Abstract
This population-based case–control study of African-American women (355 breast cancer case patients, 327 controls) examined the association between breast cancer and circulating levels of PCBs and dichlorodiphenyldichloroethene (DDE), a metabolite of DDT. Case patients were diagnosed with invasive breast carcinoma and interviewed between June 1995 and July 1998, and control subjects were identified by random digit dialing methods. Serum levels of DDE and total PCBs were adjusted for total lipid content. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable unconditional logistic regression methods. Effect modification by tumor receptor status and cancer treatment was investigated. Breast cancer risk was not associated with increasing quintiles of lipid-adjusted PCBs or DDE (highest versus lowest quintile adjusted for age, body mass index (BMI) and breastfeeding for DDE: OR = 1.02, 95% CI = (0.61, 1.72), p-trend = 0.74; for PCBs: OR = 1.01, 95% CI = (0.63, 1.63), p-trend = 0.56). Risk did not differ by strata of BMI, breastfeeding, parity, menopausal status or tumor receptor status. This study, the largest study of African-American women to date, does not support a role of DDE and total PCBs in breast cancer risk at the levels measured.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequently diagnosed cancer and the second leading cause of cancer deaths among women in the United States [1]. Reproductive histories that maximize the total number of years women are exposed to endogenous or exogenous sex hormones, such as early age at menarche, late age at first full-term pregnancy, late age at menopause, and use of hormone replacement therapy (HT) are known to increase risk [2–4]. While environmental exposure to synthetic chemicals such as organochlorines has not been established as a breast cancer risk factor in women, evidence from experimental studies demonstrating that organochlorines have weak estrogenic activity as well as carcinogenic effects has prompted consideration of their role in breast cancer [5].
Organochlorines comprise a diverse group of synthetic chemicals whose use became widespread in the United States beginning in the 1950s. The organochlorine residues present at the highest concentrations in human adipose tissue, breast milk and blood are dichlorodiphenyldichloroethene (DDE), the metabolite of the pesticide dichlorodiphenyltrichloroethane (DDT), and the non-pesticide class of polychlorinated biphenyls (PCBs) [6]. Extensive evidence exists from animal studies of the carcinogenicity of DDT, DDE and PCBs [7]. Exposure to DDT and PCBs in mice has each resulted in an increased incidence of cancers of the liver and lung, as well as of lymphoma and leukemia [6, 8, 9]. Experimental studies have also shown that DDT and some PCBs have estrogenic properties while other PCBs are antiestrogenic [5]. Several epidemiologic studies since the 1970s have examined the association between breast cancer in women and organochlorines measured either in the serum or adipose tissue. A number of these studies have been null, but others have had mixed results or findings limited to subgroups of the populations studied [10–28]. While DDT and PCBs were banned in the US in the 1970s, residues of DDT and PCBs continue to be detected in the blood of most Americans as they are persistent in the environment, are known to accumulate in tissue including the fatty tissue of the breast, and have long half lives [29–31]. Compared with Caucasians, African-Americans have higher levels of serum DDT compounds [32, 33]; in addition, their PCB levels have been higher than whites in several studies [16, 22]. Therefore they are potentially at a greater risk of associated adverse health affects.
Given the possibility of higher organochlorine exposure among African-Americans together with the limitations of previous studies to examine this population while adequately controlling for confounding and examining effect modification, we conducted a substudy within the Los Angeles component of the Women’s Contraceptive and Reproductive Experiences (CARE) Study, a population based case–control study of lifestyle factors and breast cancer risk, to determine whether serum levels of DDE and PCBs are associated with risk of breast cancer in African-American women.
Methods
Study population
Subjects for this study were identified from among participants in a larger study, the Women’s CARE Study, which has been described in detail previously [34]. Briefly, the Women’s CARE Study was a multi-center population-based case–control study of US born English-speaking African-American and Caucasian women aged 35–64 years in five US areas (Atlanta, GA; Seattle, WA; Detroit, MI; Philadelphia, PA; and Los Angeles, CA) designed to examine reproductive health and lifestyle risk factors for breast cancer. Specifically, in Los Angeles, case patients were identified by the Cancer Surveillance Program (CSP) of Los Angeles County, a population-based cancer registry established in 1970 that joined the National Cancer Institute’s Surveillance Epidemiology and End Results registry program in 1992.
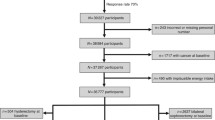
The Los Angeles Women’s CARE Study population comprised 1,255 case patients who were an age-stratified random sample of African-American and Caucasian women ages 35–64 years, with histologically confirmed invasive breast cancer, diagnosed from July 1994 through April 1998; and 1,242 control subjects who were identified through random digit dialing (RDD) using the Mitofsky–Waksberg method [35]. The control subjects were frequency matched to the age (within a 5-year range) and racial distribution of the case patients. Control subjects were eligible if they had not had a diagnosis of either in situ or invasive breast cancer. African-American case patients were oversampled to maximize their numbers in the study. The response rate for African- American case patients in the Los Angeles Center of the Women’s CARE Study was 71.9% [36]. The screening response rate was 82% for control households in the RDD process. This includes phone numbers presumed to be residential where we were unable to make contact with a person or an answering machine. Among women selected from screened households, the interview response rate was 70.6%. The estimated overall response rate for African American control subjects in the Women’s CARE Study in Los Angeles was 57.9%.
Beginning with women interviewed for the Women’s CARE Study in June 1995 and continuing through July 1998, all African-American participants residing in Los Angeles County were asked to provide a blood sample for assessment of DDE and PCBs. In total, 451 breast cancer case patients and 427 control subjects were approached. Among breast cancer patients, 381 provided a blood sample (participation rate = 84.5%), 56 refused to provide a blood sample, and 14 others were not pursued or were unable to provide a sample before the end of the study. Among control subjects, 335 provided a blood sample (participation rate = 78.5%), 82 refused to provide a blood sample, and 10 others were not pursued or were unable to provide a sample before the end of the study. Some breast cancer case patients who participated in this substudy were later excluded from the Women’s CARE Study by the coordinating center at the Centers for Disease Controls and hence, were also excluded from this substudy. One woman was not considered African-American under the rules established for the Women’s CARE Study since her mother was half African-American and half Indian, and her father was white. Fourteen case patients were excluded because the breast cancer was not histologically confirmed (n = 5), the interview reference date was more than 2 months from the date of the breast biopsy used for diagnosis (n = 3), the patient had a histologic type of breast cancer that was not considered eligible for the Women’s CARE Study (n = 5), or some other reason (n = 1). This resulted in 366 eligible breast cancer case patients for the organochlorine substudy. In addition, 11 case patients and eight controls were excluded from analyses because results from lipid assays could not be obtained for these subjects. This substudy, therefore, is based on 355 case patients and 327 control subjects. One additional case patient and one control subject were missing data on PCB levels and thus were not included in analyses of this analyte.
Data collection and blood sample
Participants in the Women’s CARE Study were interviewed using a structured questionnaire, which contained sections on demographics, reproductive history (including breastfeeding), medical history (including hormone use and body size), family history of malignancy, and several lifestyle factors (including smoking, alcohol consumption and physical activity). Information was recorded up to the date of diagnosis (month and year) for case patients and up to the date of initial household contact by RDD for control subjects. Women agreeing to participate in the organochlorine study had 20 ml of blood drawn and were asked a brief set of questions at the time of their blood draw regarding cancer treatments and surgeries (if a case patient) as well as recent weight history. Written informed consent was obtained from all study participants for participation in both the Women’s CARE Study and the organochlorine substudy.
Laboratory methods
Serum organochlorines
Serum levels of DDE and PCBs were assayed at Pacific Toxicology Laboratories (Los Angeles, California) using a protocol previously described [37]. The serum (2 ml) analyzed for DDE was extracted with hexane for 2 h, and the extract was concentrated via nitrogen evaporation [38]. The extract was analyzed with an electron-capture gas chromatograph equipped with a narrow bore fused silica column (RSL-300; 30 m, 0.25 mm, 0.25 μm thickness (Alltech Associates, Deerfield, Illinois), and Aldrin was used as the internal standard. The detection limit was 0.2 μg/l. The between-assay coefficient of variation (CV) was 10.9% at 20 μg/l and 10.1% at 10 μg/l. Recoveries ranged from 90% to 105%.
Following protein precipitation with methanol, the serum (2 ml) analyzed for PCBs was extracted with a mixed solvent (i.e., hexane and ethyl ether, 1:1) [39]. The silica gel column was cleaned, and the extract was then analyzed by an electron-capture gas chromatograph equipped with a megabore fused silica column (DB-1; 15 m, 0.53 mm internal diameter, 1-μm thick (J&W Scientific, Folsom, California). The PCBs represented by 4–16 chromatographic peaks were quantitated with the Webb–McCall method using Aroclors 1242 and 1260 with decachlorobiphenyl as an internal standard and reported as total PCBs [40]. This method of quantitating total PCBs gives values that are slightly higher, e.g., 25%, than those estimated with congener-specific methods [41]. The detection limit was 2.0 μg/l. The between-assay CV for the PCB analysis was 12.2% at 14 μg/l and 10.7% at 80 μg/l. Recoveries averaged 80%.
All subjects had levels of DDE that were above the limit of detection of the assay. A total of 145 case patients and 138 controls had no detectable PCBs; 11 case patients and six control subjects had PCB levels at the lower detectable limit of the assay (2.0 μg/l). Subjects with levels of PCBs below the limit of detection, i.e., no detectable PCBs, were categorized as “below detectable limit” (BDL) and were assigned a value of zero in analyses of this organochlorine.
Serum lipids
Measurements of the lipid components in the serum including cholesterol, triglycerides, free cholesterol and phospholipids were obtained in order to examine organochlorine levels relative to the amount of lipid in serum. Serum lipid assays were performed at Northwest Lipids Research Laboratories (Seattle, Washington) using enzymatic methods [42–45]. Three sets of quality control material were used to monitor the performance of cholesterol, triglycerides, free cholesterol and phospholipids. For assay runs to be accepted, control values must have fallen within 2.5 standard deviations of the established target mean for each pool. The between-assay CVs were ≤1.4% for total cholesterol; ≤2% for triglycerides; 5.2% for free cholesterol; and 2.9% for phospholipids.
Lipid-adjusted organochlorine levels were calculated by dividing serum levels of organochlorines by the total amount of lipids in the serum, and were expressed per gram of total serum lipid. Total serum lipids were estimated using the using the equation described by Phillips et al. [46].
Tumor characteristics
Immunohistochemical assays were performed in the laboratory of Michael Press, M.D., Ph.D. (Los Angeles, CA), to assess estrogen receptor (ER) and progesterone receptor (PR) status as well as HER-2/neu and p53 overexpression in paraffin-embedded tumor sections for 251 case patients (70.7%) for whom specimens were available. Immunohistochemistry for ER, PR and p53 was carried out as described elsewhere in detail [47–51]. Positive and negative controls (tumors with known ER/PR and p53 status) were included in each batch of staining. Only tumors that showed >10% nuclear reactivity were considered positive for ER, PR, or p53.
Immunohistochemical staining for HER-2/neu was performed as described elsewhere [52, 53] with a USC-developed 10H8 monoclonal antibody [54] that does not require antigen retrieval and has been shown to be more sensitive and accurate than commercially available, FDA-approved anti-HER2 antibody staining kits [52]. For interpretation of staining patterns using 10H8 antibody, 80% of breast cancer case patients with 2+ membrane staining of tumor cells and 99% of 3+ membrane staining have been shown to demonstrate HER-2 gene amplification [55]. Therefore, breast cancers with either 2+ or 3+ membrane staining on malignant cells were considered as positive for overexpression of HER-2/neu. All immunohistochemistry slides were examined and scored by laboratory personnel without knowledge of clinical characteristics of the case patients.
To minimize missing information on ER and PR, we were able to abstract data from their pathology reports, which are maintained by the CSP. Overall, we had ER status for 338 case patients and PR status for 333 case patients. p53 and Her-2/neu status were available for 251 case patients.
Statistical analyses
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable unconditional logistic regression while controlling for age categorized into 10-year intervals (35–44, 45–54 55–64 years). Potential confounders considered for these analyses included body mass index (BMI) (≤24.9, 24.91–28.94, 28.95–33.65, >33.65 kg/m2), lifetime physical activity (0 h/week, >0–3 h/week, > 3 h/week), breastfeeding [continuous variable for total number of weeks breastfed; and categorical variable with categories (breastfed/parous, never breastfed/parous, nulliparous)], total number of full-term pregnancies (continuous variable for completed pregnancies >26 weeks), family history of breast cancer in a mother, sister or daughter (first degree family history: yes/no), education (less than high school graduate, high school graduate, some college or technical school, college graduate) and menopausal status (pre-/post-menopausal). Cut points of continuous variables were based on the distribution of the variable among controls. BMI was obtained at the time of the subject’s blood draw, whereas all other potential confounders refer to the time period prior to the diagnosis date for case patients or prior to the date of first household contact in the RDD process for control subjects. The definition of menopausal status for the Women’s CARE Study has been described elsewhere [36]. Briefly, women were considered postmenopausal if their menstrual periods stopped naturally more than 12 months prior to the reference date or their periods ended because they had removal of both ovaries. Menopausal status was unknown if the participant had a hysterectomy prior to natural menopause with at least part of an ovary intact or began using hormones prior to her last menstrual period or during the 12 month interval following her last menstrual period. We considered a woman to be perimenopausal if her periods had stopped naturally within 12 months of her reference date and she had not taken hormone therapy. Each potential confounder was included in a model with the categorical classifications of the organochlorine and age variables and compared to a model with the organochlorine and age variables only to determine the effect of inclusion of the variable on beta coefficients. None of the potential confounders changed the age-adjusted beta coefficients by more than 15%. However, given the evidence in the literature that both BMI and breastfeeding influence organochlorine levels [6, 7] both were retained in the logistic regression models with BMI modeled in categories and breast feeding duration modeled as a continuous variable (in weeks).
The relationship between serum organochlorine levels and breast cancer was examined using quintile categories of the lipid-adjusted values of PCBs and DDE for main analyses and tertile categories for subgroup analyses. These categories were based on the distribution of lipid-adjusted organochlorine serum levels in the control subjects.
Stratified analyses were conducted to estimate separate ORs and 95% CIs for tertiles of organochlorines within categories of BMI [low (≤28.95 kg/m2) versus high (>28.95 kg/m2)], parity (parous and breastfed, parous and never breastfed, nulliparous), duration of breastfeeding (long-term: ≥26 weeks, short-term: 1–25 weeks); and menopausal status (pre-menopausal versus post-menopausal with perimenopausal women and those with unknown menopausal status excluded). A separate analysis stratified by breastfeeding compared long-term breastfeeding (≥26 weeks) to a second definition of short-term breastfeeding, which also included women who had never breastfed (0–25 weeks).
Results of immunohistochemistry for ER and PR status were available for 71% of case patients (n = 252) from the Press laboratory, and 86% (n = 305) and 74% (n = 263), respectively, for ER and PR status of case patients from the CSP SEER registry data. In the interest of maintaining consistency with a single laboratory performing all immunohistochemistry assays while minimizing missing values, the Press laboratory data were used as the primary source to assign ER and PR status, and when missing, data from the CSP were used. Among those with both CSP and Press laboratory values, there was an 86.4% concordance for ER status and an 80.0% concordance for PR status. Analyses using the Press laboratory immunohistochemistry data alone did not change the results obtained when supplementing the laboratory’s data with those from the CSP.
Analyses comparing case patients and controls were performed for each tumor marker (ER, PR, p53 and HER-2/neu) and according to whether the woman had been treated with chemotherapy.
Assessments of effect modification by tumor marker and chemotherapy were conducted. Wald tests and 95% confidence intervals for the OR were used to determine whether an interaction was statistically significant.
All analyses were performed using SAS version 9.0 (SAS Institute Inc., Cary, NC, USA.).
Results
Demographic, lifestyle and reproductive characteristics of study participants are summarized in Table 1. A substantial proportion of study participants were obese (44.9%), and the majority had never breastfed (56.9%). Case patients were, on average, 1.5 years younger than control subjects. Case patients were slightly more likely to have had a first-degree relative with breast cancer (10.4% of case patients reporting a positive family history versus 7.3% of controls). Case patients had significantly greater mean levels of serum DDE than control subjects prior to adjustment for total lipids (p = 0.03, Table 2). However, after lipid adjustment neither PCBs levels nor DDE levels differed between case patients and control subjects.
Breast cancer risk was not associated with serum DDE (OR comparing highest versus lowest exposure quintile = 1.02, 95% CI = 0.61, 1.72, p-trend = 0.74), or PCBs (OR comparing > 60 μg/l to BDL = 1.01, 95% CI = 0.63, 1.63, p-trend = 0.56) (Table 3). We did not observe any effect modification of the impact of PCB or DDE levels by BMI, parity, breastfeeding, or menopausal status (data not shown).
Neither PCBs nor DDE was associated with an increase in the risk of any subtype of breast cancer defined by PR, p53, or HER-2/neu status (Table 4). Among women who did not receive chemotherapy, those with PCB levels above 0.47 μg/l had an increased odds of breast cancer relative to women with PCB levels below the detectable limit of the assay (OR = 1.89, 95% CI = 1.06–3.37, p-trend = 0.03, p-interaction comparing those treated with chemotherapy to those not treated with chemotherapy = 0.08). ER status did not modify the results for PCBs; however, we did observe a statistically significant interaction between ER status and DDE levels, with relative odds for ER-positive breast cancer somewhat elevated and increasing with higher levels of DDE, whereas those for ER-negative tumors were less than 1.0. However, neither the result for ER-positive tumors nor the result for ER-negative tumors was statistically significant.
Discussion
In this population-based case–control study of African-American women we found no evidence that breast cancer risk was associated with PCBs or DDE at the levels measured in serum, overall, or by ER, PR, HER-2/neu, or p53 status. There was some suggestion that ER positive case patients had elevations in risk with increasing tertiles of lipid-adjusted serum DDE, although these increases were not statistically significant. Among case patients who had not been treated with chemotherapy, there was a statistically significant increasing trend in breast cancer risk with increasing serum level of PCBs.
This is the largest case–control study of African-American women to date and the first to examine interactions by p53, ER, PR, HER-2/neu and chemotherapy status in this population. However, a potential limitation of our study is the low case patient and control subject response rates achieved in the overall Women’s CARE Study in Los Angeles County. We have recently commented on the issues related to control recruitment in population-based case–control studies [56]. Nevertheless, among eligible participants in the Los Angeles portion of the Women’s CARE Study, 84.5% of case patients and 78.5% of controls who were approached participated in this substudy. All information pertaining to the study was obtained through in-person interviews using a standardized questionnaire. Furthermore, BMI was queried at the time of blood draw and was used to adjust the analyses of serum measurements. A single laboratory conducted immunohistochemical analyses for ER, PR and p53 status and HER-2/neu, maximizing consistency between methods, protocol and personnel. Missing values for ER and PR status were minimized by supplementing the primary lab’s results with those obtained from the case patients’ pathology reports. Results were highly concordant among study subjects tested in our laboratory with information also available in the CSP SEER registry pathology reports. Results restricted to the laboratory results were consistent with those presented that combined the laboratory results with those extracted from pathology reports. We were still missing data on HER-2/neu and p53 status on approximately 30% of case patients, which limited the statistical power in analyses of these biologic markers.
Several epidemiologic studies since the 1970s have examined the association between breast cancer and organochlorines measured either in serum or adipose tissue. The majority of the earliest case–control studies were null but these also tended to have small sample sizes, did not study a well-defined population or failed to control for breast cancer risk factors that could have confounded the comparison between case patients and controls [10–13]. Recent larger case–control studies that more carefully controlled for confounding factors have provided mixed results with some positive findings among subgroups of the populations defined by tumor receptor status but with limited statistical power to evaluate subgroups [14–28]. Among the positive studies, one [28] reported a significant difference in DDE between breast cancer case patients and controls, as well as an almost 4-fold increase in risk comparing the highest quintile of DDE to the lowest; however, the values for serum organochlorine levels were not lipid adjusted. PCB and DDE residue levels in serum are influenced by the amount of lipid in the serum [46] and adjustment is necessary to standardize blood residue measurements.
Among studies with positive findings among subgroups of the population studied, one reported a non-significant increased risk of breast cancer among p53 positive case patients in the highest exposure level of PCBs [26]. Another found a significant difference in adipose DDE levels restricted to ER positive case patients and controls [14]. Explanations proposed include that the expression of various tumor receptor proteins, namely ER, PR, HER2 and p53 may result in effect modification of the exposure–disease relationship in breast cancer [57–59]. Only two previous studies have looked specifically at African-American women, and neither found significant differences in serum levels of DDE or PCBs between case patients and controls [16, 22].
This study’s finding of an interaction between the association between DDE levels and breast cancer risk with ER status is in line with that of Dewailly et al. who found significant differences in mean adipose DDE between ER positive case patients and controls [14]. These results suggest the possibility that DDE may influence breast cancer risk through estrogen, mediated by its receptor [60, 61].
Treatment of breast cancer with chemotherapy has been associated with an increase in lipid-adjusted serum DDE and PCBs from pretreatment to posttreatment measures [62]. Our finding that risk increased with PCB levels among women who had not been treated with chemotherapy is thus unexplained.
This study may have missed an effect of organochlorines on breast cancer risk. It is possible that the serum organochlorine levels measured in the study population are too low to influence breast cancer risk. Our levels of PCBs and DDE, as well as those of African American women in North Carolina collected during the 1990s [16], are substantially lower than those reported by Krieger et al. [22], which were measured in the 1960s. However, compared to those measured in Caucasian women from samples taken in the 1990s, our levels and those measured among North Carolina black women are approximately 1.0 and 0.15 μg/g higher for lipid-adjusted DDE and PCBs, respectively [16]. This change in levels of African American women over time likely reflects the fact that PCB and DDE use was discontinued in the 1970s and environmental levels have continued to decay over time.
Early exposures such as those during the woman’s gestation, childhood and adolescence may have long-lasting impact on her breast cancer risk [63]. It is possible the relevant organochlorine exposure measure for breast cancer risk might be the woman’s body burden at critical periods of breast susceptibility and that current levels do not represent these exposures. Both lactation and BMI impact levels of organochlorine compounds [6, 7]. We found no evidence that either factor influenced any relationship of PCBs or DDE and breast cancer risk.
Ideally one would have preferred measuring organochlorine levels in fatty tissue since fatty tissue accumulates higher levels than blood. Levels of DDE in serum and adipose tissue have been shown to be highly correlated in many studies [64]. For PCBs this correlation has been less well studied, though it appears to be lower than for DDE [64]. However, correlations among levels of PCBs in serum and breast milk, which have been better-studied, tend to be relatively high, although again correlations for DDE are greater [65]. Overall, serum levels appear to be reasonably good biomarkers of long-term exposure, although they may function better for DDE than PCBs.
This study did not look at specific congeners of PCBs. It is therefore possible that this analysis could have missed the effect of a specific congener on risk of breast cancer. However, levels of specific congeners are highly correlated and it is difficult to differentiate their individual effects [66].
Lactation is associated with a modest reduction in breast cancer risk [67], and breastfeeding reduces a woman’s body burden of organochlorines [14]. There is evidence that BMI may also be a risk factor for breast cancer among post-menopausal women and high BMI may increase body burden and thus exposure to organochlorines. As noted above, African-Americans have higher levels of serum DDT compounds, and in some studies, of PCBs, compared with Caucasians [16, 32, 33, 68]. Whether this is explained by greater exposure to organochlorines through employment or residence, or whether it is related to higher average BMI and lower rates of breastfeeding in African-Americans relative to Caucasians [69] is not known.
In conclusion, this study did not find an increased risk of breast cancer in African-American women from PCBs or DDE at the levels measured in serum, nor was there evidence for effect modification by tumor ER or PR status, or HER-2/neu or p53 expression.
References
SEER. (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 9 Regs Public-Use, Nov 2004 Sub (1973–2002), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. Released April 2005, based on the November 2004 submission
IARC (1999) Hormonal contraception and postmenopausal hormonal therapy. IARC monographs on the evaluation of carcinogenic risks to humans, Lyon
Brinton LA, Brogan DR, Coates RJ, Swanson CA, Potischman N, Stanford JL (1998) Breast cancer risk among women under 55 years of age by joint effects of usage of oral contraceptives and hormone replacement therapy. Menopause 5(3):145–151
LM Butler NA Potischman B Newman et al. (2000) ArticleTitleMenstrual risk factors and early-onset breast cancer Cancer Causes Control 11 IssueID5 451–458 Occurrence Handle10877338 Occurrence Handle10.1023/A:1008956524669 Occurrence Handle1:STN:280:DC%2BD3cvpt1CisQ%3D%3D
Adami HO, Lipworth L, Titus-Ernstoff L, et al (1995) Organochlorine compounds and estrogen-related cancers in women. Cancer Causes Control 6(6):551–566
Longnecker MP, Rogan WJ, Lucier G (1997) The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health 18:211–244
Ahlborg UG, Lipworth L, Titus-Ernstoff L, et al (1995) Organochlorine compounds in relation to breast cancer, endometrial cancer, and endometriosis: an assessment of the biological and epidemiological evidence. Crit Rev Toxicol 25(6):463–531
Dich J, Zahm SH, Hanberg A, Adami HO (1997) Pesticides and cancer. Cancer Causes Control 8(3):420–443
Kimbrough RD, Krouskas CA (2003) Human exposure to polychlorinated biphenyls and health effects: a critical synopsis. Toxicol Rev 22(4):217–233
Unger M, Kiaer H, Blichert-Toft M, Olsen J, Clausen J (1984) Organochlorine compounds in human breast fat from deceased with and without breast cancer and in a biopsy material from newly diagnosed patients undergoing breast surgery. Environ Res 34(1):24–28
Falck F, Jr, Ricci A, Jr, Wolff MS, Godbold J, Deckers P (1992) Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch Environ Health 47(2):143–146
Wassermann M, Nogueira DP, Tomatis L, et al (1976) Organochlorine compounds in neoplastic and adjacent apparently normal breast tissue. Bull Environ Contam Toxicol 15(4):478–484
Mussalo-Rauhamaa H, Hasanen E, Pyysalo H, Antervo K, Kauppila R, Pantzar P (1990) Occurrence of beta-hexachlorocyclohexane in breast cancer patients. Cancer 66(10):2124–2128
Dewailly E, Dodin S, Verreault R, et al (1994) High organochlorine body burden in women with estrogen receptor-positive breast cancer. J Natl Cancer Inst 86(3):232–234
Aronson KJ, Miller AB, Woolcott CG, et al (2000) Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prev 9(1):55–63
Millikan R, DeVoto E, Duell EJ, et al (2000) Dichlorodiphenyldichloroethene, polychlorinated biphenyls, and breast cancer among African-American and white women in North Carolina. Cancer Epidemiol Biomarkers Prev 9(11):1233–1240
van’t Veer P, Lobbezoo IE, Martin-Moreno JM, et al (1997) DDT (dicophane) and postmenopausal breast cancer in Europe: case–control study. BMJ 315(7100):81–85
Zheng T, Holford TR, Mayne ST, et al (2000) Risk of female breast cancer associated with serum polychlorinated biphenyls and 1,1-dichloro-2,2′-bis(p-chlorophenyl)ethylene. Cancer Epidemiol Biomarkers Prev 9(2):167–74
Woolcott CG, Aronson KJ, Hanna WM, et al (2001) Organochlorines and breast cancer risk by receptor status, tumor size, and grade (Canada). Cancer Causes Control 12(5):395–404
Gammon MD, Wolff MS, Neugut AI, et al (2002) Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomarkers Prev 11(8):686–697
Engel LS, Hill DA, Hoppin JA, et al (2005) Pesticide use and breast cancer risk among farmers’ wives in the agricultural health study. Am J Epidemiol 161(2):121–135
Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J, Orentreich N (1994) Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst 86(8):589–599
Laden F, Collman G, Iwamoto K, et al (2001) 1,1-Dichloro-2,2-bis( p-chlorophenyl)ethylene and polychlorinated biphenyls and breast cancer: combined analysis of five U.S. studies. J Natl Cancer Inst 93(10):768–776
Muscat JE, Britton JA, Djordjevic MV, et al (2003) Adipose concentrations of organochlorine compounds and breast cancer recurrence in Long Island, New York. Cancer Epidemiol Biomarkers Prev 12(12):1474–1478
Raaschou-Nielsen O, Pavuk M, Leblanc A, et al (2005) Adipose organochlorine concentrations and risk of breast cancer among postmenopausal Danish women. Cancer Epidemiol Biomarkers Prev 14(1):67–74
Hoyer AP, Gerdes AM, Jorgensen T, Rank F, Hartvig HB (2002) Organochlorines, p53 mutations in relation to breast cancer risk and survival. A Danish cohort-nested case–controls study. Breast Cancer Res Treat 71(1):59–65
Wolff MS, Zeleniuch-Jacquotte A, Dubin N, Toniolo P (2000) Risk of breast cancer and organochlorine exposure. Cancer Epidemiol Biomarkers Prev 9(3):271–277
Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N (1993) Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst 85(8):648–652
Stehr-Green PA, Farrar JA, Burse VW, Royce WG, Wohlleb JC (1988) A survey of measured levels and dietary sources of selected organochlorine pesticide residues and metabolites in human sera from a rural population. Am J Public Health 78(7):828–830
Zheng T, Holford TR, Mayne ST, et al (1999) DDE and DDT in breast adipose tissue and risk of female breast cancer. Am J Epidemiol 150(5):453–458
Rogan WJ (1996) Pollutants in breast milk. Arch Pediatr Adolesc Med 150(9):981–990
Stehr-Green PA (1989) Demographic and seasonal influences on human serum pesticide residue levels. J Toxicol Environ Health 27(4):405–421
Schildkraut JM, Demark-Wahnefried W, DeVoto E, Hughes C, Laseter JL, Newman B (1999) Environmental contaminants and body fat distribution. Cancer Epidemiol Biomarkers Prev 8(2):179–183
Marchbanks PA, McDonald JA, Wilson HG, et al (2002) The NICHD women’s contraceptive and reproductive experiences study: methods and operational results. Ann Epidemiol 12(4):213–221
Waksberg J (1978) Sampling methods for random digit dialing. J Am Stat Assoc 73:40–46
Norman SA, Berlin JA, Weber AL, et al (2003) Combined effect of oral contraceptive use and hormone replacement therapy on breast cancer risk in postmenopausal women. Cancer Causes Control 14(10):933–943
Longnecker MP, Bernstein L, Bird CL, Yancey AK, Peterson JC (1996) Measurement of organochlorine levels in postprandial serum or in blood collected in serum separator tubes. Cancer Epidemiol Biomarkers Prev 5(9):753–755
Sherma J (1980) Manual of analytical methods for the analysis of pesticides in humans and environmental samples. Health Effects Laboratory; Research Triangle Park, NC
Burse VW, Korver MP, Needham LL, et al (1989) Gas chromatographic determination of polychlorinated biphenyls (as Aroclor 1254) in serum: collaborative study. J Assoc Off Anal Chem 72(4):649–659
Webb RG, McCall AC (1973) Quantitative PCB standards for electron capture gas chromatography. J Chromatogr Sci 11(7):366–373
Erickson MD (1997) Analytical chemistry of PCBs. 2nd ed. Lewis, New York
Takayama M, Itoh S, Nagasaki T, Tanimizu I (1977) A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta 79(1):93–98
McGowan MW, Artiss JD, Strandbergh DR, Zak B (1983) A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem 29(3):538–542
Beckman Instruments I (1993) CS-7 Operations Manual. Beckman Instruments, Inc. Irvine, CA
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem 19(12):1350–1356
Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL (1989) Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 18(4):495–500
Press M (1993) Estrogen and progesterone receptors in breast cancer. Adv Pathol Lab Med 6:117–148
Wen WH, Reles A, Runnebaum IB, et al (1999) p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol 18(1):29–41
Lukas J, Niu N, Press MF (2000) p53 mutations and expression in breast carcinoma in situ. Am J Pathol 156(1):183–191
Saffari B, Bernstein L, Hong DC, et al (2005) Association of p53 mutations and a codon 72 single nucleotide polymorphism with lower overall survival and responsiveness to adjuvant radiotherapy in endometrioid endometrial carcinomas. Int J Gynecol Cancer 15(5):952–963
Press MFSB, Groshen S, Kaminsky D, et al (2002) Monoclonal antibodies designed for immunohistochemical detection of progesterone receptor in archival breast cancer specimens. Steroids 67:799–813
Press MF, Slamon DJ, Flom KJ, Park J, Zhou JY, Bernstein L (2002) Evaluation of HER-2/neu gene amplification and overexpression: comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol 20(14):3095–105
Press MF, Sauter G, Bernstein L, et al (2005) Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res 11(18):6598–607
Park JM, Yang X, Park JJ, Press OW, Press MF (1999) Assessment of novel anti-p185HER-2 monoclonal antibodies for internalization-dependent therapies. Hybridoma 18(6):487–495
Press M, Spaulding B, Groshen S, et al (2002) Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids 67(9):799–813
Bernstein L (2006) Control recruitment in population-based case–control studies. Epidemiology 17(3):255–257
Coradini D, Daidone MG (2004) Biomolecular prognostic factors in breast cancer. Curr Opin Obstet Gynecol 16(1):49–55
Mass RD (2004) The HER receptor family: a rich target for therapeutic development. Int J Radiat Oncol Biol Phys 58(3):932–940
Sledge GW, Jr (2004) HERe-2 stay: the continuing importance of translational research in breast cancer. J Natl Cancer Inst 96(10):725–727
Anderson E (2002) The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4(5):197–201
Dickson RB, Stancel GM (2000) Estrogen receptor-mediated processes in normal and cancer cells. J Natl Cancer Inst Monogr 2000(27):135–145
Gammon MD, Wolff MS, Neugut AI, et al (1996) Treatment for breast cancer and blood levels of chlorinated hydrocarbons. Cancer Epidemiol Biomarkers Prev 5(6):467–471
Henderson BE, Feigelson HS (2000) Hormonal carcinogenesis. Carcinogenesis 21(3):427–433
Rusiecki JA, Matthews A, Sturgeon S, et al (2005) A correlation study of organochlorine levels in serum, breast adipose tissue, and gluteal adipose tissue among breast cancer cases in India. Cancer Epidemiol Biomarkers Prev 14(5):1113–1124
Rogan WJ, Gladen BC, McKinney JD, et al (1986) Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation. Am J Public Health 76(2):172–177
DeVoto E, Fiore BJ, Millikan R, et al (1997) Correlations among human blood levels of specific PCB congeners and implications for epidemiologic studies. Am J Ind Med 32(6):606–613
Newcomb PA (1997) Lactation and breast cancer risk. J Mammary Gland Biol Neoplasia 2(3):311–318
Kutz FW, Yobs AR, Strassman SC (1977) Racial stratification of organochlorine insecticide residues in human adipose tissue. J Occup Med 19(9):619–622
Bernstein L, Teal CR, Joslyn S, Wilson J (2003) Ethnicity-related variation in breast cancer risk factors. Cancer 97(1 Suppl):222–229
Acknowledgments
This study was supported by the National Institute of Child Health and Human Development, with additional support from the National Cancer Institute (NCI), through a contract with the University of Southern California University of Southern California (N01 HD 3–3175), by a grant from the National Institute of Environmental Health Sciences (NIEHS), ES07084, and in part by the Intramural Research Program of the National Institutes of Health, NIEHS. General support from NCI through the SEER Program contract to the Cancer Surveillance Program for Los Angeles County (N01-CN-67010) is also acknowledged. The collection of cancer incidence data for Los Angeles County used in this publication was also supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by the California Health and Safety Code Section 103885. The ideas and opinions expressed herein are those of the authors, and no endorsement by the State of California, Department of Health Services, is intended or should be inferred.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatto, N.M., Longnecker, M.P., Press, M.F. et al. Serum organochlorines and breast cancer: a case–control study among African-American women. Cancer Causes Control 18, 29–39 (2007). https://doi.org/10.1007/s10552-006-0070-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10552-006-0070-2