Abstract
Persistent organic pollutants (POPs) are a group of heterogeneous compounds of both natural and anthropogenic origin with highly persistent and bioaccumulative properties. They cause a range of adverse effects to human health and the environment around the world. There is growing concern that POPs may increase breast cancer risk due to their xenoestrogenic properties. The aim of this systematic literature review is to summarize and integrate the risks of breast cancer following environmental exposure to POPs (other than DDT) from primary epidemiological studies published between 2006 and 2015. After searching various databases, 14 case-control studies and one cohort study were included. Evidence of an association between increased breast cancer risk and environmental exposure to these chemicals is inconsistent and inadequate to conclude with certainty. However, most of the studies have examined exposure to the pollutants after diagnosis of breast cancer, overlooking exposure during critical windows of vulnerability. They have also largely focused on individual chemicals but ignored the combined effects of different chemicals. Therefore, major data gaps remain in examining exposure during critical windows of vulnerability and assessing combined effects of multiple chemicals. Development of better exposure assessment methods addressing these gaps is required for future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer among females as well as the principal cause of their death from cancer worldwide (Torre et al. 2015). Recent global cancer statistics showed that an estimated 1.7 million women were diagnosed with breast cancer and 521,900 died from this disease in 2012. These figures equate to 25 % of overall cancer incidence and 15 % of total cancer deaths in women, respectively (Torre et al. 2015). However, only 41 % of this increased number of breast cancer cases are attributable to recognized risk factors, such as later age at first birth, nulliparity, family history of breast cancer and higher socioeconomic status (Madigan et al. 1995). Environmental factors have been suggested as an important missing link for breast cancer causality.
Oestrogen exposure throughout life is considered a major risk factor for developing breast cancer (Bernstein and Ross 1993). Oestrogen exposure is influenced by factors such as age at menarche, first pregnancy and menopause, length of reproductive life, use of hormonal contraception and hormonal replacement therapy and body mass index (BMI), which are directly related to level of sex hormones (The Endogenous Hormones and Breast Cancer Collaborative Group 2011; McPherson, Steel and Dixon 2000). Persistent organic pollutants (POPs) are well known for their estrogenic effects (Soto, Chung and Sonnenschein 1994; Bonefeld-Jørgensen, Autrup and Hansen 1997; Bonefeld-Jørgensen et al. 2001; Andersen, Cook and Waldbillig 2002). These chemicals are a group of heterogeneous compounds of both natural and anthropogenic origin that possess a range of common properties (Abelsohnet al. 2002, El-Shahawiet al. 2010, Li et al. 2006). These lipophilic compounds (except perfluorinated chemicals) accumulate in fat, resulting in bioaccumulation and biomagnification up the food chain (Abelsohn et al. 2002; Damstra 2002; Li et al. 2006). They are also resistant to photolytic, biological or chemical degradation and remain in the environment for a long period (Damstra 2002). In addition, due to their semi-volatile properties, they are able to be transported long distances by air (World Health Organization [WHO] 2010). These chemicals can thus be found in distant geographical locations from their sources. Overall, their widespread distribution and long persistence in the environment makes them virtually ubiquitous.
The Stockholm Convention on POPs is a global treaty for protecting human health and the environment from exposure to POPs (Stockholm Convention n.d.). It was formed in 2001 and became effective in 2004. Initially, there were 12 chemicals (aldrin, chlordane, dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs) dieldrin, endrin, heptachlor, hexachlorobenzene, mirex, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/PCDF), hexachlorobenzene) listed under this Convention. Later, new chemicals (chlordecone, α-hexachlorocyclohexane, β-hexachlorocyclohexane, lindane, pentachlorobenzene, hexabromobiphenyl, hexabromodiphenyl ether and heptabromodiphenyl ether, perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride, tetrabromodiphenyl ether and pentabromodiphenyl ether) were added in 2009 and endosulfan and hexabromocyclododecane were added in 2011 and 2013, respectively. Among these chemicals, DDT is extensively studied and there is a recent systematic literature review on DDT and breast cancer (Park et al. 2014). There are, however, few systematic literature reviews for other POPs, with the most recent review of PCB published in 2003 (Negri et al. 2003) and cyclodienes in 2007 (Khanjani et al. 2007). There are also some narrative reviews (Brody et al. 2007, Calle et al. 2002, Golden and Kimbrough 2009, Mitra, Faruque and Avis 2004), but to our knowledge, there is no systematic literature review on breast cancer due to environmental exposure to POPs other than DDT, in the last 10 years. Hence, this study will review epidemiological studies on breast cancer due to environmental exposure of POPs other than DDT published in the last 10 years.
The aims of this systematic review are to summarize the risks of breast cancer following environmental exposure to POPs other than DDT from primary epidemiological studies, to find methodological challenges and reasons for heterogeneity among studies and to identify research gaps and, ultimately, recommend directions for future research.
Methodology
A comprehensive research strategy including key words, inclusion and exclusion criteria, databases to be searched, literature screening and quality appraisal procedure and data extraction, analysis, interpretation and documentation processes was developed and documented to avoid bias. The detailed search strategy is available in Supporting Information. Databases searched were PubMed, Scopus, CINAHL (via EBSCOhost) and Embase (via embase.com).
Types of studies
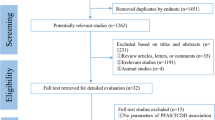
Original studies published in a peer-reviewed journal between 2006 and 2015, with a case-control, nested case-control or cohort design, that estimated risk of breast cancer (odds ratio, risk ratio or hazard ratio) associated with environmental exposure to POPs listed in the Stockholm Convention (except DDT) were included in this review. Moreover, to be included in the review, studies were required to have clear description of the methodology, including the selection criteria of cases and controls, and details of data collection and data analysis procedures. Only literature published in English was considered (Fig. 1).
Types of participants
Both female and male breast cancer patients were included.
Types of exposures
Studies of environmental exposure to POPs (other than DDT) listed in the Stockholm Convention were considered. However, studies of exposure to some derivatives of these compounds and closely related substances were also considered. For example, some articles reported exposure of oxychlordane, an oxidative metabolite of chlordane (Zheng et al. 2000) or trans-nonachlor and cis-nonachlor, which are bioaccumulating components of chlordane (Bondy et al. 2000). Furthermore, the Stockholm Convention only includes perfluorooctane sulfonic acid and its salts and perfluorooctane sulfonyl fluoride (PFOSF) while many epidemiological studies reported other perfluorinated chemicals along with perfluorooctane sulfonate (PFOS) due to their common properties. For all compounds, exposure had to be measured directly by biological sample, not by environmental data or other indirect methods.
Types of outcome measures
Histologically confirmed breast cancer.
Literature search
Databases were searched with the same keywords. However, different filters were used for different databases. In PubMed, filtering was used for publishing years (last 10 years), language (English) and human studies; in Scopus for publishing years (2006–2015), language (English), document types (articles) and source types (journal); in Embase for years (2006–2010), publication types (articles); in CINAHL for years (2006–2010), language (English) and source types (academic journal). The literature search took place between 24th August and 25th September of 2015. The title and abstracts of articles found through keyword searches were screened initially for inclusion and exclusion criteria, and full articles were subsequently further screened for inclusion and exclusion criteria. Searches were conducted by one author and checked for accuracy by another author. Any disagreement between two authors was solved by consensus.
Result of the search
After keyword searches and using relevant filters, 249 articles from PubMed, 329 from Scopus, 22 from CINAHL (via EBSCOhost) and 396 from Embase (via embase.com) were obtained. After screening of title and abstract of these articles, 17 case-control studies and 5 cohorts were selected. Of those articles, 14 case-control studies and 1 cohort study were selected after screening full articles (Tables 1, 2, 3 and 4).
Quality assessment
After screening, those articles which satisfied the inclusion and exclusion criteria were assessed for quality using the Critical Appraisal Skills Program (CASP) checklist for relevant study types (CASP).
Data extraction
Data was extracted by one author and checked by another author. Extracted data included author and year of publication, number and types of participants, exposure assessment method, risk of breast cancer for different exposure levels and significance and name of confounders considered in data analysis. Data extraction tables differed according to study design.
Results
In total, 14 case-control studies and 1 cohort study met the inclusion criteria for the literature search. Tables 1, 2, 3 and 4 summarize the findings of the included literature. Articles differed in the units of measure used to report concentrations of POPs. For this review, lipid-adjusted or lipid-based concentrations were used. Where studies reported more than one odds ratio, the odds ratio was adjusted for the highest number of covariates.
Case-control studies
Polychlorinated biphenyls
The literature search identified 8 case-control studies for polychlorinated biphenyls (PCBs), analysing the association between PCB exposure and BC risk (Rubin et al. 2006; Gatto et al. 2007; Itoh et al. 2009; Recio-Vega et al. 2011; Bonefeld-Jorgensen et al. 2011; Cohn et al. 2012; Holmes et al. 2014; Arrebola et al. 2015). Two case-control studies considering genotype in addition to exposure to PCBs for BC risk were also identified (Bräuner et al., 2014; Ghisari et al. 2014). All of these studies measured serum or plasma levels of PCBs except Bräuner et al. (2014). They used adipose tissue samples to measure 18 PCB congeners. Three studies (Cohn et al. 2012; Rubin et al. 2006; Bräuner et al. 2014) used blood samples taken before diagnosis of breast cancer, whereas the remainder used blood samples taken after the diagnosis. Cohn et al. (2012) limited their analysis to postpartum serum samples and only considered cancer incidence before 50 years of age. Most of the studies had age-adjusted healthy controls. However, in two studies, controls were women who underwent biopsies for breast conditions, with negative results (Holmes et al. 2014; Recio-Vega et al. 2011).
Only two studies which considered BC risk as a result of exposure to PCBs found a positive association between PCBs and BC risk. The first case-control study analysed 20 congeners of PCBs in 70 newly diagnosed BC patients and 70 controls (Recio-Vega et al. 2011). They found 8 congeners (118, 128, 138, 170, 180, 195, 206 and 209) positively associated with BC risk and most congeners (77 %) had more than five chlorines in their chemical structures (heavy molecular weight). The study grouped PCBs into five groups according to their structure–activity relationship. Group 1 and 2 included potentially estrogenic and antiestrogenic dioxin-like properties respectively, while group 3 included biologically persistent enzyme inducers (CYP1A & CYP1B) (Wolff and Toniolo 1995; Recio-Vega et al. 2011). Group 4 was known for environmental relevance and group 5 included neurotoxic PCBs (Recio-Vega et al. 2011). BC risk was found to be higher for groups 2b (OR = 1.90, 95 % CI: 1.25–2.88), 3 (OR = 1.81, 95 % CI: 1.08–3.04) and 4 (OR = 1.57, 95 % CI: 1.20–2.07). Moreover, after menopausal status was taken into account, significant associations were found for groups 1a, 2b and 4 in postmenopausal women and group 4 in premenopausal women. The highest OR was found for Group 1a PCBs in postmenopausal women (OR = 7.59; 95 % CI 1.12–51.38). This group is known for its potential estrogenic properties (Wolf and Toniolo 1995). In addition to PCBs, they found a positive association of BC with age, postmenopausal status, family history of BC and residence near an industrial facility (Recio-Vega et al., 2011).
The next case-control study finding a positive association between BC and PCB exposure was a prospective case-control study (Cohn et al. 2012). This nested case-control study analysed archived serum samples collected during the postpartum period for 16 congeners of PCB. The study only considered breast cancer incidence before the age of 50 years. For the 112 case-control pairs, no association was observed for total PCBs or PCB groups. However, they found a statistically significant positive association with PCB 203 and negative associations for PCB 167 and PCB 187. This result is similar to the findings of a previous study (Recio-Vega et al., 2011).
Two case-control studies examined the association of BC with PCB exposure in indigenous Alaskan women (Rubin et al. 2006; Holmes et al. 2014). Rubin et al. (2006) examined banked serum of 63 cases and 63 controls for 28 PCB congeners. They noticed a significant negative association between total PCB and BC risk in univariate analysis. The result was not significant in multivariate analysis.
Holmes et al. (2014) analysed blood collected after BC diagnosis. Seventy-five cases were newly diagnosed, histologically confirmed patients and 95 controls were diagnosed with benign breast disease. They observed no significant association between indicator PCBs (PCB 138/158, 153, 180) with BC risk in both univariate and multivariate analysis. They further analysed BC risk and oestrogen and progesterone status of 62 women with invasive tumours. Women with both oestrogen and progesterone binding capacity (ER+/PR+) had a higher geometric mean of PCBs compared to those with no binding capacity for both hormones (ER−/PR−).
Arrebola et al. (2015) also reported indicator PCBs (PCB 138, 153 and 180) in 69 breast cancer cases and 56 controls in Tunisia. PCBs were positively associated with BC risk in univariate analysis only. In contrast, Itoh et al. (2009) found a negative association for the same indicator PCBs and other congeners. The association was also negative when grouped according to PCB structure–activity. Moreover, there was significant negative association between total PCB exposure and BC risk (p = 0.008). When patients were grouped according to oestrogen and progesterone receptor and menopausal status, the negative association for total PCBs remained significant for ER+/PR−, ER+/PR+ and premenopausal and postmenopausal BC cases.
The remaining case-control studies reported total PCB level rather than individual congeners (Gatto et al., 2007; Bonefeld-Jorgensen, 2011). Gatto et al. (2007) observed no association between BC risk and PCB exposure among African-American women. Moreover, PCB exposure was not associated with any subtype of breast cancer depending on PR (progesterone receptor), p53 or HER2/neu (human epidermal growth factor receptor 2) status. However, they found a significantly increased BC risk among BC patients who had not been treated with chemotherapy. Total PCB level was also not positively associated with BC risk among cases in Greenlandic Inuit (Bonefeld-Jorgensen et al. 2011). However, when the total PCB level was divided into quartiles, the median of the highest quartile was significantly higher among cases compared to controls (p = 0.02).
Bräuner et al. (2014) tested the hypothesis that polymorphism of CYP1B1 and COMT gene modifies BC risk from PCB exposure. For 18 PCB congeners in 387 matched case-control pairs, they found no significant association between CYP1B1 genotype and breast cancer. On the other hand, there was a significant negative association between COMT and BC risk (RR = 0.65; 95 % CI 0.42–1.01, p = 0.02). However, when exposure to different functional groups of PCBs was assessed for BC risk, no significant association was found for any genotypes of CYP1B1 and COMT. The result was similar when hormonal replacement therapy was considered. Another study in Inuit women failed to find any significant association between BC risk and PCB exposure for different genotypes of CYP1A1, CYP1B1, COMT, CYP17, CYP19 and BRCA1 (Ghisari et al. 2014).
Hexachlorocyclohexane
Four studies were found that analysed the risk of BC for β-hexachlorocyclohexane (HCH) (Iwasaki et al. 2008; Itoh et al. 2009; Holmes et al. 2014; Arrebola et al. 2015) and two for γ-HCH (Boada et al. 2012; Holmes et al. 2014). Arrebola et al. (2015) found a positive association between BC and β-HCH after adjustment for selected covariates (OR = 1.10, 95 % CI: 1.03–1.18, p < 0.050). They observed a threefold increase in breast cancer risk for β-HCH concentrations above the limit of detection (LOD) compared to concentrations below LOD (OR = 3.44, p < 0.05). However, the other three case-control studies did not notice any significant association. Two of these case-control studies further analysed risk of BC among women grouped by oestrogen receptor (ER) and progesterone receptor (PR) status and found no significant result for β-HCH in subgroups (Itoh et al. 2009; Holmes et al. 2014). On the other hand, of the two case-control studies reporting γ-HCH level, neither found a positive association. For a case-control study among indigenous Alaskan women, the level of γ-HCH was higher among cases compared to controls, although not significant (8.3 ng/g lipid vs 7.7 ng/g lipid, p = 0.23) (Holmes et al. 2014). Boada et al. (2012) also found no positive association of BC risk and γ-HCH in multivariate analysis (OR = 1.097, 95 % CI: 0.420–28.412, p = 0.988). Moreover, when they considered organochlorine pesticides (OCP) mixtures as a determinant factor for breast cancer, the combination of lindane and endrin was found only among healthy women.
Hexachlorobenzene
There were four case-control studies that considered risk of breast cancer associated with hexachlorobenzene (HCB) exposure (Iwasaki et al. 2008; Itoh et al. 2009; Holmes et al.; 2014, Arrebola et al. 2015). Arrebola et al. (2015) found a positive association in an unadjusted model; however, it was no longer significant after adjustment for different covariates. For other case-control studies, the adjusted OR was less than 1, when the highest quartile was compared to the lowest.
Polybrominated diphenyl ethers
Hurley et al. (2011) and Holmes et al. (2014) examined the risk of BC and Polybrominated diphenyl ethers (PBDE) exposure. Hurley et al. (2011) examined breast cancer risk and five major PBDE congeners among Californian women known for their high level of exposure. They observed no significant association between adipose concentration of PBDEs and breast cancer risk. On the other hand, Holmes et al. (2014) measured only BDE-47 among indigenous Alaskan women. This PBDE congener was significantly high among cases (geometric mean (GM) = 38 ng/g lipid) compared to controls (GM = 25.1 ng/g lipid, p = 0.04). However, the risk was only significantly increased in univariate analysis of BDE-47 (OR = 1.79, p = 0.06).
Perfluorinated compounds
The associations between BC risk and perfluorinated compound exposure were analysed by three case-control studies in the last 10 years. Bonefeld-Jorgensen et al. (2011) collected blood samples from 31 BC patients and 115 matched controls. Serum level of perfluorooctane sulfonate (PFOS), perfluorooctane sulfonamide (PFOSA), sum PFSA (sum of PFOS, perfluorohexane sulfonate (PFHxS) and PFOSA) and sum PFCA (sum of perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), perfluorododecanoic acid (PFDoA) and perfluorotridecanoic acid (PFTrA) were measured. They also measured the sum of 12 PCB congeners (99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183 &187) and eight OCPs (p, p′-DDT, p, p′-DDE, β-HCH, aldrin, HCB, oxychlorodane, cis-nonachlor and trans-nonachlor). The serum level of these perfluorinated compounds was significantly higher among cases when compared with controls. Moreover, the risk of BC was significantly associated with serum level of PFOS (OR = 1.03, 95 % CI: 1.001–1.07, p = 0.05), sum PFSA (OR = 1.03, 95 % CI: 1.00–1.05, p = 0.02) and sum of lipophilic legacy POPs and PFCs (sum PCB + sum OCP + sum PFSA + sum PFCA) (OR = 1.02, 95 % CI: 1.01–1.04, p = 0.01) in both adjusted and unadjusted models. Using the same study population, Ghisari et al. (2014) conducted another case-control study to explore the effect of different genetic polymorphisms on the association between BC risk and perfluorinated compounds. They analysed the single nucleotide polymorphism in genes CYP1A1, CYP1B1, COMT, CYP17, CYP19 and different genotypes of BRCA1. Breast cancer risk was increased for high serum level of PFASs. After taking different polymorphisms into account, BC risk associated with PFASs was increased (Table 4). These findings strengthen the hypothesis that genetic polymorphisms might play an important role in BC risk associated with PFC exposure.
On the other hand, a prospective case-control study measured plasma level of perfluoroalkylated substances (PFAS) up to 15 years before diagnosis of breast cancer (Bonefeld-Jorgensen et al. 2014). For 250 BC cases and 233 controls, they observed a significant negative association for perfluorohexane sulfonate (PFHxS) for both unadjusted and adjusted models (RR = 0.66, 95 % CI: 0.47–0.94, p < 0.05) and positive association for perfluorooctane sulfonamide (PFOSA) in an unadjusted model (RR = 1.03, p < 0.05). However, for PFOSA, they observed a significant increased BC risk in the 5th quintile when compared to the 1st quintile (RR = 1.89, 95 % CI: 1.01–3.54, p < 0.05). Moreover, after withdrawal of 72 BC cases, they observed an increase in positive association of BC risk with PFOSA in an unadjusted model (RR = 1.04, 95 % CI: 1.00–1.08, p < 0.05) and also in BC risk for PFOSA in the 5th quintile in an unadjusted model (RR = 2.45, 95 % CI: 1.00–6.00, p < 0.05). In contrast, the negative association of PFHxS disappeared in an adjusted model (RR = 0.71, 95 % CI: 0.43–1.15, p > 0.05).
Chlordane and chlordane-associated compounds
Two case-control studies were found regarding exposure to chlordane and their associated compounds. Itoh et al. (2009) observed a negative association between BC risk and trans-nonachlor (OR = 0.49, 95 % CI: 0.22–1.06, p = 0.08), cis-nonachlor (OR = 0.41, 95 % CI: 0.19–0.91, p = 0.07) and oxychlordane (OR = 0.65, 95 % CI: 0.31–1.38, p = 0.33) when the highest quartile was compared with the lowest quartile. Holmes et al. (2014) found a similar result for oxychlordane (OR = 0.91, 95 % CI: 0.35–2.35, p = 0.84) and trans-nonachlor (OR = 0.65, 95 % CI: 0.26–1.66, p = 0.37).
Other POPs7
Only one case control study considered heptachlor and α-endosulfan exposure and BC risk (Arrebola et al. 2015). Serum levels of heptachlor were positively associated with increased risk of BC in unadjusted model (p < 0.05); however, significance was borderline in an adjusted model (p < 0.1). For mirex, two case-control studies were found (Itoh et al. 2009; Holmes et al. 2014). Though both studies found a negative association between mirex exposure and BC risk, the association was only statistically significant (p = 0.02) in the study by Itoh et al. (2009). One case control study from Spain considered aldrin and dieldrin (Boada et al. 2012). Aldrin was found at a significantly higher level among breast cancer patients (p < 0.001), although after a multivariate analysis, both aldrin and dieldrin showed no significant association with BC risk. However, this study found 24.8 % BC patients had a combination of aldrin, dichlorodiphenyldichloroethane (DDD) and DDE, while no healthy persons had this combination of chemicals. Therefore, Boada et al. (2012) hypothesised that a mixture of pollutants rather than individual pollutants could play an important role in breast cancer incidence.
Total organochlorine compounds
Two case-control studies considered total organochlorine compound levels for sum of p, p′-DDT, p, p′-DDE, β-HCH, aldrin, HCB, oxychlordane, cis-nonachlor and trans-nonachlor levels for the same population. Bonefel-Jorgenson et al. (2011) found no significant association between breast cancer and the sum OCP level. The association remained non-significant when Ghisari et al. (2014) analysed it further for different genotypes of CYP1A1, CYP1B1, COMT, CYP17, CYP19 and BRCA1.
Cohort
In this systematic literature search, only one cohort study was found that examined the association of BC risk with the dioxin-tetrachlorodibenzo-p-dioxin (TCDD) exposure among 981 women who were 0–40 years old and lived in Seveso in 1976. A chemical plant accident occurred in the town of Seveso in July 1976 and exposed the local population to a significant amount of TCDD. Over the 32 year (1976–2008) follow-up period, Warner et al. (2011) observed a non-significant increased risk of BC for a tenfold increase in serum TCDD in 33 breast cancer cases (HR = 1.44, 95 % CI: 0.89–2.33, p = 0.13). There was also a non-significant dose-response relationship (p = 0.09). However, this result is not consistent with the earlier follow-up of this cohort. After 20 years of follow-up (1976–1996), they observed a twofold increase in BC risk for a tenfold increase in serum TCDD levels (HR = 2.1, 95 % CI: 1.0–4.6, p = 0.05).
Discussion
The development of breast cancer is a complex process with multiple genetic, epigenetic and environmental factors contributing to it. Most of the risk factors of BC are directly related to the exposure of breast tissues to elevated level of sex hormones (The Endogenous Hormones and Breast Cancer Collaborative Group 2011; McPherson et al. 2000). Factors associated with elevated sex hormone levels include age at menarche, first pregnancy and menopause, parity, total lactation time, length of reproductive life, use of hormonal contraception and hormonal replacement therapy, BMI, smoking and alcohol consumption. There is now enough epidemiological evidence to demonstrate that exposure to higher level of endogenous and exogenous hormones increases the risk of breast cancer (Chen 2008). This evidence is more consistent for postmenopausal women (Kaaks et al. 2005b; Missmer et al. 2004; The Endogenous Hormones and Breast Cancer Collaborative Group 2002). Apart from circulating estrogens, these studies also observed an association of circulating androgens, such as testosterone, androstenedione and DHEA, with increased risk of breast cancer. This can be explained by the fact that androgens can be converted into estrone and estradiol by aromatase (Yager & Davidson 2006). On the other hand, for premenopausal women, epidemiological studies regarding this are inconsistent (Eliassen et al. 2006; Kaaks et al. 2005a). However, a collaborative reanalysis of seven prospective studies found that both estrogens and androgens were positively associated with the risk for breast cancer in premenopausal women (The Endogenous Hormones and Breast Cancer Collaborative Group 2013). This evidence regarding positive association of different hormones with increased breast cancer risk helped to form the hypothesis that environmental pollutants with estrogenic disruption potential might also be a risk factor for this cancer.
Endocrine-disrupting chemicals are defined by US Environmental Protection Agency [US EPA] as “an exogenous agent that interferes with synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood-borne hormones that are present in the body and are responsible for homeostasis, reproduction, and developmental process” (Knower et al. 2014). Different POPs show structural similarity with endogenous hormones and disrupt their function by competing with the binding of the same receptors (Bonefeld-Jørgensenet al. 2001). Moreover, these pollutants can trigger indirect hormonal response by binding with the aryl hydrocarbon receptor (Long and Bonefeld-Jørgensen 2012). They are also able to disrupt the endocrine system through non-receptor-mediated effects such as disrupting different hormone synthesis (Sanderson 2006). There are also action mechanisms other than hormonal disruption that might play an important role in carcinogenesis. There is growing evidence that POPs such as 2, 3, 7, 8-tetrachloridibenzo-p-dioxin (TCDD) and polychlorinated biphenyls (PCBs) are capable of influencing epigenomic landscape disruption in cancer (Knower et al. 2014). Some of these pollutants can also cause genotoxicity (Yáñez et al. 2004). Moreover, organochlorine pesticides are capable of inducing oxidative stress (Karami-Mohajeri and Abdollahi 2011). Above all, a number of recent studies observed positive association BC risk with PCB exposure when polymorphism in the CYP1A1 gene was considered (Moysich et al. 1999; Laden et al. 2002; Zhang et al. 2004; Li et al. 2005). PCB activated CYP1A1, which is involved in oestrogen metabolism and activation of procarcinogens such as polycyclic aromatic hydrocarbons (PAHs) (Bandiera et al. 1997; Moysich et al. 1999; Ghisari et al. 2014). Overall, a number of mechanisms might work behind BC occurrence due to exposure to POPs. However, the epidemiological evidence found in this systematic literature review is inconsistent.
We found two studies with significant positive association between BC risk and PCB, while the other six studies either observed no significant association or negative association. This result is consistent with other reviews undertaken for BC risk and PCB exposure (Negri et al. 2003; Brody et al. 2007). However, these two studies found positive association for heavy PCBs, which needs more investigation (Recio-Vega et al., 2011; Cohn et al., 2012). In addition, we found no study with evidence for modulation of association between BC risk and PCB exposure due to different genetic polymorphisms (Bräuner et al., 2014; Ghisari et al. 2014). A number of case-control studies published before 2006 observed higher BC risk due to PCB exposure in different genetic polymorphisms (Moysich et al. 1999; Laden et al. 2002; Zhang et al. 2004; Li et al. 2005). Therefore, the influence of genetic polymorphism on the association between PCB and BC risk requires further investigation with more epidemiological studies.
On the other hand, one case-control study found a significant negative association between total PCB and BC risk in univariate analysis (Rubin et al. 2006). The result did not remain significant with multivariate analysis. However, the controls of this study were more likely to be Eskimos (65 vs 35 %) and born in the south-western region of Alaska. Total PCB levels were highest among Eskimo women (8.57 ppb) compared to Aleut (5.61 ppb) and Indian women (2.96 ppb) and women born in south-western region (8.13 ppb) compared to north-western (7.60 ppb), south-central (4.29) and interior (5.21 ppb) regions of Alaska. Therefore, differences in ethnic distribution and birthplace between case and control could be a reason for negative association between PCB and BC risk. The association was no longer evident after adjusting for ethnicity, parity, family history of BC and triglyceride and cholesterol levels.
Though experimental studies showestrogenic properties of HCH (Steinmetz et al. 1996; Zou and Matsumura 2003), epidemiological results are conflicting. Mussalo-Rauhamaa et al. (1990) found a positive association between BC and β-HCH in 44 breast cancer patients. β and γ-HCH were found at a significantly higher level in 135 breast cancer patients in India (Mathur et al. 2002). γ-HCH was also found to be significantly associated with BC risk among postmenopausal women (OR = 1.76, 95 % CI: 1.04–2.98) in a case-control study in Spain (Ibarluzea et al. 2004). In contrast, other studies did not observe any significant association for γ-HCH (Dorgan et al. 1999) or β-HCH (Dewailly et al. 1994; Guttes et al. 1998; Høyer et al. 1998, 2000; Dorgan et al. 1999; Zheng et al. 1999b; Aronson et al. 2000; Lo’pez-Carrilloet al. 2002 and Raaschou-Nielsen et al. 2005). Demers et al. (2000) noticed breast cancer patients with increased β-HCH had large and aggressive cancer (tumour diameter ≥ 2 cm and axillary lymph node involvement). Results of epidemiological studies have been inconsistent in the last 10 years, except for a case-control study in Tunisia which observed a significant association between serum β-HCH level and increased BC risk (Arrebola et al. 2015).
Hexachlorobenzene (HCB) promotes mammary tumours in rats and has been shown to enhance tumour growth and metastasis (Randi et al. 2006; Pontillo et al. 2013). However, most of the studies found no positive association between HCB exposure and BC risk (Mussalo-Rauhamaa et al. 1990; Guttes et al. 1998; Moysich et al. 1998; Zheng et al. 1999a; Aronson et al. 2000; Høyer et al. 2001, Lo’pez-Carrillo et al. 2002, Pavuk et al. 2003; Raaschou-Nielsen et al. 2005). In contrast, Dorgan et al. (1999) observed a twofold increased risk for women in the upper three quartiles compared to the lowest quartile for women whose blood was collected within 2.7 years of diagnosis. Another case-control study observed significantly increased serum HCB levels among cases compared to controls (Charlier et al. 2004). No studies were found demonstrating significantly higher risk of BC due to HCB exposure in the last 10 years.
A number of studies calculated risk of BC associated with chlordane (Gammon et al. 2002; Raaschou-Nielsen et al. 2005), cis-nonachlor (Aronson et al. 2000; Raaschou-Nielsen et al. 2005) and trans-nonachlor (Demers et al. 2000; Aronson et al. 2000; Ward et al. 2000; Wolff et al. 2000; Zheng et al. 2000; Raaschou-Nielsen et al. 2005) or oxychlordane (Demers et al. 2000; Aronson et al. 2000; Ward et al. 2000; Zheng et al. 2000; Raaschou-Nielsen et al. 2005). However, none of these studies found a significantly increased risk associated with chlordane. Raaschou-Nielsen et al. (2005) observed an inverse association for trans-nonachlor and oxychlordane, consistent with the studies found in this review.
Cassidy et al. (2005) and Mathur et al. (2002) observed increased risk of BC for heptachlor epoxide and heptachlor, respectively; in contrast, Mussalo-Rauhamaa et al. (1990); Ward et al. (2000) and Dorgan et al. (1999) found no positive association. Arrebola et al. (2015) observed significant association in an unadjusted model, but borderline significant association in an adjusted model for association between heptachlor and BC risk.
A case-control study in Spain found a significant association of aldrin exposure and BC risk (Ibarluzea et al. 2004). When they were grouped according to menopausal status, the risk was only significant for postmenopausal women. In contrast, BC risk was non-significant and negatively associated for aldrin exposure in a case-control study; however, aldrin was detected only in three people (Ward et al. 2000).
Laboratory studies have demonstrated PBDEs’ estrogenic effect (Meerts et al. 2001; Mercado-Feliciano and Bigsby 2008), which strengthens the hypothesis that PBDE is a risk factor for hormonally related cancers such as BC. Human studies on BC risk due to PBDE exposure are limited. To our knowledge, there were no epidemiological studies published on this issue before 2006 (Brody et al. 2007). Recently, two case-control studies examined risk of BC and PBDE exposure (Hurley et al. 2011; Holmes et al. 2014) and one found a positive association for BDE-47.
Perfluorinated compounds have shown xenoestrogenic properties in MCF-7 breast cancer cells (Maras et al. 2006). However, epidemiological evidence is very limited. One cohort study did not observe any risk of BC among 32,254 residents of Mid-Ohio Valley exposed to perfluooctanoic acid in drinking water (Barry, Winquist and Steenland, 2013). There are also a number of cohort studies, which considered cancer mortality among workers exposed to different perfluorinated compounds and observed either no deaths or few deaths from breast cancer (Leonard et al. 2008; Lundin et al. 2009; Steenland and Woskie 2012). However, two studies found in this literature review showed positive association of BC risk with serum levels of perfluorooctane sulfonate (PFOS), total perfluorosulfonated acids (PFSA) (Bonefeld-Jorgensen et al. 2011) and perfluorooctanesulfonamide (PFOSA), but negative association for perfluorohexane sulfonate (PFHxS) (Bonefeld-Jorgensen et al. 2014). Another study found increase in BC risk associated with PFASs for different genetic polymorphisms (Ghisari et al. 2014). More epidemiological studies are required to confirm these preliminary results.
Experimental studies show varying evidence of toxicity and carcinogenicity due to dioxins; however, epidemiological evidence of human carcinogenicity is limited (Boffetta et al. 2011). Two case-control studies reported no increased risk of BC for TCDD; however, they both observed positive association for octachlorodibenzo-p-dioxin (OCDD) (Hardell et al. 1996; Reynolds et al. 2005). In contrast, an earlier follow-up of the Seveso Women’s Health Study (SWHS) observed a twofold increased BC risk for tenfold increase in serum TCDD levels (HR = 2.1, 95 % CI: 1.0–4.6, p = 0.05) (Warner et al. 2002). This association was not found to be significant in a recent follow-up study, which was reported above. This result is consistent with other cohort studies, which found no significant increased breast cancer incidence or mortality among the residents of this area (Bertazzi et al. 1993, 1997; Consonni et al. 2008; Pesatori et al. 2009).
Epidemiological evidence regarding breast cancer incidence due to these persistent pollutants is inconsistent. Most of the studies considered in this review are case-control studies, with too few breast cancer patients and inconclusive results. Moreover, case-control studies are more prone to biases than other analytical studies (Greenland Watson and Neutra 1981). Among these biases, selection bias is a common and significant issue in these types of studies (Mezei and Kheifets 2006). In this review, a number of articles have not always clearly reported the selection process (Arrebola et al. 2015) or response rate (Boada et al. 2012, Hurley et al. 2011). In addition, these studies showed wide variation in eligibility criteria, population selection, sample collection and laboratory procedures. Incomplete reporting of the selection process and response rate and inconsistent selection criteria make evaluation of potential selection bias and comparison of different studies difficult (Mezei and Kheifets 2006, Olson, Voigt, Begg and Weiss 2002). Apart from differences in target population and study design, there was a difference in selection of covariates and biological matrices used to measure exposure. Though most of the studies considered a large number of covariates, the possibility of missing or residual confounding cannot be ruled out completely. Moreover, studies used different biological matrices for environmental exposure. Most studies used serum level due to procedural and ethical complications regarding collection of adipose tissue. A recent study observed higher levels of heavy compounds such as PCBs in adipose tissue compared to that found in serum, but this was not the case for lighter compounds like HCB or p,p′-DDE (Artacho-Cordón et al. 2015), demonstrating the importance of choosing an appropriate biological matrix for the compounds being studied. In addition, Artacho-Cordón et al. (2015) observed that POPs in dissimilar biological matrices are affected differently by covariates. For example, they observed that recent weight loss was negatively associated with most of the pollutants in adipose tissue, but positively associated with PCB-138 concentrations in serum. These findings indicate that covariates need to be selected carefully, depending on both type of pollutant and biological matrix. Furthermore, most of the studies collected biological samples after diagnosis of breast cancer; there is growing controversy that biological samples collected after diagnosis or a few years before diagnosis do not reflect the body burden of environmental pollutants in early life. For breast cancer, exposure of environmental pollutants in early life poses more risk of disease compared to exposure after 40 years of age (Wolf and Weston 1997). Verner et al. (2008) have recently developed a physiologically based pharmacokinetic (PBPK) model to calculate the lifetime toxicokinetics of POPs. They evaluated the model by analysing thesuitability of blood PCB levels measured at the time of diagnosis of breast cancer in reflecting earlier PCB exposures (Verner et al. 2011). The results of this study suggest that PCB levels measured at the time of diagnosis was not able to reflect early life exposure, especially during the first decade of life.
Most of the epidemiological studies focused on individual chemical exposures rather than the combined effect of these chemicals (Kortenkamp 2006). Laboratory studies have shown that mixtures of weak xenoestrogenic compounds can exert a significant estrogenic effect when present at levels lower than their individual ‘no observed effect’ levels (Rajapakse, Silva and Kortenkamp 2002; Silva, Rajapakse and Kortenkamp 2002). Mixtures of organochlorines can also induce the proliferation of hormone-dependent breast cancer cell lines (Aubé, Larochelle and Ayotte 2011).A case-control study measuring total effective xenoestrogenic burden (TEXB-alpha) of environmental estrogens in adipose tissue for 260 cases and 352 controls noticed a positive association of TEXB-alpha with BC risk among women with lower BMI (Ibarluzea et al., 2004). One study found in this literature review measured variousmixtures of organochlorine pesticides and observed different mixtures in cases and controls (Boada et al. 2012). The compounds studied included aldrin, dieldrin, endrin, lindane and different isomers and metabolites of DDT. However, other POPs that were not examined could also be present and have potential effects on breast cancer risk. Simultaneous exposure of endogenous hormones and their interaction with environmental pollutants might also play an important role in carcinogenesis (Kortenkamp 2006). Hence, current epidemiological studies have a gap in the measurement of multiple chemicals and their interactions with each other and other endogenous hormones. Further, current epidemiological studies contain methodological flaws which may contribute to inconsistent results. Future studies need to address this gap by developing a better exposure method for cumulative effects of different chemicals.
Conclusion
Epidemiological studies published in the last 10 years could neither prove nor rule out the association between breast cancer risk and environmental exposure to persistent organic pollutants (other than DDT). However, most of the studies examined exposure to the pollutants after diagnosis of breast cancer, overlooking exposure during critical windows of vulnerability. The studies examined also largely focused on individual chemicals, ignoring the potential for combined effects chemicals. Therefore, major data gaps remain in examining exposure during critical windows of vulnerability and assessing combined effects of multiple chemicals. The possibility of potential interaction between environmental pollutants and endogenous and exogenous hormones also remains unaddressed. Development of better exposure assessment methods addressing these gaps is required for future research.
BC, breast cancer; BMI, body mass index; DDD, dichlorodiphenyldichloroethane; DDE, dichlorodiphenyldichloroethylene; DHEA, dehydroepiandrosterone; ER, oestrogen receptor; GM, geometric mean; HCB, hexachlorobenzene; HCH, hexachlorocyclohexane; HER2/neu, human epidermal growth factor receptor 2; HR, hazards ratio; LOD, limit of detection; OCDD, octachlorodibenzo-p-dioxin; OCP, organochlorine pesticide; OR, odds ratio; PAH, polycyclic aromatic hydrocarbon; PBDE, polybrominated diphenyl ether; PBPK, physiologically based pharmacokinetic; PCB: polychlorinated biphenyl; PCDD, polychlorinated dibenzo-p-dioxin; PCDF, polychlorinated dibenzofuran; PFAA, perfluoroalkyl acid; PFAS, perfluoroalkylated substances; PFBS, perfluorobutane sulfonate; PFC, perfluorinated contaminant; PFCA, perfluorocarboxylated acid; PFDA, perfluorodecanoic acid; PFDoA, perfluorododecanoic acid; PFPeA, perfluoro-n-pentanoic acid; PFHpA, perfluoroheptanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxA, perfluorohexanoic acid; PFHxS, pPerfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFOSA, perfluorooctane sulfonamide; PFOSF, perfluorooctane sulfonyl fluoride; PFSAs, perfluorosulfonated acids; PFTeA, perfluorotetradecanoic acid; PFTrA, perfluorotridecanoic acid; PFUnA, perfluoroundecanoic acid; POPs, persistent organic pollutants; p,p’-DDT, dichlorodiphenyltrichloroethane; p,p’-DDE, dichlorodiphenyl-dichloroethylene; PR, progesterone receptor; RR, risk ratio; TCDD, tetrachlorodibenzo-p-dioxin; TEXB, total effective xenoestrogenic burden; α-HCH, α-hexachlorocyclohexane; β-HCH, β-hexachlorocyclohexane; γ-HCH, γ-hexachlorocyclohexane.
References
Abelsohn A, GibsonBL SMD, Weir E (2002) Identifying and managing adverse environmental health effects: 5. Persistent organic pollutant. Can Med Assoc J 166(12):1549–1554
Andersen HR, Cook SJ, Waldbillig D (2002) Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol 179:1–12
Aronson KJ, Miller AB, Woolcott CG, et al. (2000) Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomark Prev 9:55–63
Arrebola JP, Belhassen H, Artacho-Cordón F, et al. (2015) Risk of female breast cancer and serum concentrations of organochlorine pesticides and polychlorinated biphenyls: a case-control study in Tunisia. Sci Total Environ 520:106–113. doi:10.1016/j.scitotenv.2015.03.045
Artacho-Cordón F, Fernández-Rodríguez M, Garde C (2015) Serum and adipose tissue as matrices for assessment of exposure to persistent organic pollutants in breast cancer patients. Environ Res 142:633–643
Aubé M, Larochelle C, Ayotte P (2011) Differential effects of a complex organochlorine mixture on the proliferation of breast cancer cell lines. Environ Res 111(3):337–347. doi:10.1016/j.envres.2011.01.010
Bandiera SM, Torok SM, Letcher RJ, Norstrom RJ (1997) Immunoquantification of cytochromes P450 1A and P450 2B and comparison with chlorinated hydrocarbon levels in archived polar bear liver samples. Chemosphere 34:1469–1479
Barry V, Winquist A, Steenland K (2013) Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 121(11–12):1313–1318. doi:10.1289/ehp.1306615
Bernstein L, Ross RK (1993) Endogenous hormones and breast cancer risk. Epidemiologic Review 5:48–65
Bertazzi A, Pesatori AC, Consonni D, Tironi A, Landi MT, Zocchetti C (1993) Cancer incidence in a population accidentally exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. Epidemiology 4:398–406
Bertazzi PA, Zocchetti C, Guercilena S, Consonni D, Tironi A, Landi MT, Pesatori AC (1997) Dioxin exposure and cancer risk: a 15-year mortality study after the “Seveso accident. Epidemiology 8:646–652
Boada LD, Zumbado M, Henríquez-Hernández LA, Almeida-González M, Alvarez-León EE, Serra-Majem L, Luzardo OP (2012) Complex organochlorine pesticide mixtures as determinant factor for breast cancer risk: a population-based case-control study in the Canary Islands (Spain). Environ Health 11:28. doi:10.1186/1476-069X-11-28
Boffetta P, Mundt KA, Adami HO, Cole P, Mandel JS (2011) TCDD and cancer: a critical review of epidemiologic studies. Crit Rev Toxicol 41(7):622–636. doi:10.3109/10408444.2011.560141
Bondy GS, Newsome WH, Armstrong CL, et al. (2000) Trans-nonachlor and cis-nonachlor toxicity in Sprague-Dawley rats: comparison with technical chlordane. Toxicological Science 58(2):386–398. doi:10.1093/toxsci/58.2.386
Bonefeld-Jorgensen EC, Long M, Bossi R, et al. (2011) Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: a case control study. Environ Health 10:88. doi:10.1186/1476-069X-10-88
Bonefeld-Jørgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM (2001) Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 158:141–153
Bonefeld-Jørgensen EC, Autrup H, Hansen JC (1997) Effect of toxaphene on estrogen receptor functions in human breast cancer cells. Carcinogenesis 18(8):1651–1654
Bonefeld-Jørgensen EC, Long M, Fredslund SO, Bossi R, Olsen J (2014) Breast cancer risk after exposure to perfluorinated compounds in Danish women: a case-control study nested in the Danish National Birth Cohort. Cancer Causes Control 25(11):1439–1448. doi:10.1007/s10552-014-0446-7
Bräuner EV, Loft S, Wellejus A, Autrup H, Tjønneland A, Raaschou-Nielsen O (2014) Adipose tissue PCB levels and CYP1B1 and COMT genotypes in relation to breast cancer risk in postmenopausal Danish women. Int J Environ Health Res 24(3):256–268. doi:10.1080/09603123.2013.809703
Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA (2007) Environmental pollutants and breast cancer. Cancer 109(12 Suppl):2667–2711. doi:10.1002/cncr.22655
Calle EE, Frumkin H, Henley SJ, Savitz DA, Thun MJ (2002) Organochlorines and breast cancer risk. CA Cancer J Clin 52:301–309. doi:10.3322/canjclin.52.5.301
Cassidy RA, Natarajan S, Vaughan GM (2005). The link between the insecticide heptachlor epoxide, estradiol, and breast cancer. Breast Cancer Res Treat 90(1):55–64
Charlier C, Foidart JM, Pitance F, Herman P, Gaspard U, Meurisse M, Plomteux G (2004) Environmental dichlorodiphenyltrichlorethane or hexachlorobenzene exposure and breast cancer: is there a risk?.Clinical. Chemistry and Laboratory Medicine 42(2):222–227
Chen WY (2008) Exogenous and endogenous hormones and breast cancer. Best Pract Res Clin Endocrinol Metab 22(4):573–585. doi:10.1016/j.beem.2008.08.001
Cohn BA, Terry MB, Plumb M, Cirillo PM (2012) Exposure to polychlorinated biphenyl (PCB) congeners measured shortly after giving birth and subsequent risk of maternal breast cancer before age 50. Breast Cancer Res Treat 136(1):267–275. doi:10.1007/s10549-012-2257-4
Consonni D, Pesatori AC, Zocchetti C, et al. (2008) Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. Am J Epidemiol 167(7):847–858
Damstra T (2002) Potential effects of certain persistent organic pollutants and endocrine disrupting chemicals on the health of children. Clin Toxicol 40(4):457–465
Demers A, Ayotte P, Brisson J, Dodin S, Robert J, Dewailly E (2000) Risk and aggressiveness of breast cancer in relation to plasma organochlorine concentrations. Cancer Epidemiol Biomark Prev 9:161–166
Dewailly E, Dodin S, Verreault R, Ayotte P, Sauvé L, Morin J, Brisson J (1994) High organochlorine body burden in women with estrogen receptor-positive breast cancer. J Natl Cancer Inst 86:232–234
Dorgan JF, Brock JW, Rothman N, et al. (1999) Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes Control 10(1):1–11
Eliassen AH, Missmer SA, Tworoger SS, et al. (2006) Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. JNCI: Journal of the National Cancer Institute 98(19):1406–1415. doi:10.1093/jnci/djj376
El-Shahawi MS, Hamzaa A, Bashammakhb AS, Al-Saggaf WT (2010) An overview on the accumulation, distribution, transformations, toxicity andanalytical methods for the monitoring of persistent organic pollutants. Talanta 80(5):1587–1597. doi:10.1016/j.talanta.2009.09.055
Gammon MD, Wolff MS, Neugut AI, et al. (2002) Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomark Prev 11(8):686–697
Gatto NM, Longnecker MP, Press MF, Sullivan-Halley J, McKean-Cowdin R, Bernstein L (2007) Serum organochlorines and breast cancer: a case-control study among African-American women. Cancer Causes Control 18(1):29–39
Ghisari M, Eiberg H, Long M, Bonefeld-Jørgensen EC (2014) Polymorphisms in phase I and phase II genes and breast cancer risk and relations to persistent organic pollutant exposure: a case-control study in Inuit women. Environ Health 13(1):19. doi:10.1186/1476-069X-13-19
Golden R, Kimbrough R (2009) Weight of evidence evaluation of potential human cancer risks from exposure to polychlorinated biphenyls: an update based on studies published since 2003. Crit Rev Toxicol 39(4):299–331. doi:10.1080/10408440802291521
Greenland S, Watson E, Neutra RR (1981) The case control method in medical care evaluation. Med Care 19(8):872–878
Guttes S, Failing K, Neuman K, Kleinstein J, Georgii S, Brunn H (1998) Chlororganic pesticides and polychlorinated biphenyls in breast tissue of women with benign and malignant breast disease. Arch Environ Contam Toxicol 35(1):140–147
Hardell L, Lindstrom G, Liljegren G, Dahl P, Magnuson A (1996) Increased concentrations of octachlorodibenzo-p-dioxin in cases with breast cancer—results from a case-control study. Eur J Cancer Prev 5(5):351–357
Holmes AK, Koller KR, Kieszak SM, et al. (2014) Case-control study of breast cancer and exposure to synthetic environmental chemicals among Alaska native women. International Journal of Circumpolar Health 73:25760. doi:10.3402/ijch.v73.25760
Høyer AP, Jørgensen T, Brock JW, Grandjean P (1998) Organochlorine exposure and risk of breast cancer. Lancet 352(9143):1816–1820
Høyer AP, Jørgensen T, Brock JW, Grandjean P (2000) Organochlorine exposure and breast cancer survival. J Clin Epidemiol 53(3):323–330
Høyer AP, Jørgensen T, Rank F, Grandjean P (2001) Organochlorine exposures influence on breast cancer risk and survival according to estrogen receptor status: a Danish cohort nested case-control study. BMC Cancer 1:8
Hurley S, Reynolds P, Goldberg D, Nelson DO, Jeffrey SS, Petreas M (2011) Adipose levels of polybrominated diphenyl ethers and risk of breast cancer. Breast Cancer Res Treat 29(2):505–511. doi:10.1007/s10549-011-1481-7
Ibarluzea JM, Fernandez MF, Santa-Marina L, et al. (2004) Breast cancer risk and the combined effect of environmental estrogens. Cancer Causes Control 15(6):591–600
Itoh H, Iwasaki M, Hanaoka T, et al. (2009) Serum organochlorines and breast cancer risk in Japanese women: a case-control study. Cancer causes and controls 20(5):567–580. doi:10.1007/s10552-008-9265-z
Iwasaki M, Inoue M, Sasazuki S, et al. (2008) Plasma organochlorine levels and subsequent risk of breast cancer among Japanese women: a nested case-control study. Sci Total Environ 402(2–3):176–183. doi:10.1016/j.scitotenv.2008.05.009
Kaaks R, Rinaldi S, Key TJ (2005a) Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocrine-Related Cancer 12(4):1071–1082. doi:10.1677/erc.1.01038
Kaaks R, Berrino F, Key T, et al. (2005b) Serum sex steroids in premenopausal women and breast cancer risk within the European prospective investigation into cancer and nutrition (EPIC). JNCI: Journal of the National Cancer Institute 97(10):755–765. doi:10.1093/jnci/dji132
Karami-Mohajeri S, Abdollahi M (2011) Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Human & experimental toxicology 30(9):1119–1140. doi:10.1177/0960327110388959
Khanjani N, Hoving JL, Forbes AB, Sim MR (2007) Systematic review and meta-analysis of Cyclodiene insecticides and breast cancer. Journal of Environmental Science and Health 25:23–52. doi:10.1080/10590500701201711
Knower KC, To SQ, Leung Y, Ho S, Clyne CD (2014) Endocrine disruption of the epigenome: a breast cancer link. Endocrine Related Cancer 21(2):T33–T55. doi:10.1530/ERC-13-0513
Kortenkamp A (2006) Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. Int J Androl 29(1):193–198
Laden F, Ishibe N, Hankinson SE, et al. (2002) Polychlorinated biphenyls, cytochrome P450 1 A1, and breast cancer risk in the nurses’ health study. Cancer Epidemiol Biomark Prev 11(12):1560–1565
Leonard RC, Kreckmann KH, Sakr CJ, Symons JM (2008) Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol 18(1):15–22
Li QQ, Loganath A, Chong YS, Tan J, Obbard JP (2006) Persistent organic pollutants and adverse health effects in humans. J Toxicol Environ Health 69(21):1987–2005. doi:10.1080/15287390600751447
Li Y, Millikan RC, Bell DA, Cui L, Tse CK, Newman B, Conway K (2005) Polychlorinated biphenyls, cytochrome P450 1 A1 (CYP1A1) polymorphisms, and breast cancer risk among African American women and white women in North Carolina: a population-based case-control study. Breast Cancer Res 7(1):R12–R18
Long M, Bonefeld-Jørgensen EC (2012) Dioxin-like activity in environmental and human samples from Greenland and Denmark. Chemosphere 89:919–928
López-Carrillo L, López-Cervantes M, Torres-Sánchez L, Blair A, Cebrián ME, García RM (2002) Serum levels of beta-hexachlorocyclohexane, hexachlorobenzene and polychlorinated biphenyls and breast cancer in Mexican women. Eur J Cancer Prev 11:129–135
Lundin JI, Alexander BH, Olsen GW, Church TR (2009) Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 20(6):921–928. doi:10.1097/EDE.0b013e3181b5f395
Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN (1995) Proportion of breast cancer cases in theUnited states explained by well-established risk factors. JNCI:Journal of the National Cancer Institute 87(22):1681–1685. doi:10.1093/jnci/87.22.168
Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, Blust R, De Coen W (2006) Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation. Environ Health Perspect 114(1):100–105
Mathur V, Bhatnagar P, Sharma RG, Acharya V, Sexana R (2002) Breast cancer incidence and exposure to pesticides among women originating from Jaipur. Environ Int 28:331–336
McPherson K, Steel CM, Dixon JM (2000) Breast cancer—epidemiology, risk factors, and genetics. Br Med J 321(7261):624–628
Meerts IA, Letcher RJ, Hoving S, et al. (2001) In vitro estrogenicity of polybrominated diphenyl ethers, Hydroxylated PBDEs, and polybrominated Bisphenol a compounds. Environ Health Perspect 109:399–407
Mercado-Feliciano M, Bigsby RM (2008) The polybrominated diphenyl ether mixture DE-71 is mildly estrogenic. Environ Health Perspect 116:605–611
Mezei G, Kheifets L (2006) Selection bias and its implications for case–control studies: a case study of magnetic field exposure and childhood leukaemia. Int J Epidemiol 35(2):397–406. doi:10.1093/ije/dyi245
Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE (2004) Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women.JNCI:journal of the. National Cancer Institute 96(24):1856–1865. doi:10.1093/jnci/djh336
Mitra AK, Faruque FS, Avis AL (2004) Breast cancer and environmental risks: where is the link? J Environ Health 66(7):24–32
Moysich KB, Ambrosone CB, Vena JE, et al. (1998) Environmental Organochiorine exposure and postmenopausal breast cancer risk. Cancer Epidemiol Biomark Prev 7:181–188
Moysich KB, Shields PG, Freudenheim JL, et al. (1999) Polychlorinated biphenyls, cytochrome P4501A1 polymorphism, and postmenopausal breast cancer risk. Cancer Epidemiol Biomark Prev 8:41–44
Mussalo-Rauhamaa HM, Hasanen E, Pyysalo H, Antervo K, Kauppila R, Pantzar P (1990) Occurrence of beta-hexachlorocyclehexane in breast cancer patients. Cancer 66:2124–2128
Negri E, Bosetti C, Fattore E, La Vecchia C (2003) Environmental exposure to polychlorinated biphenyls (PCBs) and breast cancer: a systematic review of the epidemiological evidence. Eur J Cancer Prev 12(6):509–516. doi:10.1097/01.cej.0000102559.94285.c9
Olson SH, Voigt LF, Begg CB, Weiss NS (2002) Reporting participation in case-control studies. Epidemiology 13(2):123–126
Park JH, Cha ES, Ko Y, Hwang MS, Hong JH, Lee WJ (2014) Exposure to dichlorodiphenyltrichloroethane and the risk of breast cancer: a systematic review and meta-analysis. Osong Public Health and Research Perspectives 5(2):77–84. doi:10.1016/j.phrp.2014.02.001
Pavuk M, Cerhan JR, Lynch CF, Kocan A, Petrik J, Chovancova J (2003) Case-control study of PCBs, other organochlorines and breast cancer in eastern Slovakia. J Expo Anal Environ Epidemiol 13(4):267–275
Pontillo CA, Rojas P, Chiappini F, et al. (2013) Action of hexachlorobenzene on tumor growth and metastasis in different experimental models. Toxicol Appl Pharmacol 268(3):331–342
Raaschou-Nielsen O, Pavuk M, et al. (2005) Adipose organochlorine concentrations and risk of breast cancer among postmenopausal Danish women. Cancer Epidemiol Biomark Prev 14(1):67–74
Rajapakse N, Silva E, Kortenkamp A (2002) Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environmental Health Perspective 110(9):917–921
Randi S, Cocca C, Carbone V, et al. (2006) Hexachlorobenzene is a tumor co-carcinogen and induces alterations in IGFs signaling pathway in the rat mammary gland. Toxicol Sci 89:83–92
Recio-Vega R, Velazco-Rodriguez V, Ocampo-Gómez G, Hernandez-Gonzalez S, Ruiz-Flores P, Lopez-Marquez F (2011) Serum levels of polychlorinated biphenyls in Mexican women and breast cancer risk. J Appl Toxicol 31(3):270–278. doi:10.1002/jat.1672
Reynolds P, Hurley SE, Petreas M, et al. (2005) Adipose levels of dioxins and risk of breast cancer. Cancer Causes Control 16(5):525–535
Rubin CH, Lanier A, Kieszak S, et al. (2006) Breast cancer among Alaska native women potentially exposed to environmental organochlorine chemicals. International journal of circumpolar health 65(1):18–27
Sanderson JT (2006) The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci 94(1):3–21
Silva E, Rajapakse N, Kortenkamp A (2002) Something from "nothing"—eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environmental science & technology 36(8):1751–1756
Soto AM, Chung KL, Sonnenschein C (1994) The pesticides endosulfan; toxaphene; and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environmental Health Perspect 102(4):380–383
Steenland K, Woskie S (2012) Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol 176(10):909–917. doi:10.1093/aje/kws171
Steinmetz R, Young PC, Caperell-Grant A, et al. (1996) Novel estrogenic action of the pesticide residue beta-hexachlorocyclohexane in human breast cancer cells. Cancer Res 56(23):5403–5409
Stockholm Convention (n.d.). Stockholm Convention Web http://chm.pops.int
The Endogenous Hormones and Breast Cancer Collaborative Group (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. JNCI:Journal of the National Cancer Institute 94(8):606–616. doi:10.1093/jnci/94.8.606
The Endogenous Hormones and Breast Cancer Collaborative Group (2011) Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer 105(5):709–722. doi:10.1038/bjc.2011.254
The Endogenous Hormones and Breast Cancer Collaborative Group (2013) Sex hormones and breast cancer risk in premenopausal women: collaborative reanalysis of seven prospective studies. Lancet Oncol 14(10):1009–1019. doi:10.1016/S1470-2045(13)70301-2
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012.CA. A Cancer Journal for Clinicians 65(2):87–108. doi:10.3322/caac.21262
Verner MA, Bachelet D, McDougall R, Charbonneau M, Guenel P, Haddad S (2011) A case study addressing the reliability of polychlorinated biphenyl levels measured at the time of breast cancer diagnosis in representing early-life exposure. Cancer Epidemiol Biomark Prev 20:281–286. doi:10.1158/1055-9965.EPI-10-0992
Verner MA, Charbonneau M, López-Carrillo L, Haddad S (2008) Physiologically based pharmacokinetic modeling of persistent organic pollutants for lifetime exposure assessment: a new tool in breast cancer epidemiologic studies. Environ Health Perspect 116(7):886–892. doi:10.1289/ehp.10917
Ward EM, Schulte P, Grajewski B, Andersen A, et al. (2000) Serum organochlorine levels and breast cancer: a nested case-control study of Norwegian women. Cancer Epidemiol Biomark Prev 9:1357–1367
Warner M, Eskenazi B, Mocarelli P, et al. (2002) Serum dioxin concentrations and breast cancer risk in the Seveso Women’s health study. Environ Health Perspect 110(7):625–628
Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B (2011) Dioxin exposure and cancer risk in the Seveso Women’s health study. Environ Health Perspect 119(12):1700–1705. doi:10.1289/ehp.1103720
Wolff MS, Toniolo PG (1995) Environmental organochlorine exposure as a potential etiologic factor in breast cancer. Environ Health Perspect 103(7):141–5
Wolff MS, Weston A (1997) Breast cancer risk and environmental exposures. Environ Health Perspect 105(Suppl 4):891–896
Wolff MS, Berkowitz GS, Brower S (2000) Organochlorine exposures and breast cancer risk in New York City women. Environ Res 84(92):151–161. doi:10.1006/enrs.2000.4075
World Health Organization (WHO) (2010) Persistent organic pollutants: Impact on child health. World Health Organization Web. http://whqlibdoc.who.int/publications/2010/9789241501101_eng.pdf?ua=1
Yager JD, Davidson NE (2006) Mechanisms of disease: estrogen carcinogenesis in breast cancer. N Engl J Med 354(3):270–282
Yáñez L, Borja-Aburto VH, Rojas E, et al. (2004) DDT induces DNA damage in blood cells. Studies in vitro and in women chronically exposed to this insecticide. Environ Res 94(1):18–24
Zhang Y, Wise JP, Holford TR (2004) Serum polychlorinated biphenyls, cytochrome P-450 1 A1 polymorphisms, and risk of breast cancer in Connecticut women. Am J Epidemiol 160(12):1177–1183
Zheng T, Holford TR, Mayne ST (1999a) Environmental exposure to hexachlorobenzene (HCB) and risk of female breast cancer in Connecticut. Cancer Epidemiol Biomark Prev 8(5):407–411
Zheng T, Holford TR, Mayne ST (1999b) B-Benzenehexachloride in breast adipose tissue and riskof breast carcinoma. Cancer 85(10):2212–2218
Zheng T, Holford TR, Tessari J, et al. (2000) Oxychlordane and trans-nonachlor in breast adipose tissue and risk of female breast cancer. Journal of epidemiology and biostatistics 5(3):153–160
Zou E, Matsumura F (2003) Long-term exposure to b-hexachlorocyclohexane (b-HCH) promotes transformation and invasiveness of MCF-7 human breast cancer cells. Biochem Pharmacol 66(5):831–840
Acknowledgments
LMLT is partly funded by an ARC DECRA (DE120100161).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 65 kb)
Rights and permissions
About this article
Cite this article
Mouly, T.A., Toms, LM.L. Breast cancer and persistent organic pollutants (excluding DDT): a systematic literature review. Environ Sci Pollut Res 23, 22385–22407 (2016). https://doi.org/10.1007/s11356-016-7577-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7577-1