Abstract
Purpose
Several studies have shown that aerobic exercise training improves obstructive sleep apnea (OSA) severity. However, a dose-response relationship has never been shown. This study aimed to quantify any dose-response relationships between time spent per week in aerobic exercise and key sleep apnea outcomes.
Methods
Randomized controlled trials (RCT) were selected from literature search studying the effects of supervised aerobic exercise training on patients with OSA. Dose-response meta-analyses were performed, where the ‘dose’ was the total weekly duration of aerobic exercise training. Primary outcomes were apnea hypopnea index (AHI), cardiorespiratory fitness (maximum oxygen consumption or VO2peak) and Epworth Sleepiness Scale (ESS).
Results
Analysis of data from 11 RCTs showed a non-linear dose-response relationship between the total weekly duration of aerobic exercise training and mean differences in AHI. Maximum effects on AHI (-10.92 (95%CIs: -15.57; -6.27)) were observed when the weekly duration of aerobic exercise reached 100 min/week. Similar non-linear dose-response trend was observed in the mean differences in VO2peak. Studies in which aerobic exercise training lasted ≥ 12 weeks showed greater proportional changes in mean AHI differences with maximal effects reaching a peak at ∼ 70 min/week of aerobic exercise training. ESS and total weekly duration of aerobic exercise training showed a linear dose-response relationship based on 4 RCTs.
Conclusions
Based on these analyses, aerobic exercise training of 70–100 min/week over 3 or 5 days a week should be recommended as adjunctive treatment for patients with OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous positive airway pressure (CPAP) and mandibular advancement devices have been mostly used in the treatment of obstructive sleep apnea (OSA) [1, 2]. The field is, however, continuously making progress with newer treatment options being explored based on the different phenotypes and endotypes of OSA [3]. There is now a range of options that can be considered in the personalized/precision care approach for OSA— options such as pharmacological treatment with atomoxetine-oxybutynin combination [4] to surgical procedures like the implantable device for hypoglossal nerve stimulation therapy [5].
However, just as with other chronic cardiometabolic diseases, lifestyle modification including physical activity and weight loss would remain central to the overall management of patients with OSA, especially in those who are overweight [6]. Several clinical trials exploring the effects of weight loss through lifestyle modifications in those who are overweight and have OSA, have consistently shown significant improvements in the severity of OSA and functional outcomes [7, 8]. In terms of dose-response relationship a recent study found that even a < 5% weight loss was sufficient for small but statistically significant improvement in OSA severity, and those who achieved a ≥ 10% weight loss exhibited the greatest benefits [9].
Patients with OSA also generally have lower physical activity levels as compared to those without [10] and unlike the guidelines on the optimal duration of aerobic exercise for various other cardiometabolic diseases [11, 12], there are currently no guidelines on the optimal duration of aerobic exercise training for patients with OSA. This formed the basis for this study which primarily aimed to determine if a dose-response relationship exists between the total weekly duration of aerobic exercise training and improvement in OSA severity, cardiorespiratory fitness, and subjective daytime sleepiness.
Methods
This study was conducted in accordance with the Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA) reported in (Table S1). The protocol was registered in OSF (https://doi.org/10.17605/OSF.IO/4EKVD). PubMed, Scopus and Web of Science databases were searched (independently by three authors) from inception to March 21st, 2024 (search words in Table S2). Inclusion criteria was based on PI(E)COS format, where population (P) of interest comprised of adult research participants with primarily OSA who were studied for the effects of supervised aerobic exercise training (the intervention (I)) and compared to control (C) participants who received usual care or engaged in activities like stretching but not in aerobic or non-aerobic (resistance) training. The primary outcomes (O) of interest were mean differences in apnea hypopnea index (AHI) based on polysomnogram (PSG) or home sleep apnea tests (an objective index of OSA severity), maximal oxygen consumption (VO2peak) (a measure of cardiorespiratory fitness) and Epworth Sleepiness Scale (ESS) score (ESS assesses subjective daytime sleepiness on a scale of 0 to 24; scores ≥ 11indicating excessive daytime sleepiness). Other (secondary) outcomes were explored post-hoc (described in Results). The study design (S) considered for inclusion was the randomized controlled trial (RCT) design. Studies were excluded if they studied short term effects of aerobic exercise training (< 4 weeks) or if they studied a combination of interventions in conjunction with aerobic exercise training, e.g., dietary modification or CPAP, which would not allow for isolating the effects of aerobic exercise training on the outcomes. Studies were also excluded if the data for outcomes of interest presented in median and ranges was detected to have significant skewness not allowing for calculation of means and standard deviations (SD) [13, 14].
Disagreement on any study selection or study quality was resolved by consensus. The reasons for the exclusion of studies are explained in Table S3. The studies were assessed for study quality/risk of bias (using RoB-2 tool) [15], as reported in Figure S1. Extracted data included baseline characteristics of the included participants, aerobic exercise parameters (i.e., duration of the intervention, frequency and intensity) and change scores of outcomes for each study arm according to Cochrane methodological guidelines [16].
The method introduced by Crippa and Orsini [17] was used to calculate mean differences and corresponding standard errors of change in AHI in the intervention group relative to the control group in each trial. The method requires dose (minutes/week) of aerobic exercise in each study arm, the mean and its corresponding SD of change of each outcome in each study, and the number of participants in each study. This data was extracted on the working sheets of Comprehensive Meta-analysis (CMA version 2.2.064; Biostat, Englewood, N.J. U.S.A) to get uniform measures of changes as change scores. The “dose” in this meta-analysis was the time in aerobic exercise spent per week by participants and this excluded any pre-workout/warm-up or post-workout/cool-down exercises. For studies in which the frequency (sessions per week) or the duration (minutes per session) of intervention progressed during the study, the duration of exercise in each week was calculated first and then averaged over the follow-up period to estimate the average duration of aerobic exercise (minutes/week) in each study.
Trial-specific results were pooled using a ‘one-stage random-effects model’ and dose-response curves were constructed in Stata-13 based on the methodology by Crippa and Orsini [17]. Dose-response curves characterize the relative efficacy of the dose under investigation using the placebo effect as referent (i.e. the relative efficacy for the placebo is zero by definition). Linear and nonlinear dose-response curves were plotted using restricted maximum likelihood estimation [18] using Stata packages “drmeta” and “drmeta_graph”. Nonlinear curves were reported (restricted cubic spline model graph) if the Wald test for departure from linearity was significant at p < 0.1. Data were fitted with a weighted mixed-effects model restricted cubic splines for ‘dose’ (exercise— minutes/week) with three knots located at percentiles (10th, 50th, and 90th ) of its distribution [19]. The 50% effective dose (ED50) and 95% effective dose (ED95) were also calculated [17]. Linear dose-response curves were reported if the Wald test was not significant. Conventional direct pairwise meta-analyses were also conducted, and results presented as forest plots.
Additionally, trial-sequential analyses were conducted using TSA program version 0.9.5.10 Beta (www.ctu.dk/tsa) for AHI and ESS outcomes only. Trial sequential analysis tests the credibility of the ascertained results by combining both an estimation of information size (a cumulative sample size of included trials) with an adjusted threshold of statistical significance for the cumulative meta-analysis. Meta-analysis monitoring boundaries (Trial Sequential Monitoring Boundaries) and the required-information-size (RIS) were quantified, with diversity-adjusted information size or D2 and adjusted 95% confidence intervals (CI). Diversity adjustment was performed according to an overall type I error of 5%, and power of 90%.
Heterogeneity was assessed with I2 statistics [20, 21]. I2 greater than 50% was considered to indicate substantial heterogeneity [21]. A funnel plot to assess funnel plot asymmetry was constructed reporting p-values for asymmetry (for Eggers test of intercept) [22]. Risk of bias and study quality assessment are provided in supplement (Figure S1).
Results
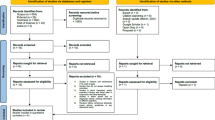
A total of 11 RCTs from 12 publications [23,24,25,26,27,28,29,30,31,32,33,34] (there were two publications [26, 27] from an RCT) were included in this meta-analysis. There were 217 participants who underwent supervised moderate to high intensity aerobic exercise training and 218 participants who formed the control population. Baseline characteristics of the study population as well as details of the aerobic exercise are reported in Table 1. Figure S2 shows the study selection process.
Dose-response meta-analysis of 11 studies [23,24,25,26, 28,29,30,31,32,33,34] showed a non-linear dose-response relationship between the total weekly duration of aerobic exercise and AHI mean difference. There was a proportional change in the AHI mean difference with the increase in the duration of aerobic exercise up to 100 min/week (mean difference = -10.92 (95%CIs: -15.57; -6.27)), with no remarkable change in effect estimate at higher duration of exercise (pdose−response< 0.001, pWald−test = 0.03, Fig. 1, Table S2). The ED50 was 26.5 min/week and the ED95 was 71 min/week. The curve (Fig. 1) peaks at 100 min/week and indicates that additional effects of exercise become trivial compared to those at 100 min/week. Pairwise meta-analysis showed that the pooled mean difference in AHI was − 9.90 (95%CIs: -14.26; -5.53), p < 0.001, I2 = 74% (Figure S3). Trial sequential analysis for mean AHI difference showed that the last line of Z-score curve crossed the conventional monitoring, the trial sequential monitoring and the RIS boundaries (Figure S4). This analysis yielded a similar pooled mean difference − 9.91 (95%CIs: -14.25; -5.56), p < 0.0001, I2 74%, as that computed with pairwise meta-analysis.
Dose-response curve for the effects of aerobic exercise on mean differences in apnea-hypopnea index. Solid and dotted lines represent the restricted maximum likelihood estimates and 95% CIs, respectively. AHI indicates apnea hypopnea index. X-axis indicates the “dose” which is aerobic exercise training in minutes/week
Restricting the analysis to only the studies [23, 24, 26, 28, 29, 31,32,33,34] where aerobic exercise training lasted ≥ 12 weeks showed the same non-linear dose-response relationship but with a greater proportional change in the AHI mean difference compared to the analysis including all studies (pdose−response< 0.0001, pWald−test = < 0.0001). Maximum effects were seen ∼ 70 min/week where the AHI mean difference was − 15.94 (95%CIs: -23.72; -8.15) and the curve appeared to flatten after 70 min/week (Fig. 2 and Table S5). The ED50 was 20.5 min/week and the ED95 was 52.5 min/week. Corresponding pairwise meta-analysis of studies where the duration of aerobic exercise lasted ≥ 12 weeks showed mean AHI difference of -9.75 (95%CIs: -14.70; -4.80) p < 0.001, I2 = 78% (Figure S5). Corresponding trial sequential analysis for mean AHI difference from these studies [23, 24, 26,27,28,29, 31,32,33,34] showed that the line of Z-score curve crossed the conventional monitoring, the trial sequential monitoring and the RIS boundaries (Figure S6) and a pooled mean AHI difference of -9.68 (95%CIs: -13.59; -5.77; p < 0.0001, I2 76%), confirming the results of the pairwise meta-analysis.
Dose-response curve for the effects of ≥ 12 weeks aerobic exercise on mean differences in apnea-hypopnea index. Solid and dotted lines represent the restricted maximum likelihood estimates and 95% CIs, respectively. AHI indicates apnea hypopnea index. X-axis indicates the “dose” which is aerobic exercise training in minutes/week
VO2peak data was reported in ‘ml/kg/min’ units in 7 studies [23,24,25, 30, 32,33,34] and in ‘ml/min’ in one study [31]. Dose-response meta-analysis of 7 studies [23,24,25, 30, 32,33,34] for VO2peak data (reported in ml/kg/min) showed a non-linear dose-response relationship between the total weekly duration of aerobic exercise and VO2peak mean difference. There was a proportional change in the VO2peak mean difference with the increase in the duration of aerobic exercise up to 90 to 100 min/week, and no significantly greater effects after that with higher duration of aerobic exercise (pdose−response< 0.001, pWald−test = 0.007, Fig. 3, Table S6). From this analysis, assuming maximum effects on VO2peak mean difference reaching 4.73 ml/kg/min, the calculated ED50 was 30 min/week and the calculated ED95 was 85 min/week. Pairwise meta-analysis for standardized mean VO2peak difference from 8 studies [23,24,25, 30,31,32,33,34] showed pooled estimate of 0.82 (95%CIs: 0.34; 1.30), p = < 0.001, I2 = 74% (Figure S7).
Dose-response curve for the effects of aerobic exercise on mean VO2 peak differences. Solid and dotted lines represent the restricted maximum likelihood estimates and 95% CIs, respectively. VO2 peak is the maximum oxygen consumption. X-axis indicates the “dose” which is aerobic exercise training in minutes/week
Data on ESS from four studies was meta-analyzed [25, 27, 31, 32]. Dose-response meta-analysis showed a linear relationship (Fig. 4) between the total weekly duration of aerobic exercise and mean ESS difference across the range of 0–200 min/week of exercise. The coefficient for slope was − 0.02 (95%CIs: -0.04; -0.007), (pdose−response = 0.006; pWald−test = 0.34). Direct pairwise meta-analysis showed pooled mean ESS difference of -3.87 (95%CIs: -5.55; -2.18), p = < 0.01, I2 = 0% (Figure S8). Trial sequential analysis for mean ESS difference showed that the line of Z-score curve crossed the conventional monitoring and RIS boundaries (Figure S9) and this analysis showed a similar pooled mean ESS difference as that obtained with pairwise meta-analysis (trial sequential analysis pooled mean ESS difference = -3.83 (95%CIs: -5.46; -2.20), p = < 0.0001, I2 = 0%.
Dose-response curve for the effects of aerobic exercise on mean differences in Epworth Sleepiness Scale scores. Solid and dotted lines represent the restricted maximum likelihood estimates and 95% CIs, respectively. ESS indicates Epworth Sleepiness Scale score. X-axis indicates the ‘dose’ which is aerobic exercise training in minutes/week
From the studies included in the meta-analysis for AHI mean difference, post-hoc exploratory meta-analyses for other outcomes were also conducted. Two RCTs [25, 27] reported data on Pittsburgh sleep quality index (PSQI) and Short Form-36 (SF-36). Pooled mean differences in PSQI and the vitality subcomponent score of SF-36 were − 1.94 (95CIs: -3.56; -0.32), p = 0.02, I2 = 0%, and 26.17 (95%CIs: 1.18; 51.16), p = 0.04, I2 = 84%, respectively (Figure S10 and S11).
PSG follow-up data was available from a few studies and pooled in separate meta-analyses. Data on oxygen desaturation index was meta-analyzed from six studies [23,24,25,26, 28, 34] and the mean difference was − 3.55 (95%CIs: -6.28; -0.82), p = 0.01, I2 = 2% (Figure S12). Sleep efficiency (total sleep time/total recording time in %) data from five studies [26, 30,31,32,33] showed mean difference in sleep efficiency of 3.88% (95%CIs: 0.39%; 7.38%), p = 0.03, I2 = 0% (Figure S13). PSG-recorded minimum oxygen saturation from 6 studies [23, 24, 26, 30, 32, 34] showed a mean difference of 1.19% (95CIs: -1.63%; 4.02%), p = 0.41, I2 = 67% (Figure S14). Based on data from 3 studies [26, 30, 32], the mean difference in stage 3 sleep was 0.68% (95%CIs: -3.29%; 4.65%), p = 0.74, I2 = 44% (Figure S15) and based on data from 7 studies [23, 25, 26, 28, 30,31,32] mean difference in total sleep time was 10.81 min (95%CIs: -4.19 min; 25.81 min), p = 0.16, I2 = 0% (Figure S16).
A few studies analyzed anthropometrics post-intervention. Pairwise meta-analyses showed mean difference in body mass index (BMI) of -1.10 kg/m2 (95%CIs: -2.21 kg/m2; 0.02 kg/m2), p = 0.05, I2 = 8% from 5 studies [23,24,25, 31, 34] (Figure S17), mean difference in neck circumference of -1.09 cm (95%CIs: -2.19 cm; 0.01 cm), p = 0.05, I2 = 16% from 5 studies [25, 26, 30, 31, 34] (Figure S18), and mean difference in body fat percentage of -2.26% (95%CIs: -4.82%; 0.30%) p = 0.08, I2 = 0%, from 2 studies [26, 31] (Figure S19).
Data on lung functions reported in a few studies was also meta-analyzed. Maximum expiratory pressure and maximum inspiratory pressure data pooled from 3 studies [26, 28, 31] showed mean differences of 13.77 (95%CIs: -13.61; 41.15), p = 0.32, I2 = 59%, and 26.65 (95%CIs: -17.11; 70.41), p = 0.23, I2 = 79%, respectively (Figure S20). Standardized mean differences in forced expiratory volume in 1 s and forced vital capacity from 2 studies [26, 31] pooled were, -0.04 (95CIs: -0.54; 0.47), p = 0.88, I2 = 0%, and − 0.19 (95%CIs: -0.69; 0.32), p = 0.47, I2 = 0%, respectively (Figure S21).
Visual inspection of funnel plot for AHI meta-analysis did not show plot asymmetry (Figure S22). Based on Egger’s test of intercept, the intercept (B0) was at -0.32 (95%CIs: -2.95, 1.95), p (1-tailed) = 0.32, which indicated no publication bias.
Discussion
To our knowledge this is the first dose-response meta-analysis and trial sequential analysis of studies on the effects of supervised moderate to high intensity aerobic exercise training in patients with OSA. Results from these analyses show significant dose-response relationships between the duration of aerobic exercise and mean differences in AHI, VO2peak and ESS. The dose-response effects for mean AHI differences were most pronounced when restricting the analysis to studies where the duration of aerobic exercise training was ≥ 12 weeks. Trial sequential analyses for AHI and ESS indicated that the observed effects from pairwise meta-analyses on AHI and ESS were conclusive and the evidence sufficient to conclude that aerobic exercise has statistically significant effects on AHI and ESS.
AHI effect-size estimates from the pairwise meta-analyses are modest in terms of the magnitude of effect size when compared to what has been shown earlier for CPAP and mandibular advancement device [35]. Nevertheless, from an epidemiological standpoint, these are significant findings, especially since these effects seem to be driven primarily by improvement in aerobic fitness with only slight decrease in BMI and do not factor in other active lifestyle interventions like dietary weight loss, which could have synergistic effects in patients with OSA. Beneficial effects of aerobic exercise training extend beyond those of improvements in OSA severity and daytime sleepiness. The 2018 Physical Activity Guidelines for Americans, recommend that adults should engage in at least 150 min of moderate intensity aerobic physical activity or at least 75 min of vigorous intensity aerobic physical activity each week, or an equivalent combination of both [36]. In a recent study with a large nationally representative sample of US adults, those who engaged in aerobic activities consistent with the recommended Physical Activity Guidelines for Americans showed a 29% reduced risk of all-cause mortality compared to those not meeting the guidelines recommended physical activity [37]. The beneficial effects on mortality risk were largely comparable between those who engaged in ≥ 150 min/week of light to moderate intensity exercise or ≥ 75 min/week of vigorous intensity exercise [37]. Considering the health risks of untreated OSA, and the generally low PAP adherence, there are strong rationales for recommending exercise training for individuals with OSA.
Accordingly, findings from these dose-response meta-analyses can be helpful to determine the optimal duration of aerobic exercise that should be recommended for those with OSA. Overall, the maximum effects on AHI (-10.92 (95%CIs: -15.57; -6.27) occurred with 100 min/week of aerobic exercise. This equates to 20 min/day of aerobic exercise 5 days a week. However, restricting the dose-response meta-analysis to studies in which aerobic exercise training lasted ≥ 12 weeks, AHI mean differences were even greater (-15.94 (95%CIs: -23.72; -8.15)) and with only 70 min/week of aerobic exercise. It is logical to assume that if aerobic exercise training can be maintained long-term one can expect sustained if not more clinically significant benefits in AHI. Aerobic exercise training for 70 min/week equates to 14 min/day of aerobic exercise 5 days a week and this excludes pre-workout warm-up and post-workout cool down exercises. Aerobic exercise training for 14–20 min a day for 5 days a week could be a time-efficient strategy for most patients with OSA with no medical contraindications otherwise for exercising and for those with no access to sophisticated training programs or resistance-training equipment. In this context, the World Health Organization guidelines [38] are noteworthy which recommend 150–300 min/week of moderate-intensity aerobic exercise for those who have hypertension and recommends 30–60 min/day of moderate aerobic physical activity at least 5 days a week for otherwise healthy adults.
Effect size estimates on ESS are generally consistent with what was observed in a previously published network comparative meta-analysis which showed ESS effect size estimate from aerobic exercise greater than that of CPAP or mandibular advancement device, though these comparisons did not reach statistical significance [35]. A growing body of evidence shows that aerobic exercise training induces beneficial effects on vigilance and cognitive functions [39, 40] and an improvement of the information processing speed, measured by a reduction in reaction time on attentional tasks [41]. The key physiological factors driving that link, however, are still a matter of scientific debate. A number of studies have examined changes in brain structure and function, as it relates to selective aspects of cognition, with aerobic exercise training. This includes evidence that aerobic exercise training leads to increase in the volume of a number of frontal and temporal gray matter and white matter regions [42, 43] and also evidence indicating increase in the hippocampal volume [44, 45]. From studies showing that aerobic exercise training leads to increase in cerebral blood flow [46] to others indicating cellular links such as increase in brain-derived neurotrophic factor [47] (in hippocampus and cerebral cortex and considered to play a central role in learning, memory and executive function), there is ample evidence to support that aerobic exercise can improve daytime sleepiness.
Aside from the analyses on AHI and ESS, most other analyses should be considered exploratory, or hypothesis-generating given the limited number of studies in each analysis. None of the studies included in the meta-analysis showed significant improvements in PSG-measured sleep efficiency but the effect size estimate pooled from these studies was statistically significant. There are, however, limitations in assessing sleep efficiency based on a single night PSG. Several other studies have shown that aerobic exercise improved insomnia symptoms as well as sleep efficiency based on either sleep diaries or multiple nights actigraphy recordings [48,49,50].
None of the other analyses in specific, the mean differences in stage 3 sleep, as well as in BMI, neck circumference, body fat percentage or lung functions showed a statistically significant difference. Therefore, none of these seem to explain the changes in AHI from aerobic exercise training. The underlying mechanisms of how aerobic exercise training exerts protective effects on OSA severity are not entirely clear with different theories and mechanisms postulated. One of these relates to the effects of aerobic exercise training on increasing stage 3 sleep [51] which is believed to protective in OSA [52, 53]. Another possible explanation is that aerobic exercise training may reduce the overnight rostral body fluid shift [54,55,56,57]. This is based on experimental observations of overnight changes in neck and calf circumferences of patients with OSA that indicate an overnight rostral body fluid shift [30, 55]. Except for one study [30], the other studies [25, 26, 31, 34] in the meta-analysis on neck circumference did not measure overnight neck circumference change. Therefore, the non-significant change in neck circumference observed in this meta-analysis does not as such disprove the overnight rostral body fluid shift hypothesis. There has also been some interest in respiratory muscles strength exercise training and whether increasing the strength of inspiratory or expiratory muscles improves upper airway patency. A recent meta-analysis did not find significant effects on AHI in participants with OSA who received training for respiratory muscles by breathing through an adjustable valve against a specific resistance [58].
The dose-response curves for AHI and VO2peak, although in opposite directions, appear similar in shape and findings. The magnitude of change and the proportionality of change in VO2peak seems to be correlated with that of AHI based on these dose-response curves. Both show maximal effects at 100 min/week of aerobic exercise— the former on AHI, and the latter on VO2peak. This suggests that the effects on AHI from aerobic exercise training are directly or indirectly a function of the improvement in cardiorespiratory fitness itself.
Findings from the dose-response meta-analyses presented here are consistent with those from similar analyses on the effects of aerobic exercise training on blood pressure in individuals with hypertension [59] and on HbA1C in individuals with type 2 diabetes mellitus [60]. Aerobic exercise training in individuals with hypertension resulted in a non-linear dose-dependent reduction in systolic and diastolic blood pressure with the greatest effects at 150 min/week of aerobic exercise training [59], and in those with type 2 diabetes mellitus, levels of HbA1C decreased proportionally with aerobic exercise training with the greatest effects at 100 min/week of aerobic exercise training [60].
It has been hypothesized that the effects of aerobic exercise on glycemic control are possibly mediated through multiple pathways— improved insulin sensitivity in the muscles, improved blood flow to tissues, weight loss, decrease in inflammatory cytokines and reduction in visceral fat [61]. However, there is no clear understanding of the exact underlying causal links or mechanistic pathways between improvements in VO2peak and in improvements in HbA1C or for that matter, in blood pressure in those with hypertension. Nevertheless, it is a finding that has been observed in several studies. As noted in this meta-analysis, the relationship between improvements in VO2peak and improvement in AHI is akin to that of VO2peak with blood pressure and HbA1C. Given the apparent link between VO2peak and AHI, possibly causal, and considering the variety of sleep apnea phenotypes and endotypes, it is likely that the protective effects of aerobic exercise against sleep apnea are not mediated by a single mechanistic pathway but involve several different pathways.
There are some limitations to this meta-analysis that warrant consideration. Firstly, the analyses on mean AHI reduction showed high statistical heterogeneity. Clinical heterogeneity of the diverse population could explain some of this. However, due to the limited number of studies, it was not possible to conduct further sub-group analyses to explore sources of heterogeneity. Additionally, most of the exploratory analyses, though with low heterogeneity in pooled estimates, contained a limited number of studies compared to the AHI meta-analysis, which limits the generalizability of these findings. Though most exercise training protocols were designed to be moderate-to-high intensity, these protocols varied across the included studies in terms of the frequency and duration, and details about intervention adherence were not uniformly reported.
Overall, in conclusion, the dose-response meta-analyses show significant beneficial effects of aerobic exercise in patients with OSA. Based on these analyses, for patients with OSA with no limitations to exercising otherwise, we recommend 70–100 min of moderate to high intensity aerobic exercise per week, spread over 3 to 5 days per week, in addition to standard treatment (such as CPAP or mandibular advancement device). More sustained effects are likely to be reached in patients after 3 months of regular aerobic exercise.
Data availability
Data will be made available on reasonable request.
References
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG (2019) Treatment of adult obstructive sleep apnea with positive Airway pressure: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2:335–343. https://doi.org/10.5664/jcsm.7640
Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD (2015) Clinical practice Guideline for the treatment of obstructive sleep apnea and snoring with oral Appliance Therapy: an update for 2015. J Clin Sleep Med 7:773–827. https://doi.org/10.5664/jcsm.4858
Martinez-Garcia MA, Campos-Rodriguez F, Barbe F, Gozal D, Agusti A (2019) Precision medicine in obstructive sleep apnoea. Lancet Respir Med 5:456–464. https://doi.org/10.1016/S2213-2600(19)30044-X
Schweitzer PK, Maynard JP, Wylie PE, Emsellem HA, Sands SA (2023) Efficacy of atomoxetine plus oxybutynin in the treatment of obstructive sleep apnea with moderate pharyngeal collapsibility. Sleep Breath 2:495–503. https://doi.org/10.1007/s11325-022-02634-x
Woodson BT, Soose RJ, Gillespie MB et al (2016) Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg 1:181–188. https://doi.org/10.1177/0194599815616618
Iftikhar IH, Kline CE, Youngstedt SD (2014) Effects of exercise training on sleep apnea: a meta-analysis. Lung 1:175–184. https://doi.org/10.1007/s00408-013-9511-3
Mitchell LJ, Davidson ZE, Bonham M, O’Driscoll DM, Hamilton GS, Truby H (2014) Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis. Sleep Med 10:1173–1183. https://doi.org/10.1016/j.sleep.2014.05.012
Carneiro-Barrera A, Diaz-Roman A, Guillen-Riquelme A, Buela-Casal G (2019) Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: systematic review and meta-analysis. Obes Rev 5:750–762. https://doi.org/10.1111/obr.12824
Georgoulis M, Yiannakouris N, Kechribari I, Lamprou K, Perraki E, Vagiakis E, Kontogianni MD (2022) Dose-response relationship between weight loss and improvements in obstructive sleep apnea severity after a diet/lifestyle interventions: secondary analyses of the MIMOSA randomized clinical trial. J Clin Sleep Med 5:1251–1261. https://doi.org/10.5664/jcsm.9834
Hall KA, Singh M, Mukherjee S, Palmer LJ (2020) Physical activity is associated with reduced prevalence of self-reported obstructive sleep apnea in a large, general population cohort study. J Clin Sleep Med 7:1179–1187. https://doi.org/10.5664/jcsm.8456
Grundy SM, Stone NJ, Bailey AL, et al. (2019) 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 25:e1082–e1143. https://doi.org/10.1161/CIR.0000000000000625.
Barone Gibbs B, Hivert MF, Jerome GJ et al (2021) Physical activity as a critical component of First-Line treatment for elevated blood pressure or cholesterol: who, what, and how? A Scientific Statement from the American Heart Association. Hypertension 2:e26–e37. https://doi.org/10.1161/HYP.0000000000000196
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 6:1785–1805. https://doi.org/10.1177/0962280216669183
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 135. https://doi.org/10.1186/1471-2288-14-135
Sterne JAC, Savovic J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ:l4898. https://doi.org/10.1136/bmj.l4898
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2019) Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons
Crippa A, Orsini N (2016) Dose-response meta-analysis of differences in means. BMC Med Res Methodol 91. https://doi.org/10.1186/s12874-016-0189-0
Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N (2019) One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res 5:1579–1596. https://doi.org/10.1177/0962280218773122
Orsini N (2021) Weighted mixed-effects dose-response models for tables of correlated contrasts. Stata J 21(2):320–347
Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2:193–206. https://doi.org/10.1037/1082-989X.11.2.193
Deeks JJHJ, Altman DG Chap. 10: Analysing data and undertaking meta-analyses. In:, Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2023) Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, www.training.cochrane.org/handbook
Sterne JA, Sutton AJ, Ioannidis JP et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ:d4002. https://doi.org/10.1136/bmj.d4002
Araujo CEL, Ferreira-Silva R, Gara EM et al (2021) Effects of exercise training on autonomic modulation and mood symptoms in patients with obstructive sleep apnea. Braz J Med Biol Res 5:e10543. https://doi.org/10.1590/1414-431X202010543
Berger M, Raffin J, Pichot V et al (2019) Effect of exercise training on heart rate variability in patients with obstructive sleep apnea: a randomized controlled trial. Scand J Med Sci Sports 8:1254–1262. https://doi.org/10.1111/sms.13447
Desplan M, Mercier J, Sabate M, Ninot G, Prefaut C, Dauvilliers Y (2014) A comprehensive rehabilitation program improves disease severity in patients with obstructive sleep apnea syndrome: a pilot randomized controlled study. Sleep Med 8:906–912. https://doi.org/10.1016/j.sleep.2013.09.023
Kline CE, Crowley EP, Ewing GB et al (2011) The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep 12:1631–1640. https://doi.org/10.5665/sleep.1422
Kline CE, Ewing GB, Burch JB, Blair SN, Durstine JL, Davis JM, Youngstedt SD (2012) Exercise training improves selected aspects of daytime functioning in adults with obstructive sleep apnea. J Clin Sleep Med 4:357–365. https://doi.org/10.5664/jcsm.2022
Lin HY, Chang CJ, Chiang CC, Su PL, Lin CY, Hung CH (2020) Effects of a comprehensive physical therapy on moderate and severe obstructive sleep apnea- a preliminary randomized controlled trial. J Formos Med Assoc 12:1781–1790. https://doi.org/10.1016/j.jfma.2020.01.011
Lins-Filho O, Germano-Soares AH, Aguiar JLP et al (2024) Effect of 12-week high-intensity interval training on hemodynamic variables at rest and during exercise in patients with obstructive sleep apnoea. J Hypertens 4:742–745. https://doi.org/10.1097/HJH.0000000000003654
Mendelson M, Lyons OD, Yadollahi A, Inami T, Oh P, Bradley TD (2016) Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur Respir J 1:142–150. https://doi.org/10.1183/13993003.01897-2015
Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B (2011) The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath 1:49–56. https://doi.org/10.1007/s11325-009-0311-1
Servantes DM, Javaheri S, Kravchychyn ACP et al (2018) Effects of Exercise training and CPAP in patients with heart failure and OSA: a preliminary study. Chest 4:808–817. https://doi.org/10.1016/j.chest.2018.05.011
Servantes DM, Pelcerman A, Salvetti XM et al (2012) Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clin Rehabil 1:45–57. https://doi.org/10.1177/0269215511403941
Yang H, Liu Y, Zheng H, Liu G, Mei A (2018) Effects of 12 weeks of regular aerobic exercises on autonomic nervous system in obstructive sleep apnea syndrome patients. Sleep Breath 4:1189–1195. https://doi.org/10.1007/s11325-018-1736-1
Iftikhar IH, Bittencourt L, Youngstedt SD et al (2017) Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med 7–14. https://doi.org/10.1016/j.sleep.2016.06.001
Services UDoHaH (2018) Physical activity guidelines for americans, 2nd edn. US Department of Health and Human Services. (Accessed 4/5/2024
Zhao M, Veeranki SP, Magnussen CG, Xi B (2020) Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ:m2031. https://doi.org/10.1136/bmj.m2031
WHO Team WHO South Pacific WWP Pacific physical activity and sedentary behaviour guidelines for everyone. https://www.who.int/publications/i/item/9789290619901 (Accessed: 3/31/2024)
Kramer AF, Colcombe S (2018) Fitness effects on the cognitive function of older adults: a Meta-Analytic Study-Revisited. Perspect Psychol Sci 2:213–217. https://doi.org/10.1177/1745691617707316
Guiney H, Machado L (2013) Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon Bull Rev 1:73–86. https://doi.org/10.3758/s13423-012-0345-4
Sanchis C, Blasco E, Luna FG, Lupianez J (2020) Effects of caffeine intake and exercise intensity on executive and arousal vigilance. Sci Rep 1:8393. https://doi.org/10.1038/s41598-020-65197-5
Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2:125–130. https://doi.org/10.1111/1467-9280.t01-1-01430
Reiter K, Nielson KA, Smith TJ, Weiss LR, Alfini AJ, Smith JC (2015) Improved Cardiorespiratory Fitness is Associated with increased cortical thickness in mild cognitive impairment. J Int Neuropsychol Soc 10:757–767. https://doi.org/10.1017/S135561771500079X
Erickson KI, Voss MW, Prakash RS et al (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 7:3017–3022. https://doi.org/10.1073/pnas.1015950108
Thomas AG, Dennis A, Rawlings NB et al (2016) Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage 162–170. https://doi.org/10.1016/j.neuroimage.2015.10.090
Pereira AC, Huddleston DE, Brickman AM et al (2007) An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A 13:5638–5643. https://doi.org/10.1073/pnas.0611721104
Wagner G, Herbsleb M, de la Cruz F et al (2017) Changes in fMRI activation in anterior hippocampus and motor cortex during memory retrieval after an intense exercise intervention. Biol Psychol 65–78. https://doi.org/10.1016/j.biopsycho.2017.01.003
Passos GS, Poyares D, Santana MG, D’Aurea CV, Youngstedt SD, Tufik S, de Mello MT (2011) Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep Med 10:1018–1027. https://doi.org/10.1016/j.sleep.2011.02.007
Baron P, Hermand E, Bourlois V, Peze T, Aron C, Lombard R, Hurdiel R (2023) Effect of Aerobic Exercise Training on Sleep and Core temperature in middle-aged women with chronic insomnia: a Randomized Controlled Trial. Int J Environ Res Public Health 8. https://doi.org/10.3390/ijerph20085452
Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC (2010) Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med 9:934–940. https://doi.org/10.1016/j.sleep.2010.04.014
Cassim TZ, McGregor KM, Nocera JR, Garcia VV, Sinon CG, Kreuzer M, Garcia PS (2022) Effects of exercise on the sleep microarchitecture in the aging brain: a study on a sedentary sample. Front Syst Neurosci 855107. https://doi.org/10.3389/fnsys.2022.855107
Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG (2009) Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 6:519–524
McSharry DG, Saboisky JP, Deyoung P et al (2013) A mechanism for upper airway stability during slow wave sleep. Sleep 4:555–563. https://doi.org/10.5665/sleep.2544
Kasai T, Motwani SS, Yumino D, Mak S, Newton GE, Bradley TD (2012) Differing relationship of nocturnal fluid shifts to sleep apnea in men and women with heart failure. Circ Heart Fail 4:467–474. https://doi.org/10.1161/CIRCHEARTFAILURE.111.965814
Redolfi S, Bettinzoli M, Venturoli N et al (2015) Attenuation of obstructive sleep apnea and overnight rostral fluid shift by physical activity. Am J Respir Crit Care Med 7:856–858. https://doi.org/10.1164/rccm.201412-2192LE
Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, Bradley TD (2009) Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med 3:241–246. https://doi.org/10.1164/rccm.200807-1076OC
Yumino D, Redolfi S, Ruttanaumpawan P et al (2010) Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 14:1598–1605. https://doi.org/10.1161/CIRCULATIONAHA.109.902452
Torres-Castro R, Solis-Navarro L, Puppo H, Alcaraz-Serrano V, Vasconcello-Castillo L, Vilaro J, Vera-Uribe R (2022) Respiratory muscle training in patients with obstructive sleep apnoea: a systematic review and Meta-analysis. Clocks Sleep 2:219–229. https://doi.org/10.3390/clockssleep4020020
Jabbarzadeh Ganjeh B, Zeraattalab-Motlagh S, Jayedi A et al (2024) Effects of aerobic exercise on blood pressure in patients with hypertension: a systematic review and dose-response meta-analysis of randomized trials. Hypertens Res 2:385–398. https://doi.org/10.1038/s41440-023-01467-9
Jayedi A, Emadi A, Shab-Bidar S (2022) Dose-dependent effect of supervised Aerobic Exercise on HbA(1c) in patients with type 2 diabetes: a Meta-analysis of Randomized controlled trials. Sports Med 8:1919–1938. https://doi.org/10.1007/s40279-022-01673-4
Silva FM, Duarte-Mendes P, Teixeira AM, Soares CM, Ferreira JP (2024) The effects of combined exercise training on glucose metabolism and inflammatory markers in sedentary adults: a systematic review and meta-analysis. Sci Rep 1:1936. https://doi.org/10.1038/s41598-024-51832-y
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Conceptualization, IHI, ASB and SDY; methodology, IHI; search strategy, IHI, EA and AR; data extraction and analysis, IHI; writing—original draft preparation, IHI; writing—review and editing, IHI, ASB, SDY, AB and AR; supervision, IHI, ASB and SDY. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
For this type of study, formal consent is not required.
Consent for publication
This article does not contain any studies with human participants performed by any of the authors.
Disclaimer
The views expressed in this article are those of the authors and do not represent the views of the affiliated institutions, or of the U.S Department of Veterans Affairs.
Conflict of interest
No Conflicts of Interest. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iftikhar, I.H., Reynolds, A.M., AlBisher, E. et al. Aerobic exercise training and obstructive sleep apnea: dose-response meta-analyses. Sleep Breath (2024). https://doi.org/10.1007/s11325-024-03158-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11325-024-03158-2