Abstract
Introduction
Regular exercise is confirmed as a lifestyle treatment option for all obstructive sleep apnea (OSA) patients. It has beneficial effects other than weight loss, although the mechanisms remain unclear. Autonomic function imbalance plays an important role in OSA, so that it is meaningful to observe the effect of exercise on autonomic function.
Methods
Seventy mild to moderate OSA patients were divided into two groups. The exercise group received a 12-week exercise program prescribed according to their first cardiopulmonary exercise tests, while the control group kept previous lifestyle. All patients underwent blood tests, cardiopulmonary exercise tests, and polysomnography studies at enrollment and at the 12-week’s follow-up.
Results
At the end of 12 weeks, three patients of the exercise group did not complete the program due to lack of adherence. The current study showed 12-week aerobic exercises could improve body mass index (27.6 ± 4.7 kg/m2 vs. 24.5 ± 4.2 kg/m2, P < 0.05), exercise capacities, apnea-hypopnea index (total AHI 20.2 ± 7.5 vs. 16.4 ± 5.2, P < 0.05; supine AHI 22.1 ± 6.3 vs. 18.3 ± 4.9, P < 0.05), average oxyhemoglobin saturation (AverSpO2), time/percentage SpO2 below 90%, and heart rate recovery (HRR) of OSA patients. Moreover, AverSpO2 change was significantly associated with HRR change in the exercise group.
Conclusions
Our findings suggested regular aerobic exercise had beneficial effects on body mass index, functional capacity, intermittent hypoxia, and parasympathetic tone of OSA patients, and whether parasympathetic tone modification plays a role in improving intermittent hypoxia or not deserves further exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common clinical condition in which repetitive episodes of partial or complete occlusion of the upper airway occur during sleep. These episodes lead to intermittent hypoxia (IH) and arousals from sleep. OSA is diagnosed by polysomnography (PSG), which is a test that allows the calculation of the apnea-hypopnea index (AHI), the ratio of the total number of apneas, and hypopneas to total sleep time. The severity of OSA is determined by the AHI: 5.0–14.9 events/h, mild OSA; 15–30 events/h, moderate OSA; and ≥ 30 events/h, severe OSA [1]. Growing evidence has indicated that OSA is independently correlated with metabolic dysregulations such as diabetes, arterial hypertension, hypercholesterolemia, obesity, increased risk of cardiovascular events as well as atherosclerosis [2,3,4]. The underlying mechanisms are still unclear, but some fundamental features have been identified, such as increased sympathetic activation, endothelial dysfunction, oxidative stress, and possibly systemic inflammation [5].
Recommended therapy, such as continuous positive airway pressure (CPAP) treatment, oral appliance therapy, and upper airway surgery can relieve symptoms; especially, CPAP still remains the first-line therapy for severe and moderate OSA patients. Data from small trials provide evidence that CPAP treatment improves not only patient-reported outcomes such as sleepiness, quality of life, and mood but also intermediate cardiovascular end points such as blood pressure, cardiac ejection fraction, vascular parameters, and arrhythmias. However, data from large-scale randomized controlled trials do not consistently support a role for reducing cardiovascular mortality [6]. The American Academy of Sleep Medicine recommends exercise as a lifestyle treatment option for all OSA patients [7]. Recent studies have focused on exercise programs because they constitute a low-cost easy-to-use treatment modality, and have been shown to be effective in mitigating several harmful consequences of OSA, including AHI severity [8], glucose intolerance [9], and inflammatory profiles [10].
The mechanisms whereby physical exercise attenuates OSA have not yet to be well defined. Several hypotheses have been proposed: increasing upper airway dilator muscle tone, increasing slow-wave sleep, reducing fluid accumulation in the neck, reducing body weight, and reducing the systemic inflammatory response [11]. As we know, autonomic dysfunction is closely associated with cardiovascular disease development. Previous research also revealed autonomic dysfunction in OSA patients who belong to a specific population with a high risk of developing cardiovascular diseases. Different indexes were used in those studies, such as sympathetic skin responses from the neck [12] and multi-unit muscle sympathetic nerve activity [13] to represent sympathetic tone, heart rate variability to represent sympathovagal modulation [14], and heart rate recovery (HRR) at 1 min after exercise termination to represent vagal tone [15]. However, there are few data to indicate whether there is improvement in autonomic dysfunction in OSA patients after regular aerobic exercise training. Moreover, personalized patient management is encouraged in OSA patients. In this respect, we prescribed a 12-week exercise program based on the patient’s cardiopulmonary exercise test (CPET) performance to observe if there was a beneficial effect on autonomic dysfunction. We adopted serum norepinephrine (NE) levels and HRR to represent sympathetic nervous tone and parasympathetic nervous tone, respectively. We also tried to investigate if there is any association with any other improvement in OSA patients, which would be very meaningful to explore underlying pathways.
Patients and methods
Study population
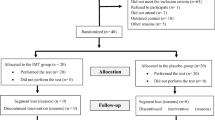
Seventy patients were consecutively enrolled as they were referred to the sleep laboratory in Tongji Hospital to be examined for suspected sleep apnea. The inclusion criteria comprised newly diagnosed mild to moderate OSA (AHI 5 to 30/h with clinical symptoms) and rejection of CPAP therapy, none of whom received surgical or mechanical ventilation treatment prior to inclusion. The exclusion criteria were patients with diabetes mellitus, hypertension, coronary artery disease (angina and/or electrocardiogram signs of ischemia on treadmill-exercise test), peripheral arterial disease, thyroid disorder, severe ventricular arrhythmia, severe reduction of LVEF (left ventricular ejection fraction, LVEF ≤ 45%), valvular disease requiring surgery, severe renal dysfunction (i.e., creatinine > 2.5 mg/dl), severe orthopedic problems that would prohibit exercise, history of psychiatric or neurodegenerative disorders, acute systemic illness, or circadian desynchrony (e.g., shift workers). The enrolled OSA patients were assigned by alternate randomization in a 1:1 ratio into two groups, stratified by age and sex. One group received 12 weeks of supervised exercise training (exercise group), and the other was the control group who maintained their previous lifestyle. Our study met the ethical standards, and it was also approved by the Ethics Committee of Tongji Hospital. All patients gave their written informed consent.
Laboratory measurement
A venous blood sample was collected from each participant under fasting condition. Fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, and creatinine were measured using standard laboratory methods. Serum NE level was determined by sandwich immunoassay with an ELISA-kit (IBL, Germany), according to the manufacturer’s methods.
Polysomnographic data collection
Polysomnographic recording was performed using Alice 6 (Philips, Netherlands). All participants underwent 7-h nighttime monitoring with PSG in bed. No alcohol or caffeine consumption was permitted during the study. Apnea was defined as being without respiration for more than 10 s. Hypopnea was defined as the deduction of at least 50% ventilation causing a drop in arterial saturation of at least 4%. OSA could be viewed as apnea or hypopnea occurring at least five times per hour, persisting for more than 10 s. The AHI documents the number of apnea-plus-hypopnea incidents every hour during sleep and was summarized by body position. Total AHI and supine AHI were recorded. The oxygen desaturation index (ODI) assesses the average number of oxygen desaturation incidents every hour during sleep. Oxyhemoglobin saturation during wakefulness (SpO2wakefulness), average oxyhemoglobin saturation (AverSpO2), minimum oxyhemoglobin saturation (MinSpO2), arousal index, and time/percentage SpO2 below 90% were collected.
Cardiopulmonary Exercise Test
All OSA patients underwent an incremental Cardiopulmonary Exercise Test (CPET) on a bicycle ergometer at the beginning of the study and 12 weeks later. To stabilize respiratory exchange, patients were asked to remain still on the ergometer for at least 3 min before starting the exercise. CPET was performed according to a symptom-limited Bruce’s protocol with continuous electrocardiographic monitoring, and cuff blood pressure was manually recorded every 2 min. Blood pressure was recorded at rest, at the end of each stress stage, at peak stress, and at recovery. The test was stopped for any of the following reasons: a rating of perceived exertion > 17 (Borg scale), achievement of > 90% of age-predicted maximum heart rate (HRmax = 220 − age), if the subject was too fatigued to continue the test safely, systolic blood pressure > 200 mmHg, typical chest discomfort, severe arrhythmias, or more than 1 mm horizontal or down-sloping ST segment depression. Respiratory gas exchange measurements were obtained breath-by-breath with the use of a computerized metabolic monitor (Innocor 5.0, Innovision Cor., Denmark). VO2peak was recorded as the mean value of VO2 during the last 20 s of the test and expressed in ml × kg−1 × min−1. Powermax was the maximal workload during exercise. The ventilatory anaerobic threshold (AT) was assessed mathematically, and VO2 at AT (VO2AT) was recorded. After achievement of peak exercise, the test was almost immediately terminated while the subjects were in a seated position. The HRR was obtained by subtracting the HR at the first minute of recovery from the peak HR obtained during the exercise. Therefore, the subjects in our CPET did not go through a “cool-down” phase. It was reported that the value of HRR might be affected by the small workload during the “cool-down” phase, decreasing its diagnostic sensitivity [16, 17]. The whole process was supervised by a cardiologist and a nurse who were unaware of the subject’s clinical history.
Training protocol
Exercise group patients attended exercise training three times per week. Training sessions, performed under continuous electrocardiogram monitoring, were supervised by a cardiologist and a nurse. Each session was preceded by a 15-min warm-up and followed by a 15-min cool-down. Exercise was performed for 30 min on a bicycle ergometer at VO2AT (using target HR as a scale), which was determined at the initial symptom-limited CPET.
Statistics
Descriptive statistics were reported as mean values ± SD. Comparison between groups for continuous variables were made using a t test or one-way analysis of variance where appropriate. Correlations were determined with the Pearson’s or Spearman’s correlation tests as appropriate. Differences between the two groups and changes over time within each group were assessed by two-way repeated measures ANOVA. A P value below 0.05 was considered statistically significant. All statistical analyses were performed using the software package SPSS, version 16.0 (SPSS Inc., Chicago, USA).
Results
During the course of the study, three patients did not complete the exercise program due to lack of adherence to the training regimen. No statistically significant differences were found at baseline between the exercise group and the control group in clinical, demographic, PSG characteristics as well as CPET indexes (Table 1).
At the end of 12 weeks, we found the patients’ exercise capacities (VO2peak, Powermax, AT, and VO2AT) elevated and body mass index decreased in the exercise group (P < 0.05, Table 2), and there was no change in the control group. Moreover, HRR in the exercise group increased significantly (P < 0.01, Table 2), while there was no significant change in NE level in either group.
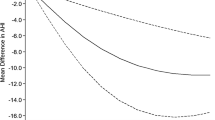
As for those PSG parameters, it was found that AHI (total AHI and supine AHI) and time percentage SpO2 below 90% decreased significantly, while AverSpO2 increased significantly in the exercise group. But there was no obvious change in ODI, MinSpO2, and arousal index in either group (Table 2). In the exercise group, there was a significant positive correlation between the change of AverSpO2 and the change of HRR (r = 0.217, P < 0.01, Fig. 1).
Discussion
A growing amount of published data has confirmed that regular physical exercise could have valid benefits to OSA patients, including improving both sleeping quality and some cardiovascular risk factors. To our knowledge, there is a lack of studies evaluating the effects of regular aerobic training on OSA patients in China. The current study showed that 12 weeks of aerobic exercise could improve the exercise capacities, body mass index, AHI, AverSpO2, time percentage SpO2 below 90%, and HRR of OSA patients. Moreover, the change in AverSpO2 was significantly associated with the change in HRR in the exercise group. Those findings suggested that the beneficial effects of exercise on parasympathetic tone might be partly responsible for improvement of IH in OSA patients, which is a vital pathway deserving further exploration.
According to the American Academy of Sleep Medicine, CPAP should be the first-line treatment for moderate to severe OSA (AHI > 15 events/h). However, many patients struggle with this therapy, for which adherence remains unacceptably low [18, 19]. Changes in lifestyle habits, including weight loss, regular physical activity, discontinuation or replacement of drugs that directly interfere with upper airway muscle function, reducing alcohol consumption, and smoking cessation are always encouraged in all OSA patients [7]. Among those lifestyle modifications, regular exercise has gradually gained great attention because clinical trials have illustrated its beneficial effects other than weight loss. Kline et al. enrolled 43 patients with moderate OSAS and carried out a 12-week supervised exercise program [20]. They found reduced AHI and ODI. Similarly, it was reported that AHI, functional outcomes of sleep questionnaire (FOSQ) scores, and short form-36 (SF-36) scores for quality of sleep and health-related quality of life were improved after 12 weeks of exercise training (1.5 h, 3 days weekly) in mild to moderate OSA patients [21]. In the current study, we found that 12 weeks of exercise training decreased AHI and increased AverSpO2. Our sample size, OSAS severity of enrolled subjects and exercise duration were similar to those previous investigations. Recently, a meta-analysis of nine studies revealed that exercise as the sole treatment for OSA could lead to reduction in the AHI and in daytime sleepiness [22].
As an optimal exercise program for OSA treatment should identify the type, frequency, and intensity of exercise; length of program; duration of individual session; and number of sessions per week, we prescribed exercise based on each OSAS patient’s CPET performance in an effort to approach personalized therapy. As we expected, the exercise capacity of the exercise group was improved at the 12-week follow-up. VO2peak is considered to be one of the best measurements of cardiovascular fitness. It was reported that supervised exercise enhanced the VO2peak/exercise capacity not only in mild to moderate OSAS patients [23] but also in severe OSA patients [24], even in OSA patients with heart failure [25]. After 12 weeks of training, we observed enhanced VO2peak, Powermax, AT, and VO2AT, which suggested that both exercise capacity and aerobic metabolism were improved, because AT represented the point at which anaerobic metabolism started with production of lactic acid. The reasons for the increases in exercise parameters were out of our study scope and we will try to explore it in subsequent studies.
Epidemiological research indicates that OSA is associated with the incidence and progression of cardiovascular diseases [6]. Although the underlying mechanisms are still not well defined, some common traits, including autonomic nervous system imbalance, endothelial dysfunction, and oxidative stress are confirmed as contributors to this increased risk [5]. As CPAP treatment is accepted as a classic therapeutic modality for moderate and severe OSA patients, earlier clinical observations have proved its beneficial effects on autonomic functions in this specific population [26,27,28]. Likewise, whether exercise training would bring about a favorable influence on autonomic nerve imbalance deserves our attention. Serum NE level, a typical sympathetic index, was used in our study. However, we did not find any change in NE levels in either of those two groups. It was reported that 1 month of CPAP treatment resulted in a significant reduction in 24-h urinary NE levels only in severe OSA patients, not in patients with mild-moderate OSA [29]. As for parasympathetic tone assessment, we used the index of HRR, which quantifies the decrease in heart rate following exercise. It is a useful straightforward method and a highly reproducible tool. Slower HRR has been identified as a powerful prognostic parameter for mortality in cardiovascular patients [30, 31]. Maeder et al. reported that the severity of OSAS, expressed as higher AHI, was independently associated with lower HRR [15]. Meanwhile, a change in HRR after exercise training was found in patients with chronic obstructive pulmonary disease [32], type 2 diabetes mellitus [33], and chronic heart failure [34]. Kline et al. reported that blunted HRR was improved following 12 weeks’ exercise training in overweight OSA patients and AHI change was associated with change in HRR at 5-min post-exercise [20]. Similarly, we found a significant increase in HRR in the exercise group at the end of the 12-week follow-up. Moreover, the enhancement of AverSpO2 was significantly associated with the increase in HRR, which suggested that the improvement of ANS induced by exercise might be an important pathway of ameliorating IH in OSA patients. As we know, IH is a key feature of OSA. Our findings were so encouraging that further studies enrolling more patients are needed to clarify the underlying mechanisms.
Certain limitations to the current study should be considered. First, this was an observational and a single-center study in which only a small number of OSA patients were available for analysis; second, we used the NE level as the measure of sympathetic activity. Although NE was previously shown to be elevated in OSA patients [35], it has limitations, in particular its nonspecificity. Therefore, we were cautious in our interpretation that sympathetic activity was unaffected after exercise training; third, we did not collect subjective sleep quality data, such as the Epworth Sleeping Scale (ESS) and Pittsburgh Sleep Quality Index (PSQI). Those subjective parameters were included to evaluate the effects of regular exercises in earlier reports; fourth, we used the subjects’ single night of laboratory PSG performances at baseline and at 12 weeks’ follow-up, which likely introduced additional variability in measures of OSA severity [36]. Another limitation of our study was that we did not observe any correlation between HRR change and any other PSG index. The causative mechanisms of the only significant correlation between HRR change and AverSpO2 change were difficult to hypothesize.
Conclusions
In conclusion, 12 weeks of aerobic exercise induced a significant improvement in the body mass index, AHI, AverSpO2, time percentage SpO2 below 90%, and HRR in OSA patients. Furthermore, the increase in AverSpO2 was positively associated with HRR change. These findings suggested that regular personalized aerobic training could reverse OSA severity to a certain extent. Modification of the automatic nervous system might be a vital pathway for the exercise effect on IH of OSA patients, which will be explored in future research.
References
Laratta CR, Ayas NT, Povitz M, Pendharkar SR (2017) Diagnosis and treatment of obstructive sleep apnea in adults. CMAJ 189(48):E1481–E1488
Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, Lavie L, Pépin JL (2015) Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 1:15015
Patinkin ZW, Feinn R, Santos M (2017) Metabolic consequences of obstructive sleep apnea in adolescents with obesity: a systematic literature review and meta-analysis. Child Obes 13(2):102–110
Schaefer CA, Adam L, Weisser-Thomas J, Pingel S, Vogel G, Klarmann-Schulz U, Nickenig G, Pizarro C, Skowasch D (2015) High prevalence of peripheral arterial disease in patients with obstructive sleep apnoea. Clin Res Cardiol 104(9):719–726
Turnbull CD (2018) Intermittent hypoxia, cardiovascular disease and obstructive sleep apnoea. J Thorac Dis 10(Suppl 1):S33–S39
Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S, INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) (2017) Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation 136(9):1840–1850
Dobrosielski DA, Papandreou C, Patil SP, Salas-Salvadó J (2017) Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur Respir Rev 26(144). https://doi.org/10.1183/16000617.0110-2016
Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE (2012) Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med 125(5):485–490
Alves ES, Lira FS, Santos RV, Tufik S, de Mello MT (2011) Obesity, diabetes and OSAS induce of sleep disorders: exercise as therapy. Lipids Health Dis 10:148
Alves Eda S, Ackel-D'Elia C, Luz GP, Cunha TC, Carneiro G, Tufik S, Bittencourt LR, de Mello MT (2013) Does physical exercise reduce excessive daytime sleepiness by improving inflammatory profiles in obstructive sleep apnea patients? Sleep Breath 17(2):505–510
Andrade FM, Pedrosa RP (2016) The role of physical exercise in obstructive sleep apnea. J Bras Pneumol 42(6):457–464
Korkmaz B, Benbir Şenel G, Kızıltan ME, Karadeniz D (2016) Demonstration of sympathetic dysfunction in patients with obstructive sleep apnea syndrome by measuring sympathetic skin responses from the neck. Sleep Med 25:13–15
Hamaoka T, Murai H, Kaneko S, Usui S, Okabe Y, Tokuhisa H, Kato T, Furusho H, Sugiyama Y, Nakatsumi Y, Takata S, Takamura M (2016) Single-unit muscle sympathetic nerve activity reflects sleep apnea severity, especially in severe obstructive sleep apnea patients. Front Physiol 7:66
Gammoudi N, Ben Cheikh R, Saafi MA, Sakly G, Dogui M (2015) Cardiac autonomic control in the obstructive sleep apnea. Libyan J Med 10:26989
Maeder MT, Münzer T, Rickli H, Schoch OD, Korte W, Hürny C, Ammann P (2008) Association between heart rate recovery and severity of obstructive sleep apnea syndrome. Sleep Med 9(7):753–761
Georgoulias P, Orfanakis A, Demakopoulos N, Xaplanteris P, Mortzos G, Vardas P, Karkavitsas N (2003) Abnormal heart rate recovery immediately after treadmill testing: correlation with clinical, exercise testing, and myocardial perfusion parameters. J Nucl Cardiol 10(5):498–505
Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS (2001) Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation 104(16):1911–1916
Libman E, Bailes S, Fichten CS, Rizzo D, Creti L, Baltzan M, Grad R, Pavilanis A, Tran DL, Conrod K, Amsel R (2017) CPAP treatment adherence in women with obstructive sleep apnea. Sleep Disord 5:1–8
Olsen S, Smith S, Oei T, Douglas J (2008) Health belief model predicts adherence to CPAP before experience with CPAP. Eur Respir J 32(3):710–717
Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, Davis JM, Youngstedt SD (2013) Blunted heart rate recovery is improved following exercise training in overweight adults with obstructive sleep apnea. Int J Cardiol 167(4):1610–1615
Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, Davis JM, Youngstedt SD (2011) The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep 34(12):1631–1640
Aiello KD, Caughey WG, Nelluri B, Sharma A, Mookadam F, Mookadam M (2016) Effect of exercise training on sleep apnea: a systematic review and meta-analysis. Respir Med 116:85–92
Norman JF, Von Essen SG, Fuchs RH, McElligott M (2000) Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online 3(3):121–129
Ackel-D'Elia C, da Silva AC, Silva RS, Truksinas E, Sousa BS, Tufik S, de Mello MT, Bittencourt LR (2012) Effects of exercise training associated with continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Sleep Breath 16(3):723–735
Servantes DM, Pelcerman A, Salvetti XM, Salles AF, de Albuquerque PF, de Salles FC, Lopes C, de Mello MT, Almeida DR, Filho JA (2012) Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clin Rehabil 26(1):45–57
Jurysta F, Kempenaers C, Lanquart JP, Noseda A, van de Borne P, Linkowski P (2013) Long-term CPAP treatment partially improves the link between cardiac vagal influence and delta sleep. BMC Pulm Med 13(1):1–11
Chrysostomakis SI, Simantirakis EN, Schiza SE, Karalis IK, Klapsinos NC, Siafakas NM, Vardas PE (2006) Continuous positive airway pressure therapy lowers vagal tone in patients with obstructive sleep apnoea-hypopnoea syndrome. Hell J Cardiol 47(1):13–20
Maser RE, Lenhard MJ, Rizzo AA, Vasile AA (2008) Continuous positive airway pressure therapy improves cardiovascular autonomic function for persons with sleep-disordered breathing. Chest 133:86–91
Pinto P, Bárbara C, Montserrat JM, Patarrão RS, Guarino MP, Carmo MM, Macedo MP, Martinho C, Dias R, Gomes MJ (2013) Effects of CPAP on nitrate and norepinephrine levels in severe and mild-moderate sleep apnea. BMC Pulm Med 13:13
Lachman S, Terbraak MS, Limpens J, Jorstad H, Lucas C, Scholte Op Reimer W, Boekholdt SM, Ter Riet G, Peters RJG (2018) The prognostic value of heart rate recovery in patients with coronary artery disease: a systematic review and meta-analysis. Am Heart J 199:163–169
Peçanha T, Silva-Júnior ND, Forjaz CL (2014) Heart rate recovery: autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin Physiol Funct Imaging 34(5):327–339
Mohammed J, Derom E, Van Oosterwijck J, Da Silva H, Calders P (2018) Evidence for aerobic exercise training on the autonomic function in patients with chronic obstructive pulmonary disease (COPD): a systematic review. Physiotherapy 104(1):36–45
Bhati P, Shenoy S, Hussain ME (2018) Exercise training and cardiac autonomic function in type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr 12(1):69–78
Dimopoulos S (2015) Abnormal heart rate recovery in patients with heart failure: an important target for exercise training treatment. Anatol J Cardiol 15(9):735–736
Chaidas K, Tsaoussoglou M, Theodorou E, Lianou L, Chrousos G, Kaditis AG (2014) Poincare plot width, morning urine norepinephrine levels, and autonomic imbalance in children with obstructive sleep apnea. Pediatr Neurol 51(2):246–251
Levendowski DJ, Zack N, Rao S, Wong K, Gendreau M, Kranzler J, Zavora T, Westbrook PR (2009) Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath 13(2):163–167
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Huan Zheng received a research grant from the National Nature Science Foundation of China (Award Number: 81400206). Other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee Review Board of Tongji Hospital (Shanghai, China).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yang, H., Liu, Y., Zheng, H. et al. Effects of 12 weeks of regular aerobic exercises on autonomic nervous system in obstructive sleep apnea syndrome patients. Sleep Breath 22, 1189–1195 (2018). https://doi.org/10.1007/s11325-018-1736-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1736-1