Abstract
Human activity in estuarine areas has resulted in pollution of the aquatic environment, but little is known about the levels of synthetic musks (SMs) in river water and sediments in estuarine areas. This study investigated the concentrations and distribution of SMs in the Jiaozhou Bay wetland, including celestolide, phantolide, traseolide, galaxolide (HHCB), tonalide (AHTN), musk xylene and musk ketone (MK). The SMs HHCB, AHTN and MK were detected at concentrations of 10.7–208, not detected (ND)–59.2 and ND–13.6 ng/L, respectively, in surface water samples and 13.1–27.3, 3.06–14.5 and 1.33–18.8 ng/g (dry weight; dw), respectively, in sediment samples. Based on the calculated total organic carbon (TOC) concentrations, there was no significant correlation between SMs and TOC in sediment samples (p > 0.05). The hazard quotients were 0.204, 0.386 and 0.059 for AHTN, HHCB and MK, respectively, which indicated no serious environmental impact, because these values are all less than 1. The concentrations of SMs decreased as the distance to the Xiaojianxi refuse landfill increased in both surface water and sediments. Compared with previous studies, the concentration of SMs in the Jiaozhou Bay wetland was relatively high. Therefore, more attention should be paid to SMs because of their persistent impact on human health and the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic musks (SMs), including nitro musks (NMs), polycyclic musks (PCMs), alicyclic musks and macrocyclic musks, are organic chemicals that are widely used as fragrance additives in many household products and consumer goods (Heberer 2003). Among the PCMs, galaxolide (1,3,4,6,7,8-hexahydro-4,6,6,7,8,8,-hexamethylcyclopenta[g]-2-benzopyran; HHCB) and tonalide (6-acetyl-1,1,2,4,4,7-hexamethyltetraline; AHTN) are the most heavily produced and used worldwide, almost completely replacing NMs. The use of musk xylene and musk ketone (MK) has decreased because of their potential toxicity and bioaccumulation (Nakata et al. 2007), but they can still be detected in the effluent from some wastewater treatment plants (WWTPs) in China (Liu et al. 2014).
HHCB and AHTN have potential oestrogen effects and low-dose effects (Yamauchi et al. 2008). The predicted no effect concentrations (PNECs) of HHCB and AHTN are 6.80 × 103 and 3.50 ×1 03 ng/L in surface water and 8.40 × 103 and 5.20 × 103 ng/g (dry weight; dw) in sediments, respectively (HERA 2004). The concentrations of HHCB and AHTN in WWTP sewage sludge reflect the production and consumption patterns of SMs in an area.
According to a toxicology report, in most parts of China, SMs were detected at concentrations ranging from below the limit of quantification (LOQ) to 4.14 × 104 ng/g for HHCB and below the LOQ to 2.20 × 104 ng/g (dw) for AHTN (Liu et al. 2014). Removal by WWTPs is one of the main elimination processes for HHCB and AHTN, with removal efficiencies of 80.0–90.0% (Luo et al. 2014). Many SMs exist in domestic sewage, and as a result of their incomplete removal by WWTPs or direct dumping, SMs are discharged into lakes and rivers (Clara et al. 2010). Due to their extensive production and use and their persistence, lipophilicity and bioaccumulation (Kannan et al. 2005), SMs have been widely detected in the environment, including surface waters (Hu et al. 2011; Reiner and Kannan 2011), snow (Hu et al. 2012), sediments and sewage sludges (Guo et al. 2010; Villa et al. 2012), aquatic biota (Moon et al. 2011) and even breast milk and blood (Moon et al. 2012).
Some studies of SMs in lakes and laboratory studies have been conducted, but little is known about SMs in wetlands, which is essential for addressing the connection between SM pollution and human health. Therefore, we analysed SM pollution in the Jiaozhou Bay wetlands. When wastewater flows through a wetland, complex migration and transformation processes may occur, including evaporation, photochemical degradation, adsorption, accumulation, plant uptake and microbial degradation (Reyes-Contreras et al. 2012). Photochemical degradation has almost no effect on HHCB, and in spring and winter, HHCB is removed mainly by the evaporation of surface water (Quednow and Puttmann 2008). HHCB has a longer photolysis half-life (~135 h) than that of AHTN (~4 h) (Buerge et al. 2003). In addition, MK undergoes strong photochemical degradation in surface waters. After exposure to light for 1 h, the MK concentration decreased to 1% of the original level (Butte et al. 1999). In sediments, AHTN is more readily absorbed than is HHCB (Wang et al. 2010). The major mechanism of HHCB and AHTN removal is absorption by organic matter in the substrate due to the high log Kow values. The reported removal rates of HHCB and AHTN exceeded 80.0% in subsurface flow constructed wetlands and ranged from 70.0% to 90.0% in a vertical flow constructed wetland (Hijosa-Valsero et al. 2011; Zhang et al. 2014).
The objectives of this study were to (1) investigate and characterise the levels of SMs in the Jiaozhou Bay wetland and compare them with global levels, (2) conduct a preliminary environmental risk assessment, (3) examine the relationships between SMs and total organic carbon (TOC) in this area, and (4) analyse the removal of SMs and the effect of wetlands on SM pollution.
Materials and methods
Sampling site
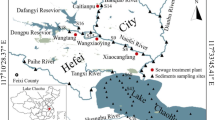
Sediment samples were collected from the Jiaozhou Bay wetland in Qingdao, Shandong Province, eastern China. This is a typical estuary wetland covered with reeds and strongly influenced by the tides. The Dagu River is the largest water system in the Jiaozhou Bay wetland, and Taoyuan River is one of its branches. As the largest estuary bay wetland in the Shandong Peninsula, the Jiaozhou Bay wetland is a heavily industrialised and urbanised region involving a large number of industries. The Xiaojianxi landfill is located in the upper reach of the Taoyuan River, and it processes approximately 1710 tons of rubbish collected from Qingdao City every day. Consequently, rubbish leaching liquor mixed with large amounts of pollutants flows into the Jiaozhou Bay wetland. The Xiaojianxi refuse landfill may be the main source of pollution in this area. It provides an ideal model to investigate the contamination and spatial distribution of SMs in water and sediments from wetlands.
Sampling
A total of 18 sediment samples and 14 surface water samples were collected in March 2014. When we collected the samples, the riverbed at sites 15–18 was bone dry, and therefore water samples could not be collected from these sites. Figure 1 is a map of the sampling sites, which are located mainly in the Taoyuan (sites 1–7) and Dagu (sites 8–18) Rivers. These rivers join downstream of site 7. Both surface water samples and sediment samples were collected at sites 1–14, while only sediment samples were collected at sites 15–18.
The sediment samples were collected using a spade (preconditioned with hexane and deionised water) at a depth of approximately 10 cm. Three parallel samples were collected at each site. After collection, the samples were immediately wrapped in several layers of aluminium foil and placed in sealed plastic bags to avoid irradiation by light. Then, the samples were placed in an icebox before transporting to the laboratory and storing at − 20 °C until analysis. When collecting the surface water samples, we divided the river section into three parts, and three samples of the same volume were collected from each part and then mixed as a composite sample for each water sampling site.
Methanol was added to all surface water samples to prevent decomposition of organic material by microorganisms in the water. Samples were extracted within 5 days of collection. The samples were filtered through glass-fibre film (0.45 μm) and stored at 4 °C before treatment. The sediment samples were frozen for 12 h and dried in a vacuum freeze drier for at least 48 h. Then, the samples were crushed and sieved through a 60-mesh screen and stored at − 20 °C until analysis.
Sample preparation and analysis
Chemicals
Five PCMs standards, including celestolide (ADBI), phantolide (AHMI), traseolide (ATII), HHCB, AHTN, and two NMs standards, including MX, MK, and the internal standard, C13 isotope labelled hexachlorobenzene (HCB-C13), were all purchased from Dr. Ehrenstorfer, (Augsburg, Germany). Surrogate standard, Deuterated fluoranthene (d10-fluoranthene) and solid phase extraction (SPE) column (C-18) were purchased from Supeclo (Bellefonte, PA, USA). All solvents were of the high-performance liquid chromatography (HPLC) grade, including methanol, n-hexane (HEX), dichloromethane (DCM) and methanol. Anhydrous sodium sulphate was baked at 450 °C for 4 h. Silica gel (100–200 mesh) and neutral Al2O3 were extracted with HEX/DCM (1:1, V/V) for 24 h, baked at 180 and 250 °C for 12 h, respectively, and wetted with distilled water to reduce their activation prior to use. Copper powder was activated by 35%–37% hydrochloric acid.
Surface water samples
The SPE columns were preconditioned with 5 mL DCM/methanol (1:1, V/V), 5 mL methanol and then 5 mL distilled water. Surface water samples (400 mL) were spiked with 5 μL of 1 μg/mL d10-fluoranthene, and loaded onto the SPE column at a flow rate of 5–10 mL/min. Thereafter, the SPE columns were dried suing nitrogen, before target analytes were eluted with 6 mL HEX and 4 mL HEX/DCM (1:1, V/V). The eluates were combined and concentrated to 0.5 mL. Finally, 5 μL of 1 μg/mL HCB-C13 was added to the extract before analysis.
Sediment samples
The pretreatment procedure of sediment samples is similar to the methods that had been reported (Hu et al., 2011). Sediment samples (2.50 g, dry weight) were spiked with 5 μL of 1 μg/mL d10-fluoranthene and mixed with 15 g anhydrous sodium sulphate. The samples were then extracted with 105 mL HEX/DCM (1:1 v/v) in a Soxhlet extractor at 60 °C for 24 h. Thereafter, copper was added to the extract to remove sulphur. Then all the extract liquor was concentrated to 1 mL, and silica gel/neutral Al2O3 was applied to clean up columns. The columns were eluted with 5 mL HEX, 20 mL HEX/DCM (2:1, V/V), 30 mL HEX/DCM (1:2, V/V) and 30 mL HEX/DCM (1:3, V/V). The combined eluents were concentrated to 0.5 mL and added 5 μL of 1 μg/mL HCB-C13 before analysis. During the experimentation, three parallel samples were analysed from each sampling site.
Analysis
All samples were analysed using GC-MS (Agilent 7890A-5975C) in the selective ion monitoring mode and using electron-impact (EI) ionisation source. The mass spectrometer quadrupole, source temperature and transfer line temperature were 150, 230 and 280 °C, respectively. The target compounds were separated by HP-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm) and injected splitless (1 μL) with the temperature of injector port was 250 °C. The temperature programme was as follows: the start temperature was 90 °C, holding 90 °C for 2 min, reached to 170 °C with the speed of 10 °C/min, then the temperature increased to 180 °C with the speed of 1 °C/min, holding 180 °C for 2 min and temperature increased to 270 °C with the speed of 30 °C/min, holding 270 °C for 5 min.
Determination of TOC
The TOC contents were determined by the potassium bichromate volumetry-external heating method (Bao 1999). During the experimentation, three parallel samples were analysed in each site, and two procedural blanks were processed with each batch of samples at the same time.
Calculation of the hazard quotients (HQ)
We used hazard quotients (HQ) to make a preliminary environmental risk assessment. According to the European Guidelines (European Commission 2003), we can calculate the HQs.
MEC is the measured environmental concentration, PNEC is the predicted no effect concentration. L(E)C50 is the median lethal dose or the half maximal effective concentration, NOEC is the no observed effect concentration. If HQ > 1, pollutants may be harmful to the environment, then additional tests need to be performed to quantify the real risk (Hernando et al. 2006).
Quality control and assurance
In order to avoid the contamination during experimentation, skin care products were not used and nitrile gloves were worn to avoid direct contact with samples and all the instruments. All the glassware were soaked in K2Cr2O7-H2SO4 solution then baked at 300 °C for 12 h, all glassware were rinsed with HEX before use. The blank samples were processed in the experimentation, all the SMs contaminations were below detection limits in every blank sample. HCB-C13 was used as internal standard to quantify the concentrations of SMs and d10-fluoranthene was used as surrogate standard.
The recoveries of d10-fluoranthene in surface water samples were 81.6–111% and were 93.9–106% in sediment samples. All the experimental results were corrected with the recoveries of surrogate standards. The different concentrations of SMs standards were analysed, all of the working curves had good linear range, and the correlation coefficients were larger than 0.999 (R 2 > 0.999). The limits of detection (LOD), as well as the LOQ of SMs were determined by a signal-to-noise ratio of 3 and 10. In surface water and sediment samples, the LODs were in the range of 0.04–0.34 ng/L, 0.03–0.27 ng/g, respectively; and the LOQs were in the range of 0.13–1.13 ng/L, 0.10–0.92 ng/g, respectively. The recoveries of SMs in surface water and sediment samples were 80.9–97.5% and 92.4–109%, respectively. At the spiking level of 5 ng, repeatability, the relative standard deviation (% RSD) were 3.40–8.20% in surface water samples and 5.30–9.21% in sediment samples. And the linear equations, correlation coefficients, LOD and LOQ of SMs in samples are in the Table 1.
Statistical analyses
The concentrations of SMs in surface water and sediment samples, as well as the TOC in sediment samples were examined using the non-parametric Kolmogorov-Smirnov test (K-S) (Lou et al. 2016). Pearson correlation analysis was used to assess correlations among the different kinds of SMs and TOC in sediment samples (Lou et al. 2016). The p value below 0.05 was considered to be significant. All statistical analyses were performed using the software of SPSS 17.0 and Origin 7.5.
Results and discussion
Levels of SMs in the Jiaozhou Bay wetland
Concentrations, composition profiles and correlation analysis of SMs in samples
HHCB was detected in all samples, accounting for 53–100% of the total SMs in surface water samples (Table 2) and 48–71% in the sediment samples (Table 3). AHTN and MK were detected in 57% and 71% of the water samples, respectively, while no other SM residues were detected in any water sample. All of the sediment samples also contained AHTN and MK but no other reportable SM residues. The respective concentrations of HHCB, AHTN and MK were in the ranges of 10.7–208 (mean 42.2), not detected (ND)–59.1 (mean 10.4) and ND–13.6 (mean 3.60) ng/L in the surface water samples and 13.1–27.4 (mean 19.2), 3.06–14.5 (mean 5.03) and 1.33–18.8 (mean 9.05) ng/g dw in the sediment samples.
HHCB and AHTN may have common sources and similar environmental fates, because the concentrations of HHCB and AHTN were significantly correlated (p < 0.05) in both the surface water and sediment samples. However, the AHTN-to-HHCB ratios were not significantly correlated (p > 0.05), implying that the production and use of AHTN are lower or that AHTN is degraded more easily compared with HHCB in the wetland. There was also a significant correlation between MK and HHCB concentrations in the sediment samples (p < 0.05). The difference between MK and PCMs may be caused by different modes of transport and enrichment in water–sediment environments. Further investigation is needed.
Global comparison of SM levels
The concentrations of HHCB and AHTN detected in the surface water of Jiaozhou Bay (Table 4) are similar to those in Suzhou Creek (Zhang et al. 2008) and the Haihe River in China (Hu et al. 2011), the Hudson River in the USA (Reiner and Kannan 2011) and Ruhr River and Hessen (four small freshwater river systems in Hessen) in Germany (Bester 2005; Quednow and Puttmann 2008). However, the levels are slightly higher than those in Meiliang Bay, China and the Michigan River, Canada (Ma et al. 2014; Peck and Hornbuckle 2004), but slightly lower than those detected in Korea, the Molgora River in Italy (Lee et al. 2010; Villa et al. 2012) and the Someș River in Romania (Moldovan 2006). The level of MK pollution was higher in this area than in other regions, except for Korea (Table 4).
The contamination of sediments by HHCB and AHTN was similar to that reported for the Haihe River, Liangtan River and Suzhou Creek in China (Hu et al. 2011; Sang et al. 2012; Zhang et al. 2008) and the Lippe River in Germany (Kronimus et al. 2004). However, the concentrations were higher than those in Taihu Lake, Meiliang Bay and the intertidal zone of Jiaozhou Bay in China (Che et al. 2010; Ma et al. 2014; Wang et al. 2015) and in Lake Erie and Lake Ontario in the USA (Peck et al. 2006). However, they were lower than the concentrations in the Hudson River in the USA and Molgora River in Italy (Reiner and Kannan 2011; Villa et al. 2012). The level of MK pollution in the sediments was similar to those in the Liangtan River (Sang et al. 2012) and intertidal zone of Jiaozhou Bay and Haihe River in China (Che et al. 2010; Hu et al. 2011), but higher than those in other areas evaluated. The difference in concentrations was largely due to the different consumption patterns of SMs in different areas: MK is used in cosmetics and soap, HHCB to treat myocardial infarction, and AHTN in top-grade cosmetics, detergents and fabric softeners (Zhou 2016). MK was detected in almost all samples, which suggested that MK is used widely in many household products in this area (Sang et al. 2012; Ma et al. 2014).
Preliminary environmental risk assessment and source identification
Studies of the toxicity of musk to organisms (Vera et al. 2017) indicated that PNECAHTN = PNECHHCB = 71 ng/g dw, and PNECMK = 320 ng/g dw. Considering the worst-case scenario, we took the maximum concentration that we detected as the maximum estimated concentration (MEC); thus, the results represent the worst possible contamination in the area. We then derived hazard quotients (HQs) of 0.204, 0.386 and 0.059 for AHTN, HHCB and MK, respectively.
According to the method used by the European Commission (2003), it does not seem likely that our target chemicals will cause much environmental damage theoretically, because their HQs are all less than 1. Nevertheless, more relevant toxicology data for estuarine organisms are needed to confirm this conclusion.
For hydrophobic organic compounds in sediments, TOC is also required for ecological risk assessment (Hu et al. 2011). In this study, the TOC in the sediment samples was in the range of 0.264 – 1.38% (mean 0.894%) (Table 3). There were no significant correlations between SMs and TOC (p > 0.05); however, this relationship requires further research.
Distributions patterns of SMs in the Jiaozhou Bay wetland
The highest concentration of total SMs was detected at site 2 (Fig. 2: 248 ng/L in surface water and Fig. 3: 53.6 ng/g in sediment samples). The lowest concentration of total SMs was detected at site 8 for the surface water samples and at site 16 for the sediment samples (13.1 ng/L and 18.8 ng/g, respectively). In general, the concentration of SMs in the Jiaozhou Bay wetland decreased in the direction of flow, except at the sites near landfill sites or older villages. This phenomenon occurred in both surface water and sediment samples, which indicated the presence of point source pollution of SMs in the Jiaozhou Bay wetland.
In the Taoyuan River, the concentrations of SMs tended to be higher at sites 1 and 2 because of the continuous input of landfill leachate. Perhaps the accumulation effect was greater than the degradation effect in this area. At sites 3 and 4, the pollutant concentrations dropped rapidly; the flow may be greater there, and the effects of erosion on pollutants were obvious. Because the concentration of pollutants in sediments did not change much, there was no accumulation in sediments at sites 3 and 4 (Fig. 3). Due to the purification effect and current scouring, the concentrations of SMs decreased gradually with the flow, except at sites 4 and 5, which are near villages.
Conclusions
SMs were detected in surface water and sediment samples from the Jiaozhou Bay wetland, China. HHCB, AHTN and MK were the main pollutants detected in both the surface water and sediment samples. The sources of HHCB and AHTN were similar. The distribution of SMs conformed to the characteristics of the population density and sewage drainage. Based on the HQ and TOC content, there may be no significant harm to the environment. More relevant toxicology data for estuarine organisms are still needed for accurate assessment of the environmental risk. The relationships between TOC and SMs require further research. The concentrations of SMs discharged from the landfill were lower in the Dagu River estuary. Therefore, the wetland is a natural barrier in Jiaozhou Bay, as it prevented contaminants from entering the sea.
References
Bao SD (1999) Soil and agricultural chemistry analysis. China Agriculture Press (Bei Jing)
Bester K (2005) Polycyclic musks in the Ruhr catchment area-transport, discharges of waste water, and transformations of HHCB, AHTN and HHCB-lactone. J Environ Monit 7(1):43–51. https://doi.org/10.1039/B409213A
Buerge IJ, Buser HR, Mueller MD, Poiger T (2003) Behavior of the polycyclic musks HHCB and AHTN in lakes, two potential anthropogenic markers for domestic wastewater in surface waters. Environ Sci Technol 37(24):5636–5644. https://doi.org/10.1021/es0300721
Butte W, Schmidt S, Schmidt A (1999) Photochemical degradation of nitrated musk compounds. Chemosphere 38(6):1287–1291. https://doi.org/10.1016/S0045-6535(98)00529-3
Che JS, Yu RP, Wang LP, Ran GX, Song QJ (2010) Investigation on content distribution of synthetic musks in Taihu Lake. Flavour Fragrance Cosmetics 6:12–16
Clara M, Gans O, Windhofer G, Krenn U, Hartl W, Braun K, Scharf S, Scheffknecht C (2010) Occurrence of polycyclic musks in wastewater and receiving water bodies and fate during wastewater treatment. Chemosphere 82(8):1116–1123. https://doi.org/10.1016/j.chemosphere.2010.11.041
European Commission (EC) (2003) Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) N.1488/94 on Risk Assessment for Existing Substances, Directive 98/8/EC of the European Parliament and of theCouncil Concerning the Placing of Biocidal Products on the Market. Part II: Environmental Risk Assessment. Office for Official Publications of the European Communities, Luxembourg
Guo R, Lee IS, Kim UJ, Oh JE (2010) Occurrence of synthetic musks in Korean sewage sludges. Sci Total Environ 408(7):1634–1639. https://doi.org/10.1016/j.scitotenv.2009.12.009
HERA (Human and Environmental Risk Assessment on ingredients of household cleaning products) (2004) Polycyclic musks AHTN (CAS 1506-02-1) and HHCB (CAS 1222-05-05). Available at http://www.Heraproject.com/RiskAssessment.cfm (accessed November 10, 2014)
Heberer T (2003) Occurrence, fate, and assessment of polycyclic musk residues in the aquatic environment of urban areas-a review. Acta Hydrochim Hydrobiol 30(5–6):227–243
Hernando MD, Mezcua M, Fern’andez-Alba AR, Barcel’o D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69(2006):334–342. https://doi.org/10.1016/j.talanta.2005.09.037
Hijosa-Valsero M, Matamoros V, Pedescoll A, Martin-Villacorta J, Becares E, Garcia J, Bayona JM (2011) Evaluation of primary treatment and loading regimes in the removal of pharmaceuticals and personal care products from urban wastewaters by subsurface-flow constructed wetlands. Int J Environ Anal Chem 91(7–8):632–653. https://doi.org/10.1080/03067319.2010.526208
Hu ZJ, Shi YL, Cai YQ (2011) Concentrations, distribution, and bioaccumulation of synthetic musks in the Haihe River of China. Chemosphere 84(11):1630–1635. https://doi.org/10.1016/j.chemosphere.2011.05.013
Hu ZJ, Shi YL, Niu HY, Cai YQ (2012) Synthetic musk fragrances and heavy metals in snow samples of Beijing urban area, China. Atmos Res 104:302–305
Kannan K, Reiner JL, Yun SH, Perrotta EE, Tao L, Johnson-Restrepo B, Rodan BD (2005) Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States. Chemosphere 61(5):693–700. https://doi.org/10.1016/j.chemosphere.2005.03.041
Kronimus A, Schwarzbauer J, Dsikowitzky L, Heim S, Littke R (2004) Anthropogenic organic contaminants in sediments of the Lippe river, Germany. Water Res 38(16):3473–3484. https://doi.org/10.1016/j.watres.2004.04.054
Lee IS, Lee SH, Oh JE (2010) Occurrence and fate of synthetic musk compounds in water environment. Water Res 44(1):214–222. https://doi.org/10.1016/j.watres.2009.08.049
Liu NN, Shi YL, Li WH, Xu L, Cai YQ (2014) Concentrations and distribution of synthetic musks and siloxanes in sewage sludge of wastewater treatment plants in China. Sci Total Environ 476:65–72. https://doi.org/10.1016/j.scitotenv.2013.12.124
Lou YH, Wang J, Wang L, Shi L, Yu Y, Zhang MY (2016) Determination of synthetic musks in sediments of Yellow River Delta wetland. China Bull Environ Contam Toxicol DOI 97(1):78–83. https://doi.org/10.1007/s00128-016-1814-7
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473:619–641. https://doi.org/10.1016/j.scitotenv.2013.12.065
Ma L, Jing Y, Zhou J, Zeng XY, Zhang XL, Yu YX (2014) Distribution of synthetic musk in surface water and sediments from Meiliang Bay, Taihu Lake. Environ Chem 33(4):630–635
Moldovan Z (2006) Occurrences of pharmaceutical and personal care products as micropollutants in rivers from Romania. Chemosphere 64(11):1808–1817. https://doi.org/10.1016/j.chemosphere.2006.02.003
Moon HB, An YR, Park KJ, Choi SG, Moon DY, Choi M, Choi HG (2011) Occurrence and accumulation features of polycyclic aromatic hydrocarbons and synthetic musk compounds in finless porpoises (Neophocaena phocaenoides) from Korean coastal waters. Mar Pollut Bull 62(9):1963–1968. https://doi.org/10.1016/j.marpolbul.2011.06.031
Moon HB, Lee DH, Lee YS, Choi SG, Moon DY, Choi M, Choi HG (2012) Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere 86(5):485–490. https://doi.org/10.1016/j.chemosphere.2011.10.008
Nakata H, Sasaki H, Takemura A, Yoshioka M, Tanabe S, Kannan K (2007) Bioaccumulation, temporal trend, and geographical distribution of synthetic musks in the marine environment. Environ Sci Technol 41(7):2216–2222. https://doi.org/10.1021/es0623818
Peck AM, Hornbuckle KC (2004) Synthetic musk fragrances in Lake Michigan. Environ Sci Technol 38(2):367–372. https://doi.org/10.1021/es034769y
Peck AM, Linebaugh EK, Hornbuckle KC (2006) Synthetic musk fragrances in Lake Erie and Lake Ontario sediment cores. Environ Sci Technol 40(18):5629–5635. https://doi.org/10.1021/es060134y
Quednow K, Puettmann W (2008) Organophosphates and synthetic musk fragrances in freshwater streams in Hessen/Germany. Clean-soil Air Water 36(1):70–77. https://doi.org/10.1002/clen.200700023
Reiner JL, Kannan K (2011) Polycyclic musks in water, sediment, and fishes from the upper Hudson River, New York, USA. Water Air Soil Pollut 214(1–4):335–342. https://doi.org/10.1007/s11270-010-0427-8
Reyes-Contreras C, Hijosa-Valsero M, Sidrach-Cardona R, Bayona JM, Becares E (2012) Temporal evolution in PPCP removal from urban wastewater by constructed wetlands of different configuration: a medium-term study. Chemosphere 88(2):161–167. https://doi.org/10.1016/j.chemosphere.2012.02.064
Sang WJ, Zhang YL, Zhou XF, Ma LM, Sun XJ (2012) Occurrence and distribution of synthetic musks in surface sediments of Liangtan River, West China. Environ Eng Sci 29(1):19–25. https://doi.org/10.1089/ees.2010.0241
Vera H, Ines M, Arminda A (2017) Assessing seasonal variation of synthetic musks in beach sands from Oporto coastal area: a case study. Environ Pollut 226(2017):190–197
Villa S, Assi L, Ippolito A, Bonfanti P, Finizio A (2012) First evidences of the occurrence of polycyclic synthetic musk fragrances in surface water systems in Italy: spatial and temporal trends in the Molgora River (Lombardia Region, Northern Italy). Sci Total Environ 416:137–141. https://doi.org/10.1016/j.scitotenv.2011.11.027
Wang F, Zhou Y, Guo Y, Zou L, Zhang X, Zeng X (2010) Spatial and temporal distribution characteristics of synthetic musk in Suzhou Creek. J. Shanghai Univ (English Edition) 14(4):306–311. https://doi.org/10.1007/s11741-010-0649-3
Wang J, Lou YH, Wang L, Zhao YY, Shi L, Zheng MG (2015) Research about the synthetic musks in intertidal sediments in the north of Jiaozhou Bay. Environ Chem 34(2):384–385
Yamauchi R, Ishibashi H, Hirano M, Mori T, Kim JW, Arizono K (2008) Effects of synthetic polycyclic musks on estrogen receptor, vitellogenin, pregnane X receptor, and cytochrome P4503A gene expression in the livers of male medaka (Oryzias latipes). Aquat Toxicol 90(4):261–268. https://doi.org/10.1016/j.aquatox.2008.09.007
Zhang DQ, Gersberg RM, Ng WJ, Tan SK (2014) Removal of pharmaceuticals and personal care products in aquatic plant-based systems: a review. Environ Pollut 184:620–639. https://doi.org/10.1016/j.envpol.2013.09.009
Zhang XY, Yao Y, Zeng XY, Qian GR, Guo YW, Wu MH, Sheng GY, Fu JM (2008) Synthetic musks in the aquatic environment and personal care products in Shanghai, China. Chemosphere 72(10):1553–1558. https://doi.org/10.1016/j.chemosphere.2008.04.039
Zhou J (2016) Synthetic musk in daily chemicals. China Surfactant Detergent&Cosmetics Vol. 46 No.9 Sept.2016
Funding
This work was supported by the Basic Scientific Fund for National Public Research Institutes of China (No. 2016Q07), National Key R&D Program of China (Grant 20177YFC1404504), the Open Fund of Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China (No.OF2015NO14) and the National Natural Science Foundation of China (No. 21307063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Jiang, S., Wang, L., Zheng, M. et al. Determination and environmental risk assessment of synthetic musks in the water and sediments of the Jiaozhou Bay wetland, China. Environ Sci Pollut Res 25, 4915–4923 (2018). https://doi.org/10.1007/s11356-017-0811-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0811-7