Abstract
Purpose
To determine the possible associations between total sleep time spent with arterial oxygen saturation < 90% (T90) and comorbid cardiometabolic diseases (CMDs) in patients with severe obstructive sleep apnea (OSA).
Methods
A retrospective review of the chart was conducted in patients with severe OSA diagnosed by in-lab polysomnography (PSG) between January 2018 and December 2019 at Siriraj Hospital. The patients were divided into two groups: hypoxic (T90 ≥ 10%) and nonhypoxic (T90 < 10%). The association between common CMDs including hypertension (HT), type 2 diabetes mellitus (T2DM), and impaired fasting glucose (IFG) was investigated and compared between the two groups.
Results
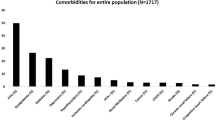
Data were collected from 450 patients with severe OSA, 289 males/161 females with a mean age of 53.5 ± 14.2 years and an apnea-hypopnea index (AHI) of 49.6 events/h. Among these, 114 patients (25.3%) were defined as the hypoxic group (T90 ≥ 10%). When compared between the hypoxic and nonhypoxic groups, the patients in the hypoxic group were significantly younger and more obese, and had a higher proportion of male patients. The majority of patients (80%) had at least one CMD; however, the most common comorbidities significantly associated with hypoxic OSA (T90 ≥ 10%) were HT and IFG.
Conclusion
Hypoxic burden is significantly associated with an increased prevalence of HT and IFG in patients with severe OSA. T90 may be potentially useful for predicting CMDs in these patients. However, prospective studies are still required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a highly prevalent sleep disorder characterized by frequent or dynamic collapses of the upper airways while the patient sleeps. OSA can have a negative effect on a patient’s health and quality of life, such as persistent morning headache, dizziness, excessive daytime sleepiness (EDS), mood instability, and an increased risk of traffic accidents. Without proper management, OSA can eventually become a significant contributor to metabolic diseases [1], particularly hypertension (HT) and type 2 diabetes mellitus (T2DM) [2], cardiac arrhythmia [3], coronary artery disease (CAD) [4], and neurocognitive impairment.
Although the apnea-hypopnea index (AHI), calculated from polysomnography (PSG) [5], continues to be the parameter most used in clinical practice as a diagnostic and severity grading tool for OSA, it is not an ideal marker to visualize the clinical status of patients [6]. Several studies have demonstrated that AHI does not accurately reflect OSA severity, OSA phenotypes, or comorbid diseases [6]. One reason for this inaccuracy is that the AHI does not capture the extent and duration of oxygen desaturation [7]. To better characterize the clinical presentation of patients with OSA, various studies [8, 9] have attempted to identify and use other new measures in addition to AHI. These included arousal intensity, odds ratio product, cardiopulmonary coupling [10], hypoxic burden [9], and others. Recently, a consensus has emerged that hypoxia at night or hypoxic burden, especially when severe, has detrimental consequences on cardiometabolic function [9, 11].
Sleep time with arterial oxygen saturation below 90% (T90), which can be obtained regularly from an overnight sleep test, is one of the metrics that may be useful to represent the hypoxic burden. However, due to the lack of compelling data and the T90 duration cut-off percentage, the most recent guidelines do not highlight the usefulness of this measure [12, 13].
Although there have been studies [14,15,16] examining the relationship between T90 and CMD, most of the studies have investigated patients with moderate to severe OSA. The current study was designed to focus on hypoxic burden in patients with severe OSA (AHI ≥ 30 events/h) since they were the majority (>60%) in our sleep center and previous evidence did not support a strong association between the mild-to-moderate OSA and common CMDs [17, 18]. Furthermore, the presence of significant hypoxic burden (T90 > 10%) in patients with mild-to-moderate OSA is uncommon. However, employing AHI as the only criterion for OSA severity is knowingly controversial due to heterogeneous characteristics of the disorders; utilizing hypoxic load, particularly T90 as an addition, is of interest [14, 19]. In addition, the T90 cut-off value used in prior studies has varied and has been inconsistent. Therefore, the objective of this study was to investigate relationships between OSA-related hypoxic burden as reflected by T90 ≥ 10% and the risk of common CMD in adult patients with newly diagnosed severe OSA.
Materials and methods
Study design
This retrospective study was conducted after the protocol was approved by the Siriraj Institutional Review Board (SIRB). Data from relevant electronic medical records including demographic data (age and sex), body mass index (BMI), underlying diseases [impaired fasting glucose (IFG), T2DM, HT, CAD, dyslipidemia (DLP), stroke, congestive heart failure (CHF), atrial fibrillation (AF), cardiomegaly], and important PSG parameters were obtained between January 2018 and December 2019.
Selection criteria
The inclusion criteria were patients aged ≥ 18 years old who were diagnosed with severe OSA (AHI ≥ 30 events/h) by PSG in the lab. The exclusion criteria were patients with sleep efficiency (SE) < 50%, total sleep time (TST) < 2 h, severe technical error during the sleep test, significantly incomplete data, and patients with history of active or uncontrolled lower respiratory tract diseases such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary emphysema, pneumonia, pulmonary edema, severe bronchiectasis, and pulmonary embolism.
Polysomnography
PSG standard protocol recording channels consisted of electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), abdominal and chest wall movements, pulse oxygen saturation, nasal flow, and nasal thermistor. The scoring was performed by certified sleep technicians and sleep specialists with criteria following the guidelines of the American Academy of Sleep Medicine (AASM) scoring manual update in 2017 (version 2.4) criteria; an apnea was defined as a reduction in airflow of ≥ 90% lasting for 10 s of pre-event baseline using oronasal thermistor and a hypopnea was defined as a reduction in airflow of ≥ 30% lasting for 10 s of pre-event baseline using nasal pressure with an associated oxygen desaturation of ≥ 3% or EEG arousals. Relevant PSG data collected included TST, SE, AHI, apnea index (AI), hypopnea index (HI), percentage of T90, oxygen desaturation index (ODI), mean peripheral oxygen saturation (meanSpO2), and minimum SpO2 (minSpO2).
Cardiometabolic diseases
The CMDs in this study included HT, T2DM, IFG, DLP, AF, CAD, CHF, cardiomegaly, and stroke. These diagnoses were determined by participant interviews and medical records. Both involved test findings, information from the International Statistical Classification of Diseases (ICD)-10 (with or without a record of a specific condition in the disease), and information on medications used to treat underlying diseases. The definitions of CMDs used in this study were as follows: HT: systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg for two or more days; T2DM: fasting plasma glucose (FPG) ≥ 126 mg/dL, or glycated hemoglobin (HbA1c) ≥ 6.5%; IFG: FPG 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L); AF: irregular supraventricular tachyarrhythmia diagnosed from ECG; DLP: following the 2016 Clinical Practice Guideline on Pharmacologic Therapy of Dyslipidemia for the Prevention of Atherosclerotic Cardiovascular Disease Prevention; cardiomegaly: chest radiographs that demonstrate a cardiothoracic ratio > 0.5 or from ECG; CAD: angiography showing > 50% stenosis in the coronary arteries; stroke: ischemic or hemorrhagic stroke diagnosed by clinical, neurological examination and computed tomography (CT) or magnetic resonance imaging (MRI) of the brain; and CHF: chest radiographs revealing pulmonary venous congestion or interstitial edema.
Statistical analysis and outcome measurement
Continuous data are presented as mean ± standard deviation (SD) for parametric data, and median with 25th–75th percentile for nonparametric data. Categorical data are presented as number and percentage. The association between hypoxic burden (T90) in severe OSA and common CMDs in each group together with comparing between two groups were evaluated by Chi-square test, T-test, or Fisher’s exact test. Logistic regression analysis or multiple regression analysis was used depending on the types of results. A significant difference was established at the P-value < 0.05. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 18.
Results
Data from 450 patients with severe OSA were collected. These included 289 males and 161 females, with a mean age of 53.5 ± 14.2 years and a mean BMI of 30.6 ± 7.3 kg/m2. The majority of patients in this study (80%) had at least one CMD, the most prevalent of which was HT. There were 114 patients (25.3%) who had T90 ≥ 10% and 293 patients (65.1%) with 3%ODI ≥ 30 events/h. Additional information on PSG data of these patients are shown in Table 1. When compared between the hypoxic and nonhypoxic groups (Table 2), the patients in the hypoxic group were significantly younger and more obese, and had a higher proportion of males. Several PSG parameters were also significantly different between these two groups. When comparing prevalence of CMDs between the two groups, those of the overall CMDs, HT, and IFG were significantly lower in the nonhypoxic group (Table 2).
Discussion
In addition to AHI, sleep parameters related to hypoxic burden [9, 20] or hypoxia at night may be useful to characterize OSA that could be associated with a worsening of cardiometabolic function. The findings of this study showed that patients with severe OSA and T90 levels above 10% (hypoxic group) tended to be younger, more obese, male-dominated, and more abnormal in PSG parameters that may be related to concurrent CMD. These findings were consistent with previous studies showing that obesity is associated with an increased prevalence of prolonged duration of T90 [15] and more severe OSA [21], and that men were found more frequently in the group with severe desaturation [22]. Our findings were also consistent with a study by Labarca and colleagues [14, 15] which suggested that patients with OSA and T90 ≥ 10% had a sensitivity of 48.7% and a specificity of 72.7% to predict the potential risk of HT.
Although it appears that the T90 threshold cut-off points for clinical application were somewhat arbitrary and heterogeneous, the majority of the research findings [19, 20] showed that T90 with certain specified cut-off points was still potentially useful for anticipating common CMDs. In this study, we chose T90 of ≥ 10% as the cut-off criterion due to the following reasons. First, there were supporting evidences, such as that of Martinez et al. [19] and Quan et al. [23], which demonstrated that the levels of T90 close to this cut-off point have clinical significance. Second, the results of subsequent analysis in our study were consistent when we used higher cut-off levels of T90 (10%, 15%, and 20%); however, utilizing the lower T90 cut-off level (5%) failed to detect a significant association between OSA and common CMDs. The prevalence of T90 greater than 15% or 20% in our population is quite low, less than 15% compared to over 25% in T90 ≥ 10%; as a result, if we utilized these levels, we may have risked omitting some people who would benefit from appropriate therapies.
After using logistic regression analysis adjusted for confounders related to CMD, T90 ≥ 10% was the only independent parameter associated with IFG, while BMI and older age were considered risk factors for HT in this study. Most previous studies focused only on T2DM that was already known as a risk factor for CVD and mortality, but often ignored IFG, a phase between the individual with normal blood glucose and T2DM even though it may also increase the risk of CVD development [24]. The results of this study showed that T90 ≥ 10% may be one of the sensitive metrics to predict the risk of IFG in patients with severe OSA, supporting that OSA may be one of the important independent risk factors for the development of T2DM [25, 26].
The potential mechanisms by which OSA contributes to blood pressure elevation are multi-factorial. One of the most commonly described pathophysiology is that hypoxia induced by OSA causes systemic inflammation and the production of reactive oxygen species [27], which increased endothelin-1 production while decreasing nitric oxide production in endothelial cells, increasing arterial peripheral resistance, and ultimately raising blood pressure. Chronic hypoxia, in particular hypoxia with prolonged duration (greater degree of T90 severity), may play an essential role to provoke structural and biochemical abnormalities in pulmonary arteries and vascular cells [28] leading to pulmonary vasoconstriction, pulmonary arterial pressure elevation, and increased thickness of arterial walls. These alterations of arterial vessels are commonly found in patients with hypoxic OSA phenotype, whereas chronic intermittent hypoxia especially in severe OSA with prolonged T90 can considerably stimulate the carotid body (CB) or induce pancreas and adipose tissue to promote hyperinsulinemia, hyperleptinemia, and inflammation process. Signaling from CB to the sympathetic nervous system also generates an activation that leads to metabolic abnormality in insulin sensitive organs or tissue such as the pancreas, liver, skeletal muscle, and adipose tissue. This creates a vicious cycle that leads to metabolic diseases such as obesity, IFG, T2DM, and metabolic syndrome [11].
Two large cohort studies from Azarbarzin et al. [8], the Sleep Heart Health Study (SHHS) and the Osteoporotic Fractures in Men Study (MrOS), found that sleep apnea-specific hypoxic burden (SASHB) predicted incident HF in approximately 10 years of follow-up a fully adjusted model of untreated patients with moderate to severe OSA (HR 1.14, 95% CI 1.03–1.25). In contrast, AHI was not significantly predictive of cardiovascular mortality. According to our study, the prevalence of first diagnosed HF in the hypoxic group was also higher than in the nonhypoxic group but without statistical significance.
Recent post hoc analyses of the SAVE study (Sleep Apnea Cardiovascular Endpoints) [29] demonstrated that the pattern of oxygen desaturation during sleep was associated with heart failure and myocardial infarction but not AHI. Some other studies determining T90 also showed that it is associated with overall mortality [30]. Therefore, our findings support that the important parameters of OSA are not only the frequency of obstruction of the upper airways during sleep (as represented by AHI) but also the duration of sleep time associated with oxygen desaturation (as represented by T90), a simple indicator of hypoxic burden that needs attention.
There are limitations of this study. First, some risk factors for CMDs, such as a history of alcohol consumption or smoking, were not included due to incomplete data from the medical record. Second, other confounding factors such as concurrent subclinical upper and lower respiratory disorders in patients with CMDs that can increase hypoxemia during PSG cannot be completely excluded from this study. Third, well-known conditions, including night-to-night variation and first-night effect, may affect the accuracy of PSG implementation. Fourth, the diagnosis of each CMD was derived from electronic medical records described by physicians, but some were not confirmed by their original diagnostic criteria. Fifth, nearly half of the patients in our study underwent split-night sleep studies, so their TST was inherently less than 4 h, which could have an effect on the percentage of T90. Sixth, this research is a retrospective study with inherent biases. For future studies, prospective cohort investigation about the hypoxic burden in patients with moderate and mild forms of OSA should also be considered.
Conclusion
The results of this study demonstrated that there was a significant association between T90 ≥ 10% and comorbid IFG, HT in patients with severe OSA. This suggests that T90 may be another useful parameter in predicting high-risk patients who may have CMDs and planning patient monitoring. However, more well-designed prospective studies are needed.
Data availability
Raw data for dataset are not publicly available to preserve individuals’ privacy under the European General Data Protection Regulation.
References
Fallahi A, Jamil DI, Karimi EB, Baghi V, Gheshlagh RG (2019) Prevalence of obstructive sleep apnea in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Syndr 13(4):2463–2468
Lam JC, Mak JC, Ip MS (2012) Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology. 17(2):223–236
Dhakal SS, Neupane A, Bhattarai M, Karki DB (2020) Prevalence of atrial fibrillation in obstructive sleep apnea patients in a tertiary care center. JNMA J Nepal Med Assoc 58(222):80–83
Sterz U, Buchner S, Hetzenecker A, Satzl A, Debl K, Luchner A et al (2018) Erratum to: resolution of ST deviation after myocardial infarction in patients with and without sleep-disordered breathing. Somnologie (Berl) 22(2):130
Qaseem A, Dallas P, Owens DK, Starkey M, Holty JE, Shekelle P et al (2014) Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 161(3):210–220
Bailly S, Destors M, Grillet Y, Richard P, Stach B, Vivodtzev I et al (2016) Obstructive sleep apnea: a cluster analysis at time of diagnosis. PloS One 11(6):e0157318
Punjabi NM (2016) COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No. Chest 149(1):16–19
Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW et al (2020) The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest. 158(2):739–750
Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI et al (2019) The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 40(14):1149–1157
Thomas RJ, Weiss MD, Mietus JE, Peng CK, Goldberger AL, Gottlieb DJ (2009) Prevalent hypertension and stroke in the Sleep Heart Health Study: association with an ECG-derived spectrographic marker of cardiopulmonary coupling. Sleep. 32(7):897–904
Almendros I, Basoglu OK, Conde SV, Liguori C, Saaresranta T (2022) Metabolic dysfunction in OSA: is there something new under the sun? J Sleep Res 31(1):e13418
Akashiba T, Inoue Y, Uchimura N, Ohi M, Kasai T, Kawana F et al (2022) Sleep apnea syndrome (SAS) clinical practice guidelines 2020. Respir Investig 60(1):3–32
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG (2019) Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 15(2):335–343
Labarca G, Campos J, Thibaut K, Dreyse J, Jorquera J (2019) Do T90 and SaO2 nadir identify a different phenotype in obstructive sleep apnea? Sleep Breath 23(3):1007–1010
Labarca G, Dreyse J, Salas C, Letelier F, Schmidt A, Rivera F et al (2020) Clinical utility of oximetric parameters to identify a high-risk phenotype of moderate-severe obstructive sleep apnea (OSA). Clin Respir J 14(12):1166–1175
Andre S, Andreozzi F, Van Overstraeten C, Ben Youssef S, Bold I, Carlier S et al (2020) Cardiometabolic comorbidities in obstructive sleep apnea patients are related to disease severity, nocturnal hypoxemia, and decreased sleep quality. Respir Res 21(1):35
Brown LK (2007) Mild obstructive sleep apnea syndrome should be treated. Pro J Clin Sleep Med 3(3):259–262
Silva LOE, Guimaraes TM, Luz GP, Coelho G, Badke L, Almeida IR et al (2018) Metabolic profile in patients with mild obstructive sleep apnea. Metab Syndr Relat Disord 16(1):6–12
Martinez-Garcia MA, Campos-Rodriguez F, Barbe F, Gozal D, Agusti A (2019) Precision medicine in obstructive sleep apnoea. Lancet Respir Med 7(5):456–464
Chen F, Chen K, Zhang C, Chen X, Huang J, Jia P et al (2018) Evaluating the clinical value of the hypoxia burden index in patients with obstructive sleep apnea. Postgrad Med 130(4):436–441
Venkatnarayan K, Krishnaswamy UM, Rajamuri NKR, Selvam S, Veluthat C, Devaraj U, Ramachandran P, D'Souza G (2022) Identifying phenotypes of obstructive sleep apnea using cluster analysis. Sleep Breath. https://doi.org/10.1007/s11325-022-02683-2
Lin CM, Davidson TM, Ancoli-Israel S (2008) Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 12(6):481–496
Quan W, Zheng D, Douglas McEvoy R, Barbe F, Chen R, Liu Z et al (2018) High risk characteristics for recurrent cardiovascular events among patients with obstructive sleep apnoea in the SAVE study. EClinicalMedicine. 2-3:59–65
Emerging Risk Factors C, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S et al (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 375(9733):2215–2222
Muraki I, Wada H, Tanigawa T (2018) Sleep apnea and type 2 diabetes. J Diabetes Investig 9(5):991–997
Nagayoshi M, Punjabi NM, Selvin E, Pankow JS, Shahar E, Iso H et al (2016) Obstructive sleep apnea and incident type 2 diabetes. Sleep Med 25:156–161
Olea E, Agapito MT, Gallego-Martin T, Rocher A, Gomez-Nino A, Obeso A et al (2014) Intermittent hypoxia and diet-induced obesity: effects on oxidative status, sympathetic tone, plasma glucose and insulin levels, and arterial pressure. J Appl Physiol (1985) 117(7):706–719
Fagan KA, Fouty BW, Tyler RC, Morris KG Jr, Hepler LK, Sato K et al (1999) The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103(2):291–299
Linz D, Loffler KA, Sanders P, Catcheside P, Anderson CS, Zheng D et al (2020) Low prognostic value of novel nocturnal metrics in patients with OSA and high cardiovascular event risk: post hoc analyses of the SAVE study. Chest. 158(6):2621–2631
Rahangdale S, Yeh SY, Novack V, Stevenson K, Barnard MR, Furman MI et al (2011) The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J Clin Sleep Med 7(2):172–178
Acknowledgements
The authors thank Jeerapa Kerdnoppakhun, our assistant researcher, for all the help and support regarding documents and working process formalities and Chulaluk Komoltri and Saowalak Hunnangkul for providing sample size calculation and statistical analysis. We also thank all the medical staff at the Department of Otorhinolaryngology and Siriraj Sleep Center for their kind cooperation.
Author information
Authors and Affiliations
Contributions
MU, conception and design, data acquisition, collection, analysis, interpretation, drafting the article, final approval; WB, conception and design, data acquisition, interpretation, critical revisions, drafting the article, final approval, and being the corresponding author; WC, PK, SR, and CP, data acquisition, interpretation, critical revisions, final approval.
Corresponding author
Ethics declarations
Ethical approval
This retrospective study was conducted after the protocol was approved by the Siriraj Institutional Review Board (SIRB). For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uataya, M., Banhiran, W., Chotinaiwattarakul, W. et al. Association between hypoxic burden and common cardiometabolic diseases in patients with severe obstructive sleep apnea. Sleep Breath 27, 2423–2428 (2023). https://doi.org/10.1007/s11325-023-02860-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02860-x