Abstract
Human-induced perturbations such as crude-oil pollution can pose serious threats to aquatic ecosystems. To understand these threats fully it is important to establish both the immediate and evolutionary effects of pollutants on behaviour and cognition. Addressing such questions requires comparative and experimental study of populations that have evolved under different levels of pollution. Here, we compared the exploratory, activity and social behaviour of four populations of Trinidadian guppies (Poecilia reticulata) raised in common garden conditions for up to three generations. Two of these populations originated from tributaries with a long history of human-induced chronic crude-oil pollution with polycyclic aromatic hydrocarbons due to oil exploitation in Trinidad, the two others originating from non-polluted control sites. Laboratory-raised guppies from the oil-polluted sites were less exploratory in an experimental maze than guppies from the non-polluted sites and in a similar manner for the two independent rivers. We then compared the plastic behavioural responses of the different populations after an acute short-term experimental exposure to crude oil and found a decrease in exploration (but not in activity or shoaling) in the oil-exposed fish compared to the control subjects over all four populations. Taken together, these results suggest that both an evolutionary history with oil and an acute exposure to oil depressed guppy exploratory behaviour. We discuss whether the behavioural divergence observed represents adaptation to human-induced pollutants, the implications for conservation and the possible knock-on effects for information discovery and population persistence in fish groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contemporary human-induced perturbations such as fragmentation, climate change and pollution can pose severe challenges for natural populations in terrestrial and aquatic ecosystems (Vitousek et al. 1997; Palumbi 2001; Schwarzenbach et al. 2006; Sih et al. 2011; Arnold et al. 2014). Particularly significant are organic pollutants entering aquatic ecosystems, which, by impacting a wide array of physiological and behavioural traits, can severely reduce fitness and the persistence of vertebrate populations such as fish (Scott and Sloman 2004; Arnold et al. 2014). Organic pollutants interfere with neurophysiological pathways, behaviour and cognition in numerous taxa, and in fish such pollutants cause impairment in foraging, sexual, antipredator and social behaviour, as well as impacting ecosystem function (Brown et al. 1985; Scott and Sloman 2004; Weis and Candelmo 2012; Zala and Penn 2004; Brodin et al. 2014). With this growing body of literature documenting the behavioural effects and impacts of contaminants, there is some recognition of the value of an evolutionary perspective, since evolutionary history and evolved responses may be important determinants of impact (Sih et al. 2011; Montiglio and Royauté 2014). However, few studies have examined the role of evolutionary history in shaping behavioural responses to anthropogenic pollution.
Behavioural responses to oil pollution
One major source of persistent organic pollutants (POPs) in aquatic ecosystems is polycyclic aromatic hydrocarbons (PAHs). Environmental releases of PAHs, which are found in oil, tar and coal, have increased in recent decades, becoming one of the most prominent toxic stressors worldwide (Samanta et al. 2002; Shen et al. 2013). Major PAH contamination of aquatic environments can occur in acute instances, like the well-known Exxon Valdez, Erika and Deepwater Horizon oil spills, as well as over long time periods from continuous seepage following drilling activities. Both have alarming effects on aquatic biodiversity (Peterson et al. 2003; Sojinu et al. 2010; Fodrie and Heck 2011; Incardona et al. 2014). PAHs have toxic effects on fish growth (Vignet et al. 2015) and cause gill and internal organ damage (Akaishi et al. 2004; Incardona et al. 2004; Oziolor et al. 2014) as well as immune depression (Reynaud and Deschaux 2006). In addition, severe behavioural alterations have been observed (Akaishi et al. 2004; Gonçalves et al. 2008; Vignet et al. 2014). PAHs are believed to disrupt normal behaviour and cognition in vertebrates through neurotoxic effects on neurotransmitter levels and synapse functioning (Gonçalves et al. 2008). As a consequence, fish exposed to PAHs generally display altered activity, social interactions (Schwarzenbach et al. 2006) and exploratory behaviour (Correia et al. 2007; Vignet et al. 2014). Such changes could affect their likelihood of discovering new information through individual and social learning and their ability to escape predators (Scott and Sloman 2004; Weis and Candelmo 2012), but the evolutionary consequences of such behavioural alterations are still poorly understood.
In the natural environment, low levels of pollution over long periods of time (i.e. chronic exposure) can create environmental gradients, generating spatial variation in selection pressures on populations (Klerks and Weis 1987; Rolshausen et al. 2015). If selection is sufficiently strong and gene flow between populations limited, local adaptation to polluted environments could theoretically occur (Kawecki and Ebert 2004). Accordingly, some chronically exposed populations of vertebrates have evolved particular physiological responses to organic pollutants (Meyer and Di Giulio 2003; Williams and Oleksiak 2008; Oziolor et al. 2014; Clark et al. 2014). Local adaptation to pollutants may also result in different abilities to cope with transient, acute exposures. For instance, chronic exposure to metal pollution leads to increased tolerance to en experimental exposure to methylmercury in killifish Fundulus heteroclitus (Weis et al. 1981). However, much work remains to be done to test whether pollution could select for particular behavioural traits or acute behavioural responses in natural populations (Breckels and Neff 2010; Montiglio and Royauté 2014). In particular, few studies have documented parallel evolution of behaviour in response to the same pollutants in replicate natural populations, i.e. whether the same pollution regime in independent evolutionary lineages results in the fixation of the same behavioural traits. In this study, we thus addressed two main questions: Does long-term exposure to oil pollution in wild fish populations select (1) for similar alterations in behavioural traits in independent evolutionary lineages, and/or (2) for particular behavioural responses to an acute experimental oil exposure?
Study system and questions
To address the above questions, we investigated behavioural divergence in response to chronic oil pollution across different populations of wild Trinidadian guppies (Poecilia reticulata) having evolved in oil-polluted versus non-oil-polluted conditions. The Trinidadian guppy provides some of the best evidence for trait divergence in response to different selective regimes such as predation (Endler 1995; Reznick et al. 1996; Magurran 2005) and parasitism (van Oosterhout et al. 2007; Dargent et al. 2013; Pérez-Jvostov et al. 2015, Jacquin et al. 2016). Oil drilling in southern Trinidad is well documented (Agard et al. 1993), causing variation in crude-oil pollution both across the entire island (absent in the north, common in the south) and within rivers (for a given river, some tributaries are heavily polluted, while others are not polluted; Rolshausen et al. 2015). Indeed, several independent tributaries have been chronically polluted with crude oil due to intense oil exploitation, yet bear dense populations of guppies, allowing a comparative study design well suited to investigate adaptive responses to selection in ecologically similar environments (Rolshausen et al. 2015). Furthermore, previous studies have documented numerous effects of acute exposure to anthropogenic pollutants on guppy behaviour (e.g. Colgan et al. 1982; Brown et al. 1985; Schröder and Peters 1988; Baatrup and Junge 2001). However, measures of survival and growth revealed limited evidence for local adaptation in guppies from crude-oil-polluted and non-polluted sites from southern Trinidad (Rolshausen et al. 2015). In this study, we investigated (1) whether there were long-lasting differences in exploratory behaviour, activity and shoaling tendency in laboratory-reared offspring of populations of wild guppies having evolved in two polluted sites versus two unpolluted sites. We also tested (2) their behaviour after exposure to control (non-polluted water) and oil-polluted (experimental exposure to crude oil) conditions to compare plastic behavioural responses to an acute experimental oil contamination.
Behaviours investigated and predictions
We focused on exploration, activity and sociability because these behaviours are known to be affected by pollution, and are tightly linked to individual performance and fitness (Scott and Sloman 2004). First, we compared exploratory behaviour, which reflects an individual’s reaction to a new situation or new environment (Réale et al. 2007; Reader 2015). Exploration allows animals to gather information about the environment and to map their habitat, which might be especially important in shallow rivers such as those in Trinidad. Exploration has been demonstrated to differ between individuals and species and is linked to learning, problem-solving performance and behavioural innovation (Archer and Birke 1983; Renner 1988; Griffin and Guez 2014; Laland and Reader 1999; Reader 2015). Thus, while exploration itself may be underpinned by non-cognitive processes, it predicts cognitive performance and may be a locus of selection for changes in processes such as problem solving. Moreover, animals are known to gather adaptive information during exploration, and exploration is shaped by both evolutionary and developmental influences (e.g. Archer and Birke 1983; Renner 1988; McCabe et al. 2015). The impact of human activities on individual and population differences in exploratory behaviour and exploratory propensities is thus highly relevant to understanding impacts on cognition. Second, we measured activity, the rate of individual locomotory movement (Réale et al. 2007). Activity rate is often impacted by organic pollutants, which can affect the foraging and mating opportunities of individuals, as well as the probability of escaping predators (Scott and Sloman 2004). Third, we compared grouping tendencies between populations. In the wild, guppies usually group with others (shoal), but shoaling tendency varies strongly between populations and ecological contexts such as high- and low-predation regimes, with enlarged group size an antipredator adaptation to predatory fish (Magurran 2005). Grouping can facilitate information discovery through social information use and social learning, but can also hamper individual exploration of the environment (Day et al. 2001; Reader et al. 2003; Chapman et al. 2008; Reader 2015).
Given that behavioural traits are central to fitness in guppies, we hypothesized that chronic oil pollution in the polluted sites of Trinidad would select for particular cognitive and behavioural traits in these wild populations. If this divergence is genetically based, we expect (1) differences in exploratory behaviour, activity and shoaling tendency among populations to persist in laboratory–raised guppies. More precisely, if our assumption that pollution is the major selective force driving divergence in behaviour, we expect to see similar differences in behavioural traits among laboratory-reared guppies originating from the two oil-polluted sites compared to guppies originating from the two non-oil-polluted sites. In addition, if oil pollution in the wild selects for plastic abilities to buffer the behavioural effects of an acute oil exposure, we expect (2) guppies originating from oil-polluted sites to display a lower response to an experimental acute exposure to crude oil compared to guppies originating from non-oil-polluted sites.
Methods
Overview
To investigate behavioural differences among populations, we measured exploration, activity and shoaling tendency. We measured these three behaviour patterns twice, with a first set of tests to determine any population differences under standard ‘baseline’ conditions and a second set of tests to compare responses to an acute exposure to oil. In this second set of tests fish were either exposed to oil (oil-exposed group) or to conditioned water (control group). The results of the first ‘baseline’ tests would thus reflect any evolutionary divergence in behaviour due to contrasting environmental conditions in the wild. Any behavioural differences between oil-exposed and control groups in the second set of tests would reflect divergence in the acute immediate response to the experimental exposure to oil (i.e. short-term plastic responses; e.g. Breckels and Neff 2010).
Study populations and housing conditions
In 2011 and 2012, 30 sexually mature individuals were collected from each of four populations of wild Trinidadian guppies P. reticulata, with at least 20 females collected per population. Study populations and sites are described in Rolshausen et al. (2015). Two populations originated from oil-polluted areas, originating from oil-polluted parts of the Morne River (OIL-MR) and the Vance River (OIL-VR). Two populations originated from areas with no crude-oil pollution, originating from unpolluted parts of the Morne River (NOIL-MR) and from the unpolluted Paria River (NOIL-P).
The Morne (MR) and Vance Rivers (VR) were chosen because crude oil is commercially exploited nearby, and hence soil leakage and spillage lead to high levels of chronic crude-oil pollution (Fig. 1, Agard et al. 1993; Rolshausen et al. 2015). Organic contaminants of the polluted sites are high molecular weight, non-volatile petroleum hydrocarbons in the range of C10–C34 such as naphthalene, pyrene and carbazole (see Rolshausen et al. 2015 for details). In the Morne River, only some tributaries are polluted. In the Vance River, there are non-polluted tributaries too, but we were not able to successfully breed F1s from these tributaries in the laboratory. Moreover, these two rivers flow in different directions, which lead to independent evolutionary histories between the rivers, confirmed by neutral genetic markers (Rolshausen et al. 2015). In addition to populations from the Morne and Vance Rivers, we tested one additional control non-polluted population from the Paria River, located in the northern part of Trinidad. The motivation for including this river was to add a northern site that was genetically independent from the other rivers, one which had never been exposed to crude-oil pollution, therefore constituting an additional control site with independent evolutionary history (Rolshausen et al. 2015). All four sampling sites lack predatory cichlids and are considered low-predation environments (Magurran 2005; Rolshausen et al. 2015). The Morne and Vance Rivers contain an impoverished fish assemblage, with few other fish species, namely Rivulus hartii and characins Astyanax sp. (Rolshausen et al. 2015), while the Paria River contains Rivulus hartii and the predatory prawn Macrobrachium crenulatum (Magurran 2005).
Photographs of oil pollution due to crude-oil seepage at polluted field sites OIL-VR (1, 3) and OIL-MR (2, 4, 5) (pictures from Rolhausen et al. 2015)
Fish were transported to McGill University and were raised in the laboratory for one to three generations (F1 to F3 generation) in common garden standardized conditions. Fish were housed in 2.5-gal tanks (2 replicate tanks per population and per generation) at 23 °C with 10:14-h light/dark period and fed with brine shrimp twice daily, for at least 2 months before experiments began. Due to unexpected mortality in captivity (around 50 %), we obtained around 10 founder females and 10 founder males per population. Fish were then free to breed within each population but fry were moved to new tanks shortly after birth. In total, we obtained 77 F1–F3 laboratory-reared individuals. We tested 38 male and 39 female guppies with the tests described below. 23 individuals originated from the oil-polluted region of the Morne River (OIL-MR: 11 females, 10 males; 8 F1, 13 F2), 23 from the oil-polluted region of the Vance River (OIL-VR: 12 females, 11 males; 7 F1, 12 F2, 4 F3), 21 from the non-oil-polluted region of the Morne River (NOIL-MR: 11 females, 10 males; 8 F1, 13 F2) and 10 from the non-oil-polluted river of Paria (NOIL-P: 5 females, 5 males; 8 F1, 2 F2). No significant differences in sex ratio (χ 2 = 0.0011, P = 0.97), generation composition (χ 2 = 4.61, P = 0.10), body mass (χ 2 = 5.07, P = 0.17), body length (χ 2 = 5.35, P = 0.15) or body condition (Fulton index: χ 2 = 0.11, P = 0.73) were found between populations.
Experimental exposure to crude oil
To mimic a transient crude-oil spillage in Trinidadian rivers, we exposed half of the fish, selected at random, with a 50 % water-soluble fraction (50 % WSF) of crude oil for 60 min. To prepare the 50 % WSF of oil, one part of commercial crude oil (diesel gasoil, Shell, Calgary, Canada) was diluted in 4 parts of conditioned water and stirred for 20 h at room temperature under a fume hood. The upper insoluble phase was then eliminated, and the watery phase was diluted 50 % in conditioned water to obtain a 50 % WSF (Alkindi et al. 1996; Vanzella et al. 2007; Simonato et al. 2008). This crude-oil WSF preparation has been shown to result in a highly reproducible mix of aromatic hydrocarbons (Anderson et al. 1974; Alkindi et al. 1996). Diesel gasoil is directly extracted from natural oil and contains a variety of toxic monoaromatic hydrocarbons (benzene, toluene and xylene) and small polyaromatic hydrocarbons (PAHs) in the range of C9–C25, which were also found in the oil-polluted sites of Trinidad that we sampled (Rolshausen et al. 2015). For instance, WSF preparations can contain naphthalene, a toxic neuro-disruptor known to affect fish behaviour (e.g. Gesto et al. 2006) and that was also documented at OIL-MR and OIL-VR (Rolshausen et al. 2015). Oil pollution intensity varies across polluted Trinidadian sites and is also likely to vary across time, for example as a result of variable water temperatures and flow (Rolshausen et al. 2015). We thus based the oil dilution on previous studies that have demonstrated detrimental impacts on fish (Alkindi et al. 1996; Vanzella et al. 2007; Simonato et al. 2008), although our period of exposure was shorter to trigger behavioural changes while avoiding deleterious physiological effects such as organ damages. The experimental oil mixture chosen in this study thus contains a number of similar toxic molecules as Trinidadian oil spills and is likely to trigger correspondingly similar behavioural responses.
Oil-exposed fish were exposed in a 5-gal exposure tank for 60 min, following protocols validated by previous studies (e.g. Alkindi et al. 1996; Simonato et al. 2008). The remaining fish were submitted to the same procedure, but conditioned water was added instead of oil. A total of 49 fish were tested (oil-exposed group, N = 22: 8 NOIL-MR, 3 NOIL-P, 5 OIL-MR, 6 OIL-VR; control group, N = 27: 10 NOIL-MR, 3 NOIL-P, 5 OIL-MR, 9 OIL-VR). Immediately after this exposure, fish were transferred to the test tanks and their behaviours were measured. All behaviours were thus assessed twice: before exposure and after exposure to either oil or control water.
We exposed the fish for a short period of time (1 h) for two reasons. First, since drilling intensity and water flow vary across time in Trinidadian rivers, fish in natural populations might be exposed to repeated but short flushes of oil (Agard et al. 1993). Second, we wanted to trigger transient behavioural changes while avoiding detrimental or lethal physiological effects that can occur after 3 h (Alkindi et al. 1996), because we were interested in the immediate response to pollutants rather than potentially longer-lasting and debilitating effects. Whether fish were exposed to oil or not for 1 h had no effect on changes in body mass (effect of exposure group on mass change immediately after the exposure and the test: χ 2 = 0.13, P = 0.71).
Exploration test
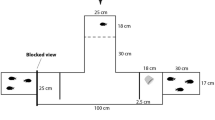
Exploration was assessed in a maze task (Laland and Reader 1999) in a 5-gallon (19l) tank with a dark green background and homogeneous light conditions to minimize stress. The tank contained 1 cm of white sand and 10 cm of conditioned water, with three 10 cm × 12 cm green partitions placed 10 cm apart to form three maze compartments (Fig. 2a). The maze set-up followed apparatus used by Mikheev and Andreev (1993), Girvan and Braithwaite (1998) and Nomakuchi et al. (2009) to measure exploration. Pilot experiments with domestic guppies had found they entered the maze but that only some of them reached the final compartment (Sims P. personal communication), suggesting that our apparatus was suitable for measuring differences in exploratory behaviour.
a Top view of the experimental Z maze for the exploration test. Exploratory tendency was measured as the maximum number of different zones explored by the focal fish. b Top view of the shoaling experimental tank set-up. Shoaling tendency was measured as the proportion of time spent within 4 body lengths of the shoaling compartment (proportion of time spent within the shoaling zone). Activity was measured as the total time spent swimming
Two female companion fish (mean body length 2.25 cm) in a transparent 5-cm-diameter plastic cup were placed in the starting zone of the maze in order to minimize stress for the focal fish (following Mikheev and Andreev 1993). We wanted to ensure that the exploratory behaviour of the focal fish would be tested in a new environment but not in a highly stressful environment, which could largely reflect risk taking rather than exploratory behaviour (Réale et al. 2007). Companion fish were kept in a separate container to ensure that no additional olfactory cues were present. Companion fish used during the experiments were randomly selected and replaced after testing every 20 focal individuals. Water in the experimental tank was changed regularly to remove potential olfactory cues.
The focal fish was selected at random and placed in the starting zone of the experimental tank (Fig. 1) and allowed to acclimate for 2 min within a transparent plastic cup (Seghers and Magurran 1995). After 2 min, the plastic cup was gently lifted from the tank (using a string on a reel to avoid disturbance from the observer) and the fish was allowed to explore the maze for 10 min. After 10 min, we recaptured the fish with a net, placed it back in the plastic cup and transferred it into another experimental tank for the other behavioural tests. All populations took a similar amount of time to enter the maze (χ 2 = 3.63, P = 0.30). We measured the number of zones explored as an indicator of exploratory tendency (Mikheev and Andreev 1993; Girvan and Braithwaite 1998; Nomakuchi et al. 2009).
Activity and shoaling test
Shoaling tendency was measured as time spent within four body lengths of a stimulus shoal in a two-choice experiment according to standard procedures commonly used for small fish (Wright and Krause 2006) (Fig. 2b). Such tests have been demonstrated to reliably reflect grouping behaviour in fish (e.g. Chapman et al. 2008). Each focal fish was placed in a transparent cylinder in the middle of a 5-gallon tank (40 cm × 20.5 cm × 10 cm water depth, 1 cm white sand) containing two shoaling compartments (5 cm length) on each side of the tank. One compartment contained a stimulus shoal of 4 size-matched and randomly selected wild-type females (mean body length = 2.71 cm) of mixed origin, while the other compartment was left empty. Compartments were enclosed by clear Plexiglas partitions with 1.0-cm mesh sides to allow odour cues to pass through. Eighty per cent of the water was changed every day, and the remaining water was filtered with carbon, to remove most chemical cues in the tanks. The stimulus shoal was changed every 20 focal fish to reduce any shoal preference biases, and the side of the shoaling compartment was changed every 5 fish to avoid any confounding effect of a spatial preference, as recommended by Wright and Krause (2006). After 2 min of acclimation for the focal fish the cylinder was gently removed, using a string on a reel to minimize disturbance. Activity was recorded as the time spent swimming and shoaling tendency as the proportion of time spent within four body lengths of the stimulus shoal, over 5 min, using JWatcher software (Blumstein et al. 2006). At the end of the shoaling test, a round weight (ca. 42 g) was dropped into the tank, 1–2 cm away from the focal fish to mimic a predator attack (e.g. Tytell and Lauder 2008). We hoped to measure variation in freezing, a measure of fear response (Budaev 1997), but all fish responded strongly and similarly to the stimulus, and thus, this measure was not analysed further.
Each fish was anesthetised with 0.02 % tricaine methanesulfonate (MS-222, solution buffered to a neutral pH), and body mass was measured to the nearest mg before and after the exposure to oil. Body standard length was measured to the nearest mm.
Statistical analyses
Exploration (number of zones explored), activity (time spent swimming) and shoaling (time spent near conspecifics) were not significantly correlated (Spearman’s correlation test, all P > 0.10) and hence were analysed separately. We first compared the behaviour of guppies from different populations in the first set of tests, before the experimental exposure, using generalized linear models (GLM) with population (NOIL-MR, NOIL-P, OIL-MR or OIL-VR) as an independent variable. We then compared the acute response to oil exposure among populations, using GLM, with the independent variables population and exposure group (control or oil-exposed) and the population-by-exposure interaction. Sex and body size were included as covariates in all models. All generations (F1, F2 and F3) were analysed together. Differences among F1 individuals could stem from maternal effects, which would be expected to be diminished or abolished in later generations (Mousseau and Fox 1998). Although our sample sizes per generation are low, making it difficult to compare generations, our results are qualitatively similar to or without the F1 generation. We thus present only models including all generations in the results. We began with a full model containing all variables and used backward selection, successively eliminating non-significant terms (P > 0.10), starting with interactions. Post hoc tests between each population and treatment group were performed using Wilcoxon tests with false recovery rate adjustment to account for multiple testing (Pike 2011). All statistical analyses were carried out using R version 2.15.3 (R Development Core Team 2008).
Ethical statement
All procedures were carried out in accordance with the Canadian Council on Animal Care and ASAB guidelines and were approved by the Animal Care Committee of McGill University (Protocol #7133). Field sampling was approved by the Ministry of Agriculture, Land and Marine Resources of the Republic of Trinidad and Tobago, and fish import was approved by the Canadian government. At the conclusion of the study fish were placed into breeding populations at McGill University.
Results
Exploration
In the first ‘baseline’ exploration test before exposure, populations differed significantly in the number of zones explored, and large fish tended to explore more zones than small fish (Table 1). Sex was not retained in the final model (Table 1). Fish from both the oil-polluted sites of OIL-VR and OIL-MR explored significantly less zones than fish from the non-oil-polluted sites of NOIL-P and NOIL-MR (Fig. 3; Wilcoxon post hoc tests with FDR adjustment to account for multiple comparisons, OIL-VR vs. NOIL-MR: W = 337, P = 0.037; vs. NOIL-P: W = 166.5, P = 0.056; OIL-MR vs. NOIL-MR: W = 360, P = 0.021; vs. NOIL-P: W = 173, P = 0.019).
Mean ± SE number of zones explored in a Z maze of laboratory-reared guppies originating from different Trinidadian populations before (dark grey) and after an experimental exposure to control water (mid-grey) or to a 50 % water-soluble fraction of crude oil (light grey). NOIL-MR non-oil-polluted population of Morne River, NOIL-P non-oil-polluted population of Paria River, OIL-MR = oil-polluted population of Morne River, OIL-VR = oil-polluted population of Vance River. Significant differences between populations were found before exposure (different a and b letters above bars), as well as after exposure, whatever their exposure group (different x and y letters above bars). Numbers within bars represent sample sizes. The effect of the experimental exposure to oil was not significantly different across populations, as the population-by-exposure interaction was not retained in the final model (see Table 1). Hence, no post hoc comparison of exposure effect across populations was performed and only post hoc comparisons of population differences are presented
In the second exploration test, after the experimental exposure to oil or water, fish that had been experimentally exposed to oil explored less than control fish, over all four populations of origin (Table 1). Population was also retained in the best model (Table 1), and fish from the OIL-MR population explored less than fish from the NOIL-P population whatever their exposure group (Wilcoxon post hoc test: W = 7, P = 0.028; Fig. 3). The effect of the experimental exposure to oil was not significantly different across populations, since the population-by-exposure interaction was not retained in the final model (Table 1). Hence, no post hoc comparison of the effect of exposure across populations was performed and only post hoc comparisons of population differences are presented in Fig. 3.
Activity
In both the first and second activity tests, larger fish swam more than smaller individuals (Table 1). Population, experimental exposure to oil and sex had no significant effect on the time spent swimming and were not retained in the final models (Table 1).
Shoaling
In the first shoaling test, populations were found to differ in shoaling tendency (proportion of time spent near conspecifics; Table 1), with fish from NOIL-MR shoaling more than fish from NOIL-P (Fig. 4; Wilcoxon post hoc test: W = 171, P = 0.025). This population difference was not detected in the second shoaling test, after the experimental exposure to oil or water, since population was not retained in the final model (Table 1; Fig. 4). The experimental exposure to oil had no effect on shoaling propensity: exposure group, sex, body length and interaction effects were also not retained in the final model (Table 1).
Mean ± SE shoaling tendency (proportion of time spent shoaling) of laboratory-reared guppies originating from each population before (dark grey) and after an experimental exposure to control water (mid-grey) or to a 50 % water-soluble fraction of crude oil (light grey). NOIL-MR non-oil-polluted population of Morne River, NOIL-P non-oil-polluted population of Paria River, OIL-MR oil-polluted population of Morne River, OIL-VR oil-polluted population of Vance River. Significant differences between populations were found before exposure (different a and b letters above bars), but not after exposure, whatever their exposure group (x letters above bars). Number within bars represent sample sizes. The effect of the experimental exposure to oil was not significantly different across populations, as the population-by-exposure interaction was not retained in the final model (see Table 1). Hence, no post hoc comparison of exposure effect across populations was made and only post hoc comparisons of population differences are presented
Discussion
We found that (1) laboratory-raised fish originating from polluted sites explored less than fish from non-polluted sites and that (2) acute oil exposure decreased exploratory behaviour regardless of population origin. More specifically, laboratory-reared guppies from the two polluted populations (OIL-MR and OIL-VR) explored less of a novel environment compared to guppies from non-polluted sites (NOIL-MR and NOIL-P). Since all fish were reared in common garden conditions in the laboratory, i.e. experienced the same environmental conditions during their lifetime, behavioural differences in exploration likely reflect persistent genetic differences among populations, although we cannot completely rule out non-genetic sources of inheritance. For example, pollution could cause epigenetic changes which would be passed on over generations (although the standardized rearing environment might abolish such trans-generational epigenetic effects). Another possibility is long-lasting behavioural ‘traditions’, say if offspring learned exploratory strategies from adults (although our breeding design, which separated fry from adults, would reduce the time available for learning). Further work is now needed to explore the mechanisms underpinning the observed long-lasting behavioural differences among populations.
Both polluted sites are located in independent rivers and represent two independent evolutionary lineages of guppies (Rolshausen et al. 2015). Despite this, both oil-polluted lineages showed a similar decrease in exploration that persisted in laboratory-raised generations, suggesting chronic oil pollution as a potential cause of this population divergence. Our findings suggest that oil pollution has resulted in a lower exploratory tendency in the wild, which may be the result of selection on exploratory behaviour or on traits that impact exploration. Moreover, we found that oil exposure had similar acute and evolutionary effects in depressing exploratory tendency.
Several alternative explanations could account for our results. First, decreased exploratory tendencies might be driven by poor condition and health of fish born to parents originating from polluted habitats, which may impair their energetic reserves and/or ability to swim, and therefore their exploration. However, we found no evidence for a lower body mass, body condition or activity of fish from polluted sites. Second, chance founder effects could potentially explain our results, particularly because breeding was uncontrolled so we cannot be certain how many fish from the founder populations bred. However, similar, parallel findings across pollution regimes reduce the likelihood that the results are due to founder effects, while the finding that our results are similar to or without the F1 generation suggests that inbreeding or domestication during captive breeding does not account for our findings. Third, it might be that the differences in exploration are driven by differences in sociability or risk taking between the populations, given that those behaviours are often linked to exploration and may be difficult to separate (Réale et al. 2007; Reader 2015). However, shoaling levels among populations were not reliably associated with a particular pollution regime. Similarly, the populations did not differ in their latency to leave the companion fish and enter the maze (arguably largely reflecting risk taking) but did differ in the number of zones explored of the maze once they entered. This suggests that differences in exploratory behaviour found between the populations from different regimes (oil or non-oil) were not due to differences in condition, tendency to remain with conspecifics or initial avoidance of the maze, but rather resulted from differences in the propensity to explore new environments.
A potential explanation for the observed population differences in exploratory tendencies is that pollution favours a lower exploration tendency, perhaps because the costs of exploration are increased or the benefits of exploration are decreased in polluted environments. ‘Intrinsic’ exploration refers to information-gathering activity that is not motivated by an immediate goal (Archer and Birke 1983; Reader 2015), and the knowledge gained can be utilized later to find rich food patches or to avoid risky areas. For intrinsic exploration to be advantageous, the information gathered at one time point should have value later on. Gathering information would not be beneficial in either highly uniform or highly unstable environments (Dunlap and Stephens 2009), both situations which could characterize polluted habitats. In addition, sensory, olfactory and chemical cues emitted by food or conspecifics are likely disturbed by organic toxic molecules (Lürling and Scheffer 2007). If the fish do not acquire any additional information about the surroundings (food resources, conspecifics or danger) when exploring polluted areas because of such altered chemical cues, we can hypothesize that this information-poor environment would select for a lower exploratory behaviour. Moreover, the chance of encountering toxins may itself increase the costs of exploration. Additional studies manipulating the amount of information conveyed by polluted environments are needed to test this hypothesis.
Another potential factor determining the pay-offs of exploration is the ability of individuals to gather and remember information during habitat exploration, i.e. their learning abilities. For example, even if exploration provides useful information, individuals with poor spatial memory may benefit little since they might not be able to remember the spatial cues encountered during exploration. Organic pollutants are known to strongly impair learning abilities and to cause deficits in spatial memory and learning (e.g. Schantz et al. 1995). We could thus hypothesize that the pathological effects of chronic pollution could cause deficits in learning and consequently select for a lower tendency for guppies to explore their habitat. Under this hypothesis, individuals are unable to benefit significantly from their exploration. Further studies comparing the learning performance of guppies originating from different habitats will help test this hypothesis.
Interestingly, the experimental exposure to a standard water-soluble fraction of oil in the laboratory also plastically reduced the exploration tendency. Again, such differences in exploration could not be explained by a lower health condition of oil-exposed individuals, since no effects of exposure on body mass, body condition or activity were found. This result is consistent with a previous study in zebrafish Danio rerio showing that an experimental exposure to PAHs has an immediate negative effect on exploration tendency, for instance through the disruption of neurological pathways (Vignet et al. 2014). Contrary to our predictions, the observed acute decrease in exploration in oil-exposed individuals was not significantly different across populations, suggesting similar plastic responses across populations, although differences may have been revealed with a larger sample size. We expected a smaller response in fish originating from polluted sites, on the basis that they would be less sensitive to pathological effects of oil. Here, our result is consistent with the limited evidence for local adaptation to oil in these populations (Rolshausen et al. 2015). Further studies with more individuals and different oil doses would help us to test the differences in the plastic response to an acute experimental oil exposure across populations.
Shoaling tendency also varied among populations, but differences in shoaling tendency among populations were not reliably associated with pollution regime. Fish from the non-polluted site of Morne River (NOIL-MR) shoaled more than fish from the non-polluted Paria (NOIL-P) and intermediate levels of shoaling were observed in OIL-MR and OIL-VR populations. The low shoaling in Paria-origin fish has been previously described and is thought to be a response to predatory prawns found in the Paria River (Magurran 2005). In the absence of consistent differences in shoaling in replicate oil-polluted and non-polluted populations no firm conclusions can be drawn. Moreover, no acute effects of oil exposure on shoaling were detected. Oil pollution may be a weak evolutionary force on grouping behaviours compared to other factors in our study system, such as predation (e.g. Seghers 1974; Jacquin et al. 2016). All sites were considered low predation in this study, but subtle, unmeasured differences in predation regime may explain the pattern of variation in shoaling we observed. We also note that our sample sizes were low in our study, due to difficulties in breeding these populations in captivity, and thus limited our power to detect differences.
Whether the reduced exploration we observe is the results of benefits, costs or constraints of exploration or associated traits in polluted habitats is an open question. Understanding the context in which exploration occurs (e.g. foraging or finding mates) is an important next step in investigating the reduced exploratory behaviour of the crude-oil-polluted populations. Further experiments manipulating the context (e.g. foraging or mating) of exploration as well as the learning performance of fish from polluted versus non-polluted sites would help in the understanding of how crude-oil pollution influences exploration in wild guppies. Moreover, it would be highly relevant to compare predator and prey communities between oil-polluted and non-polluted sites, given that both factors are likely to affect both the risks and costs of exploration.
Finally, it is important to understand how the differences in exploration can be translated to the population level. The current study suggests that behavioural adaptations might occur in response to human-driven disturbances, which might in turn be expected to affect ecological processes, such as foraging, predator–prey and host–parasite interactions. Indeed, lower exploratory behaviour may lead to decreased information discovery and use. This could decrease fish foraging efficiency and resource-use diversity in oil-polluted rivers, which might weaken the viability of the populations, especially in situations that might further expose the fish to environmental risks. This calls for further work investigating the consequences of pollution-driven behavioural alterations for biotic interactions and ecosystem functioning.
References
Agard JBR, Gobin J, Warwick RM (1993) Analysis of marine macrobenthic community structure in relation to pollution, natural oil seepage and seasonal disturbance in a tropical environment (Trinidad, West Indies). Mar Ecol Prog Ser 92:233–243
Akaishi FM, de Assis HCS, Jakobi SCG et al (2004) Morphological and neurotoxicological findings in tropical freshwater fish (Astyanax sp.) after waterborne and acute exposure to water soluble fraction (WSF) of crude oil. Arch Environ Contam Toxicol 46:244–253
Alkindi AYA, Brown JA, Waring CP, Collins JE (1996) Endocrine, osmoregulatory, respiratory and haematological parameters in flounder exposed to the water soluble fraction of crude oil. J Fish Biol 49:1291–1305. doi:10.1111/j.1095-8649.1996.tb01796.x
Anderson JW, Neff JM, Cox BA et al (1974) Characteristics of dispersions and water-soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish. Mar Biol 27:75–88. doi:10.1007/BF00394763
Archer J, Birke LIA (eds) (1983) Exploration in animals and humans. Van Nostrand Reinhold, Wokingham
Arnold K, Brown R, Ankley G, Sumpter J (2014) Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Phil Trans R Soc B 369:20130569
Baatrup E, Junge M (2001) Antiandrogenic pesticides disrupt sexual characteristics in the adult male guppy Poecilia reticulata. Environ Health Perspect 109:1063–1070
Blumstein DT, Daniel JC, Evans CS (2006) JWatcher
Breckels RD, Neff BD (2010) Pollution-induced behavioural effects in the brown bullhead (Ameiurus nebulosus). Ecotoxicology 19:1337–1346
Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M (2014) Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Phil Trans R Soc B 369:20130580
Brown JA, Johansen PH, Colgan PW, Mathers RA (1985) Changes in the predator-avoidance behaviour of juvenile guppies (Poecilia reticulata) exposed to pentachlorophenol. Can J Zool 63:2001–2005
Budaev SV (1997) ‘‘Personality’’ in the guppy (Poecilia reticulata): a correlational study of exploratory behaviour and social tendency. J Comp Psychol 111:399–411
Chapman BB, Ward AJW, Krause J (2008) Schooling and learning: early social environment predicts social learning ability in the guppy, Poecilia reticulata. Anim Behav 76:923–929. doi:10.1016/j.anbehav.2008.03.022
Clark BW, Bone AJ, Di Giulio RT (2014) Resistance to teratogenesis by F1 and F2 embryos of PAH-adapted Fundulus heteroclitus is strongly inherited despite reduced recalcitrance of the AHR pathway. Environ Sci Pollut Res 21:13898–13908. doi:10.1007/s11356-013-2446-7
Colgan PW, Cross JA, Johansen PH (1982) Guppy behaviour during exposure to a sub-lethal concentration of phenol. Bull Environ Contam Toxicol 28:20–27. doi:10.1007/BF01608407
Correia AD, Gonçalves R, Scholze M et al (2007) Biochemical and behavioural responses in gilthead seabream (Sparus aurata) to phenanthrene. J Exp Mar Biol Ecol 347:109–122. doi:10.1016/j.jembe.2007.03.015
Dargent F, Scott ME, Hendry AP, Fussmann GF (2013) Experimental elimination of parasites in nature leads to the evolution of increased resistance in hosts. Proc R Soc B Biol Sci 280:20132371. doi:10.1098/rspb.2013.2371
Day RL, MacDonald T, Brown C, Laland KN, Reader SM (2001) Interactions between shoal size and conformity in guppy social foraging. Anim Behav 62:917–925
Dunlap AS, Stephens DW (2009) Components of change in the evolution of learning and unlearned preference. Proc R Soc B 276:3201–3208
Endler JA (1995) Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol Evol 10:22–29. doi:10.1016/S0169-5347(00)88956-9
Fodrie FJ, Heck KL Jr (2011) Response of coastal fishes to the Gulf of Mexico oil disaster. PLoS One 6:e21609. doi:10.1371/journal.pone.0021609
Gesto M, Tintos A, Soengas JL, Míguez JM (2006) Effects of acute and prolonged naphthalene exposure on brain monoaminergic neurotransmitters in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol Part C Toxicol Pharmacol 144:173–183. doi:10.1016/j.cbpc.2006.08.002
Girvan JR, Braithwaite VA (1998) Population differences in spatial learning in three–spined sticklebacks. Proc R Soc Lond B Biol Sci 265:913–918
Gonçalves R, Scholze M, Ferreira AM et al (2008) The joint effect of polycyclic aromatic hydrocarbons on fish behaviour. Environ Res 108:205–213. doi:10.1016/j.envres.2008.07.008
Griffin AS, Guez D (2014) Innovation and problem solving: a review of common mechanisms. Behav Process 109:121–134
Incardona JP, Collier TK, Scholz NL (2004) Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196:191–205. doi:10.1016/j.taap.2003.11.026
Incardona JP, Gardner LD, Linbo TL et al (2014) Deepwater horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc Natl Acad Sci USA 111:E1510–E1518. doi:10.1073/pnas.1320950111
Jacquin L, Reader SM, Boniface A, Matelunna J, Patalas I, Perez-Jvostov F, Hendry AP (2016) Parallel and nonparallel behavioural evolution in response to predation and parasitism in Trinidadian guppies. J Evol Biol 29:1406–1422. doi:10.1111/jeb.12880
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241. doi:10.1111/j.1461-0248.2004.00684.x
Klerks PL, Weis JS (1987) Genetic adaptation to heavy metals in aquatic organisms: a review. Environ Pollut Bark Essex 45:173–205
Laland KN, Reader SM (1999) Foraging innovation in the guppy. Anim Behav 57:331–340
Lürling M, Scheffer M (2007) Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol Evol 22:374–379. doi:10.1016/j.tree.2007.04.002
Magurran AE (2005) Evolutionary ecology: the Trinidadian guppy. Oxford University Press, Oxford
McCabe CM, Reader SM, Nunn CL (2015) Infectious disease, behavioural flexibility and the evolution of culture in primates. Proc R Soc B Biol Sci 282:20140862
Meyer JN, Di Giulio RT (2003) Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol Appl 13:490–503
Mikheev VN, Andreev OA (1993) Two-phase exploration of a novel environment in the guppy, Poecilia reticulata. J Fish Biol 42:375–383. doi:10.1111/j.1095-8649.1993.tb00340.x
Montiglio P-O, Royauté R (2014) Contaminants as a neglected source of behavioural variation. Anim Behav 88:29–35. doi:10.1016/j.anbehav.2013.11.018
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, USA
Nomakuchi S, Park PJ, Bell MA (2009) Correlation between exploration activity and use of social information in three-spined sticklebacks. Behav Ecol 20:340–345. doi:10.1093/beheco/arp001
Oziolor EM, Bigorgne E, Aguilar L et al (2014) Evolved resistance to PCB- and PAH-induced cardiac teratogenesis, and reduced CYP1A activity in Gulf killifish (Fundulus grandis) populations from the Houston Ship Channel, Texas. Aquat Toxicol 150:210–219. doi:10.1016/j.aquatox.2014.03.012
Palumbi SR (2001) Humans as the world’s greatest evolutionary force. Science 293:1786–1790. doi:10.1126/science.293.5536.1786
Pérez-Jvostov F, Hendry AP, Fussmann GF, Scott ME (2015) Testing for local host–parasite adaptation: an experiment with Gyrodactylus ectoparasites and guppy hosts. Int J Parasitol 45:409–417. doi:10.1016/j.ijpara.2015.01.010
Peterson CH, Rice SD, Short JW et al (2003) Long-term ecosystem response to the Exxon Valdez oil spill. Science 302:2082–2086
Pike N (2011) Using false discovery rates for multiple comparisons in ecology and evolution: false discovery rates for multiple comparisons. Methods Ecol Evol 3:278–282
R Development Core Team (2008) R: a language and environment for statistical computing
Reader SM (2015) Causes of individual differences in animal exploration and search. Top Cogn Sci 7:451–468. doi:10.1111/tops.12148
Reader SM, Kendal JR, Laland KN (2003) Social learning of foraging sites and escape routes in wild Trinidadian guppies. Anim Behav 66:729–739. doi:10.1006/anbe.2003.2252
Réale D, Reader SM, Sol D et al (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. doi:10.1111/j.1469-185X.2007.00010.x
Renner MJ (1988) Learning during exploration: the role of behavioural topography during exploration in determining subsequent adaptive behaviour. Int J Comp Psychol 2:43–56
Reynaud S, Deschaux P (2006) The effects of polycyclic aromatic hydrocarbons on the immune system of fish: a review. Aquat Toxicol 77:229–238. doi:10.1016/j.aquatox.2005.10.018
Reznick DN, Rodd FH, Cardenas M (1996) Life-history evolution in guppies (Poecilia reticulata: Poeciliidae). IV. Similarism in life-history phenotypes. Am Nat 147:319. doi:10.1086/285854
Rolshausen G, Phillip DAT, Beckles DM et al (2015) Do stressful conditions make adaptation difficult? Guppies in the oil-polluted environments of southern Trinidad. Evol Appl 8:854–870. doi:10.1111/eva.12289
Samanta SK, Singh OV, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 20:243–248. doi:10.1016/S0167-7799(02)01943-1
Schantz SL, Moshtaghian J, Ness DK (1995) Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundam Appl Toxicol Off J Soc Toxicol 26:117–126
Schröder JH, Peters K (1988) Differential courtship activity and alterations of reproductive success of competing guppy males (Poecilia reticulata Peters; Pisces: Poeciliidae) as an indicator for low concentrations of aquatic pollutants. Bull Environ Contam Toxicol 41:385–390
Schwarzenbach RP, Escher BI, Fenner K et al (2006) The challenge of micropollutants in aquatic systems. Science 313:1072–1077. doi:10.1126/science.1127291
Scott GR, Sloman KA (2004) The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68:369–392. doi:10.1016/j.aquatox.2004.03.016
Seghers BH (1974) Schooling behaviour in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution 28:486. doi:10.2307/2407174
Seghers BH, Magurran AE (1995) Population differences in the schooling behaviour of the Trinidad guppy, Poecilia reticulata: adaptation or constraint? Can J Zool 73:1100–1105
Shen H, Huang Y, Wang R et al (2013) Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ Sci Technol 47:6415–6424. doi:10.1021/es400857z
Simonato JD, Guedes CLB, Martinez CBR (2008) Biochemical, physiological, and histological changes in the neotropical fish Prochilodus lineatus exposed to diesel oil. Ecotoxicol Environ Saf 69:112–120. doi:10.1016/j.ecoenv.2007.01.012
Sih A, Ferrari M, Harris D (2011) Evolution and behavioural responses to human-induced rapid environmental change: behaviour and evolution. Evol Appl 4:367–387
Sojinu OSS, Wang J-Z, Sonibare OO, Zeng EY (2010) Polycyclic aromatic hydrocarbons in sediments and soils from oil exploration areas of the Niger Delta, Nigeria. J Hazard Mater 174:641–647. doi:10.1016/j.jhazmat.2009.09.099
Tytell ED, Lauder GV (2008) Hydrodynamics of the escape response in bluegill sunfish, Lepomis macrochirus. J Exp Biol 211:359–369
van Oosterhout C, Mohammed RS, Hansen H et al (2007) Selection by parasites in spate conditions in wild Trinidadian guppies (Poecilia reticulata). Int J Parasitol 37:805–812. doi:10.1016/j.ijpara.2006.12.016
Vanzella TP, Martinez CB, Colus IM (2007) Genotoxic and mutagenic effects of diesel oil water soluble fraction on a neotropical fish species. Mutat Res 631:36–43
Vignet C, Menach KL, Lyphout L et al (2014) Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish—part II: behaviour. Environ Sci Pollut Res 21:13818–13832. doi:10.1007/s11356-014-2762-6
Vignet C, Joassard L, Lyphout L et al (2015) Exposures of zebrafish through diet to three environmentally relevant mixtures of PAHs produce behavioural disruptions in unexposed F1 and F2 descendant. Environ Sci Pollut Res 22:16371–16383. doi:10.1007/s11356-015-4157-8
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of earth’s ecosystems. Science 277:494–499. doi:10.1126/science.277.5325.494
Weis JS, Candelmo A (2012) Pollutants and fish predator/prey behaviour: a review of laboratory and field approaches. Curr Zool 58:9–20
Weis JS, Weis P, Heber M, Vaidya S (1981) Methylmercury tolerance of killifish (Fundulus heteroclitus) embryos from a polluted vs non-polluted environment. Mar Biol 65:283–287
Williams LM, Oleksiak MF (2008) Signatures of selection in natural populations adapted to chronic pollution. BMC Evol Biol 8:282. doi:10.1186/1471-2148-8-282
Wright D, Krause J (2006) Repeated measures of shoaling tendency in zebrafish (Danio rerio) and other small teleost fishes. Nat Protoc 1:1828–1831. doi:10.1038/nprot.2006.287
Zala SM, Penn DJ (2004) Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim Behav 68:649–664. doi:10.1016/j.anbehav.2004.01.005
Acknowledgments
We thank G. Daggupati, C. LeBlond, L. Ljungberg, J Mateluna and PQ Sims for help at different stages of the study. We thank two anonymous referees for their helpful comments. L. Jacquin was supported by a postdoctoral grant from the Fyssen fellowship and an ATER fellowship from the Pau University UPPA, INRA Ecobiop. SM Reader thanks CFI and NSERC, and A Hendry thanks NSERC for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Special Issue Animal cognition in a human dominated world.
Rights and permissions
About this article
Cite this article
Jacquin, L., Dybwad, C., Rolshausen, G. et al. Evolutionary and immediate effects of crude-oil pollution: depression of exploratory behaviour across populations of Trinidadian guppies. Anim Cogn 20, 97–108 (2017). https://doi.org/10.1007/s10071-016-1027-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-016-1027-9