optimum removal efficiency. The optimum adsorption conditions using 1 g of raw shells (< 0.15 mm) in a 250-mL volume were: an initial oil concentration of 400 mg L−1; shaking at 150 rpm; 10-min extraction time; at 30 °C. As this combination of factors and levels (A4B4C5D2E1F2) was not included in the 25 experiments conducted under the orthogonal array, the conditions were assessed to validate the model. A 75.6% crude oil removal was obtained, which was greater than the carbonized X. sorbifolia shell of 54.4% and confirmed data obtained in the initial assessment. The variances of the factors were analyzed via ANOVA and the results are summarized in Table S3.
Temperature is an important parameter to investigate to ensure robust oil removal efficiencies. It changes seasonally and impacts oil viscosity and the oil sorption capacity (and removal efficiencies). In general, oil sorption capacity increases with increasing temperature, until an upper threshold where the surface characteristics (e.g., particle size, porosity) of sorbent become limiting (Abdelwahab et al. 2017). Using higher sorbent concentrations or smaller particles can improve removal, but this has practical limitations as well as issues with secondary contamination (Aslam and Choudhary 2017). Similarly, adsorption capacity increases with contact time—increasing until it reaches a plateau. Upon reaching the plateau, there is a dynamic equilibrium between adsorption and desorption with greater contact times (Deschamps et al. 2003). In the present research, the six interacting factors of sorbent dosage, particle size, shaking speed, sorption time, oil concentration, and temperature significantly (P < 0.01) influenced oil removal by raw X. sorbifolia shell. Similar effects were observed with carbonized X. sorbifolia shell, except for temperature. The carbonized material was less sensitive to the effects of temperature. Worth noting is factors such as aqueous oil concentration, flow rate, and presence of different organics would significantly affect performance of both materials when used in aqueous systems (Wahi et al. 2013).
Kinetics for oil sorption
Kinetic studies were conducted using pseudo-first-order (2), pseudo-second-order (3), and intra-particle diffusion (4) kinetic models to predict the data obtained from the adsorption experiments and to investigate the efficiencies of crude oil adsorption of the shells and their equilibrium contact time (Fig. 5).
Pseudo-first and pseudo-second-order kinetic models by linear method are mostly used to check the hypothesis of adsorption kinetics at the liquid/solid interface of an adsorbent (Kumar 2006; Yousef et al. 2011; Simonin 2016). The values associated with the binding models (qe, k1, k2, kd, and R2) were calculated and are listed in Table 3. The R2 values of pseudo-first order kinetic model were 0.9450 and 0.9042, respectively, for raw and carbonized sorbent. These were lower than those of the pseudo-second-order model (R2 = 0.9996 and R2 = 0.9949). In addition, the qe values of pseudo-first-order kinetic model did not agree with the values in the linear plots (> 80 mg g−1 of adsorbed oil) at equilibrium, further indicating that the first-order reaction was not a suitable model for oil adsorption to X. sorbifolia shells (Hameed et al. 2008). The theoretical values (qe,cal) calculated from the pseudo-second-order kinetic model were close to the experimental data (qe,exp), indicating that oil adsorption followed a pseudo-second-order model. In addition, the intra-particle diffusion model was assessed to determine the limit of intra-particle diffusion in oil adsorption—as shown in Eq. (4) (Wang et al. 2015). However, there was no linear relationship between residue oil after being adsorbed by sorbent against t0.5 as shown in the Fig. 5. Therefore, the pseudo-second-order model best fitted the adsorption process of both raw shell and carbonized X. sorbifolia shells as indicated by the high correlation coefficient (R2 values). These data provide a theoretical basis for the chemical reaction mechanism of these biosorbents for crude oil adsorption from water, based on the lower adsorption rate constant (k2) of carbonized material (Yao et al. 2009).
Fourier transformed infrared spectroscopy
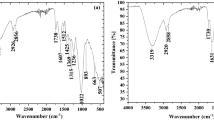
FTIR spectra of the raw and carbonized materials were obtained before and after crude oil adsorption (Fig. 3). Similar broad and strong adsorption peaks at 3410 cm−1 for raw and carbonized materials were assigned to O–H stretching vibration (Wuana et al. 2015). There was a significant decrease of band intensities at 1735 and 1515 cm−1 for the carbonized shell material, suggesting cleavage of a C=O bond and the aromatic ring in lignin. These two functional groups may play an important role in adsorption of organic pollutants due to their interaction with aliphatic groups in crude oil (Wang et al. 2012; Chand et al. 2017). The disappearance of the peak at 1054 cm−1 after carbonization (attributed to the CH or C–O–C adsorption) was likely caused by the high-temperature process altering the cellulosic structure and chemical groups (such as saponins), thus decreasing oil sorption capacity (Nidhina and Muthukumar 2015; Mutairi 2016). Bands around 1373 cm−1 (attributed to C–H stretching of C–CH3) and 1444 cm−1 (bending vibrations of –CH2–) decreased in the carbonized shell, indicating that both cellulose and lignin components were reduced during carbonization (Sun et al. 2003). The weaker carboxylate asymmetric stretching in the carbonized material (indicated at 1584 cm−1) demonstrated the importance of the carboxylic group in adsorption (Matuana et al. 2001; Gupta and Rastogi 2009). The oil-saturated samples displayed more intense peaks around 2920 and 2851 cm−1 (attributed to C–H asymmetric stretching vibrations), providing direct evidence for the adsorption of a hydrocarbon to the material. The relatively greater peak for the raw material at 1631 cm−1 (attributed to C=C stretching: Sidik et al. 2012) after oil adsorption, verified a greater oil adsorption capacity than the carbonized material. For the carbonized material, the band at 1259 cm−1 (attributed to Si–O stretching vibrations) disappeared after oil adsorption (Fig. 3c, d), which suggests that the signal from the surface Si–OH groups was quenched by organophilic CH– groups in crude oil, forming Si–O–[CH–] groups (Banerjee et al. 2006). These spectra qualitatively demonstrated why the oil sorption capacity of raw shell was higher than that of carbonized material.
Morphology analyses
To further prove the phenomenon of crude oil adsorption by raw and carbonized X. sorbifolia shell, the surfaces were viewed using SEM (Fig. 4). The SEM micrographs of the shells before adsorption are shown in Fig. 4a, c. The raw shell had clear and bumpy lamellar structures with irregular holes, whereas the carbonized shell had patchy honeycomb structures with uneven pores. Both raw and carbonized material differed with the respect to structural changes after oil adsorption. The raw shell layers and pores were less sharp because of the adsorption of oil molecules, while almost no change was observed on the surface of the carbonized shell after oil adsorption (Fig. 4d). Carbonization increased the porosity on the surface of the shell material. Although the increased surface area could theoretically improve oil adsorption (Sidik et al. 2012), little oil was observed in the micrographs. These results, in accordance with FTIR spectra results, corroborate the presence of functional groups on the outer surface of raw shell material that improve physical adsorption of crude oil in water. The intermolecular interactions between oil and the lipophilic groups contribute to oil adsorption into the fiber lumen (Deschamps et al. 2003). High-temperature treatment degraded surface functional groups associated with hydrophobicity, which rendered the microstructure less attractive to oil sorption (Wahi et al. 2013). In the present study, although the decomposition of saponins and other compounds increased the porosity of the carbonized material, the oil adsorption capacity for oil to carbonized shell was markedly decreased (Fig. 5).
Conclusions
This study found X. sorbifolia shell, without any modification, demonstrated highly efficient oil adsorption compared to other biomaterials. Better adsorption was only achieved using commercial activated carbon. The adsorption mechanism differed from activated carbon—where surface adsorption and intra-particle diffusion predominate. In the raw shell material, the saponin components significantly affected its oil adsorption capacity. This study indicates a broad potential application of this material for the removal of oil pollutants from aqueous environments and provides a theoretical basis for further development of this economical and environmentally friendly biosorbent.
References
Abdelwahab O, Nasr SM, Thabet WM (2017) Palm fibers and modified palm fibers adsorbents for different oils. Alex Eng J 56:749–755. https://doi.org/10.1016/j.aej.2016.11.020
Abdullah MA, Rahmah AU, Man Z (2010) Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. As a natural oil sorbent. J Hazard Mater 177:683–691. https://doi.org/10.1016/j.jhazmat.2009.12.085
Ahmad AL, Sumathi S, Hameed BH (2005) Residual oil and suspended solid removal using natural adsorbents chitosan, bentonite and activated carbon: a comparative study. Chem Eng J 108:179–185. https://doi.org/10.1016/j.cej.2005.01.016
Altmann J, Ruhl AS, Zietzschmann F, Jekel M (2014) Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res 55:185–193. https://doi.org/10.1016/j.watres.2014.02.025
Aslam AM, Choudhary A (2017) Removal of oil from seawater using charcoal and rice hull. IOP Conf Ser Mater Sci Eng 263:032007. https://doi.org/10.1088/1757-899X/263/3/032007
Bandura L, Franus M, Józefaciuk G, Franus W (2015) Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 147:100–107. https://doi.org/10.1016/j.fuel.2015.01.067
Banerjee SS, Joshi MV, Jayaram RV (2006) Treatment of oil spills using organo-fly ash. Desalination 195:32–39. https://doi.org/10.1016/j.desal.2005.10.038
Chand P, Bokare M, Pakade YB (2017) Methyl acrylate modified apple pomace as promising adsorbent for the removal of divalent metal ion from industrial wastewater. Environ Sci Pollut Res 24:10454–10465. https://doi.org/10.1007/s11356-017-8658-5
Cheng Y, Wang L, Faustorilla V, Megharaj M, Naidu R, Chen ZL (2017) Integrated electrochemical treatment systems for facilitating the bioremediation of oil spill contaminated soil. Chemosphere 175:294–299. https://doi.org/10.1016/j.chemosphere.2017.02.079
Deschamps G, Caruel H, Borredon ME, Bonnin C, Vignoles C (2003) Oil removal from water by selective sorption on hydrophobic cotton fibers. 1. Study of sorption properties and comparison with other cotton fiber-based sorbents. Environ Sci Technol 37:1013–1015. https://doi.org/10.1021/es020061s
El-Naas MH, Alhaija MA, Al-Zuhair S (2017) Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. Environ Sci Pollut Res 24:7511–7520. https://doi.org/10.1007/s11356-017-8469-8
Gunatilake UB, Bandara J (2017) Fabrication of highly hydrophilic filter using natural and hydrothermally treated mica nanoparticles for efficient waste oil-water separation. J Environ Manag 191:96–104. https://doi.org/10.1016/j.jenvman.2017.01.002
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402. https://doi.org/10.1016/j.jhazmat.2008.06.104
Hameed BH, Tan IAW, Ahmad AL (2008) Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J 144:235–244. https://doi.org/10.1016/j.cej.2008.01.028
Hao WM, Björkman E, Lilliestråle M, Hedin N (2013) Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl Energy 112:526–532 https://doi.org/10.1016/j.apenergy.2013.02.028
Hassanshahian M, Emtiazi G, Caruso G, Cappello S (2014) Bioremediation (bioaugmentation/biostimulation) trials of oil polluted seawater: a mesocosm simulation study. Mar Environ Res 95:28–38. https://doi.org/10.1016/j.marenvres.2013.12.010
Ho Y, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Jacquin L, Dybwad C, Rolshausen G, Hendry AP, Reader SM (2017) Evolutionary and immediate effects of crude-oil pollution: depression of exploratory behaviour across populations of Trinidadian guppies. Anim Cogn 20:97–108. https://doi.org/10.1007/s10071-016-1027-9
Kumar KV (2006) Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J Hazard Mater 137:1538–1544. https://doi.org/10.1016/j.jhazmat.2006.04.036
Li J, Zu YG, Fu YJ, Yang YC, Li SM, Li ZN, Wink M (2010) Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innovative Food Sci Emerg Technol 11:637–643. https://doi.org/10.1016/j.ifset.2010.06.004
Li J, Luo M, Zhao CJ, Li CY, Wang W, Zu YG, Fu YJ (2013) Oil removal from water with yellow horn shell residues treated by ionic liquid. Bioresour Technol 128:673–678. https://doi.org/10.1016/j.biortech.2012.11.009
Liu LN, Wang LH, Yin LM, Song WH, Yu JH, Liu Y (2014) Effects of different solvents on the surface acidic oxygen-containing functional groups on Xanthoceras sorbifolia shell. BioResources 9:2248–2258. https://doi.org/10.15376/biores.9.2.2248-2258
Matuana LM, Balatinecz JJ, Sodhi RNS, Park CB (2001) Surface characterization of esterified cellulosic fibers by XPS and FTIR spectroscopy. Wood Sci Technol 35:191–201. https://doi.org/10.1007/s002260100097
Mutairi MSA (2016) Development and evaluation of a remediation strategy for the oil lakes of Kuwait. School of civil engineering and surveying. Doctoral Thesis University of Portsmouth. United Kingdom
Nidhina N, Muthukumar SP (2015) Antinutritional factors and functionality of protein-rich fractions of industrial guar meal as affected by heat processing. Food Chem 173:920–926. https://doi.org/10.1016/j.foodchem.2014.10.071
Osin OA, Yu TY, Lin SJ (2017) Oil refinery wastewater treatment in the Niger Delta, Nigeria: current practices, challenges, and recommendations. Environ Sci Pollut Res 24:22730–22740. https://doi.org/10.1007/s11356-017-0009-z
Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12:633–654. https://doi.org/10.3390/ijms12010633
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid-water interface. Adv Colloid Interf Sci 110:75–95. https://doi.org/10.1016/j.cis.2004.03.001
Pekdemir T, Copur M, Urum K (2005) Emulsification of crude oil-water systems using biosurfactants. Process Saf Environ Prot 83:38–46. https://doi.org/10.1205/psep.03176
Rajakovic V, Aleksic G, Radetic M, Rajakovic L (2007) Efficiency of oil removal from real wastewater with different sorbent materials. J Hazard Mater 143:494–499. https://doi.org/10.1016/j.jhazmat.2006.09.060
Ribeiro TH, Smith RW, Rubio J (2000) Sorption of oils by the nonliving biomass of a Salvinia sp. Environ Sci Technol 34:5201–5205. https://doi.org/10.1021/es991139g
Said AE, Ludwick AG, Aglan HA (2009) Usefulness of raw bagasse for oil absorption: a comparison of raw and acylated bagasse and their components. Bioresour Technol 100:2219–2222. https://doi.org/10.1016/j.biortech.2008.09.060
Sewu DD, Boakye P, Jung H, Woo SH (2017) Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica. Bioresour Technol 244:1142–1149. https://doi.org/10.1016/j.biortech.2017.08.103
Shi MJ, Tang CG, Yang XD, Zhou JL, Jia F, Han YX, Li ZY (2017) Superhydrophobic silica aerogels reinforced with polyacrylonitrile fibers for adsorbing oil from water and oil mixtures. RSC Adv 7:4039–4045. https://doi.org/10.1039/C6RA26831E
Sidik SM, Jalil AA, Triwahyono S, Adam SH, Satar MAH, Hameed BH (2012) Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem Eng J 203:9–18. https://doi.org/10.1016/j.cej.2012.06.132
Simonin JP (2016) On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem Eng J 300:254–263. https://doi.org/10.1016/j.cej.2016.04.079
Singh V, Kendall RJ, Hake K, Ramkumar S (2013) Crude oil sorption by raw cotton. Ind Eng Chem Res 52:6277–6281. https://doi.org/10.1021/ie4005942
Skouteris G, Saroj D, Melidis P, Hai FI, Ouki S (2015) The effect of activated carbon addition on membrane bioreactor processes for wastewater treatment and reclamation – a critical review. Bioresour Technol 185:399–410. https://doi.org/10.1016/j.biortech.2015.03.010
Srinivasan A, Viraraghavan T (2008) Removal of oil by walnut shell media. Bioresour Technol 99:8217–8220. https://doi.org/10.1016/j.biortech.2008.03.072
Sun XF, Sun RC, Sun JX (2003) A convenient acetylation of sugarcane bagasse using NBS as a catalyst for the preparation of oil sorption-active materials. J Mater Sci 38:3915–3923. https://doi.org/10.1023/A:1026189911651
Sun XY, Shan RF, Li XH, Pan JH, Liu X, Deng RN, Song JY (2017) Characterization of 60 types of Chinese biomass waste and resultant biochars in terms of their candidacy for soil application. GCB Bioenergy 9:1423–1435. https://doi.org/10.1111/gcbb.12435
Urum K, Pekdemir T (2004) Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 57:1139–1150. https://doi.org/10.1016/j.chemosphere.2004.07.048
Urum K, Grigson S, Pekdemir T, McMenamy S (2006) A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 62:1403–1410. https://doi.org/10.1016/j.chemosphere.2005.05.016
Vollaard B (2017) Temporal displacement of environmental crime: evidence from marine oil pollution. J Environ Econ Manag 82:168–180. https://doi.org/10.1016/j.jeem.2016.11.001
Wahi R, Chuah LA, Choong TSY, Ngaini Z, Nourouzi MM (2013) Oil removal from aqueous state by natural fibrous sorbent: an overview. Sep Purif Technol 113:51–63. https://doi.org/10.1016/j.seppur.2013.04.015
Wang JT, Zheng YA, Wang AQ (2012) Effect of kapok fiber treated with various solvents on oil absorbency. Ind Crop Prod 40:178–184. https://doi.org/10.1016/j.indcrop.2012.03.002
Wang JT, Zheng YA, Wang AQ (2013) Coated kapok fiber for removal of spilled oil. Mar Pollut Bull 69:91–96. https://doi.org/10.1016/j.marpolbul.2013.01.007
Wang ZX, Barford JP, Hui CW, Mckay G (2015) Kinetic and equilibrium studies of hydrophilic and hydrophobic rice husk cellulosic fibers used as oil spill sorbents. Chem Eng J 281:961–969. https://doi.org/10.1016/j.cej.2015.07.002
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng 89:31–59
Wei QF, Mather RR, Fotheringham AF (2005) Oil removal from used sorbents using a biosurfactant. Bioresour Technol 96:331–334. https://doi.org/10.1016/j.biortech.2004.04.005
Wu W, Li J, Lan T, Müller K, Niazi NK, Chen X, Xu S, Zheng L, Chu Y, Li J, Yuan G, Wang H (2017) Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: a microscopic and spectroscopic investigation. Sci Total Environ 576:766–774. https://doi.org/10.1016/j.scitotenv.2016.10.163
Wuana RA, Nnamonu LA, Idoko JO (2015) Sorptive removal of phenol from aqueous solution by ammonium chloride-treated and carbonized moringa oleifera seed shells. Int J Sci Res 4:594–602
Yang X, Lu K, McGrouther K, Che L, Hu G, Wang Q, Liu X, Shen L, Huang H, Ye Z, Wang H (2017) Bioavailability of cd and Zn in soils treated with biochars derived from tobacco stalk and dead pigs. J Soils Sediments 17:751–762. https://doi.org/10.1007/s11368-015-1326-9
Yao ZY, Wang LH, Qi JH (2009) Biosorption of methylene blue from aqueous solution using a bioenergy forest waste: Xanthoceras sorbifolia seed coat. CLEAN - Soil Air Water 37:642–648. https://doi.org/10.1002/clen.200900093
Yao Q, Zhao PH, Li R, Li CZ, Luo Y, Zhou GZ, Yang ML (2017) Fabrication of recyclable carbonized asphalt-melamine sponges with high oil-absorption capability. J Chem Technol Biotechnol 92:1415–1420. https://doi.org/10.1002/jctb.5137
Yousef RI, El-Eswed B, Al-Muhtaseb AH (2011) Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: kinetics, mechanism, and thermodynamics studies. Chem Eng J 171:1143–1149. https://doi.org/10.1016/j.cej.2011.05.012
Zadaka-Amir D, Bleiman N, Mishael YG (2013) Sepiolite as an effective natural porous adsorbent for surface oil-spill. Microporous Mesoporous Mater 169:153–159. https://doi.org/10.1016/j.micromeso.2012.11.002
Zhang WB, Qian XB, Ma LF (2009) Adsorption properties of bamboo charcoal under different carbonized temperatures for heavy metal ions. J Nanjing Forestry Univ 33:20–24 http://www.cnki.net
Zhang S, Zu YG, Fu YJ, Luo M, Liu W, Li J, Efferth T (2010) Supercritical carbon dioxide extraction of seed oil from yellow horn (Xanthoceras sorbifolia Bunge.) and its anti-oxidant activity. Bioresour Technol 101:2537–2544. https://doi.org/10.1016/j.biortech.2009.11.082
Zhang XT, Hao YN, Wang XM, Chen ZJ, Li C (2016) Competitive adsorption of cadmium(II) and mercury(II) ions from aqueous solutions by activated carbon from Xanthoceras sorbifolia Bunge hull. J Chem 1:1–10. https://doi.org/10.1155/2016/4326351
Zhu L, Wang Y, Wang YX, You LJ, Shen XQ, Li SJ (2017) An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater 241:285–292. https://doi.org/10.1016/j.micromeso.2016.12.033
Funding
The authors are grateful for the financial support provided by the National “Twelfth Five-Year” Plan for Science & Technology Support (2012BAD32B08) of China and the Natural Science Foundation of Guangdong Province, China (2017A030311019).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Wang, L., Song, W. et al. Crude oil removal from aqueous solution using raw and carbonized Xanthoceras sorbifolia shells. Environ Sci Pollut Res 25, 29325–29334 (2018). https://doi.org/10.1007/s11356-018-2895-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2895-0