Abstract
Including population genetic aspects into the selection of planting material within the framework of conservation and restoration measures is of vital importance for the long-term persistence of populations. This is especially true facing climate change since genetic diversity and the spread of potentially beneficial alleles are important for the adaptability of populations. Therefore, knowledge about genetic variability within and between populations is a critical aspect when determining provenance regions. In our study, we investigated the population genetic structure of a widespread, insect-pollinated and mainly bird-dispersed shrub species, Frangula alnus, on the basis of seven microsatellites and two chloroplast DNA markers throughout Germany. The aim was to determine the spatial, temporal and ecological processes genetically structuring populations to critically revise existing provenance regions. Therefore, we conducted analyses on different spatial scales (country-wide, regional and local) using the two different marker sets in addition to environmental variables. We detected distinct patterns on all spatial scales which indicated influences of historic recolonization processes, regional differences of seed dispersal across the landscape, as well as small-scale spatial genetic structures attributable to local dispersal processes. No relation of underlying environmental gradients such as temperature or precipitation and genetic patterns was found. We conclude that different aspects of historic and more recent gene flow shape population genetic structures and that a thorough analysis on a variety of spatial, temporal and environmental scales is necessary to appropriately select planting material for conservation and restoration measures. Correspondingly, management advice regarding provenance delineations will be provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In species conservation and restoration projects, considering the role of genetic aspects for the success and sustainability of planting measures becomes more and more an issue (Mijangos et al. 2015). This is because populations’ adaptation to local environmental conditions on the one hand and populations’ adaptability to changes in conditions on the other hand play a vital role for species’ persistence (McKay et al. 2005). Hence, gene flow is considered an important evolutionary force (Ellstrand 2014). It is a fundamental source of genetic diversity and enables the spread of beneficial alleles which can reinforce selective processes, both important aspects in the face of environmental change (Booy et al. 2000; Pautasso 2009; Pluess et al. 2016). Thus, if gene flow is too much restricted, populations can lack adaptive potential and can additionally suffer from inbreeding depressions (Booy et al. 2000; Hufford et al. 2012). On the other hand, however, gene flow can also be detrimental by counteracting local adaptation if selective forces are weak (Ellstrand 2014).
In restoration projects, it is often stated to use planting material of local origin in order to prevent maladaptation (McKay et al. 2005; De Kort et al. 2014; Beatty et al. 2015; Mijangos et al. 2015). Consequently, specifying so-called provenance regions (also seed zones or seed transfer zones) is a common strategy to select suitable planting material (Ying and Yanchuk 2006; Malaval et al. 2010; Barsch et al. 2012; Azpilicueta et al. 2013). These regions/zones are defined as distinct geographic areas of a limited range. Since patterns of gene flow and the strength and direction of gene-environment interactions are species-specific, it is, however, not clear which geographic and environmental scale defines “locality” considering both aspects of local adaptation and adaptability. Hence, knowing patterns of gene flow is an important prerequisite and stresses the need for genetic research.
One possibility to delineate provenance regions is to use eco-regions that differ regarding physiographic conditions (i.e. climate, soil, landforms, distribution of vegetation types, etc.). This approach is certainly the easiest to attain since information on related variables is usually readily available. Consequently, it is often employed (Miller et al. 2011; Potter and Hargrove 2012; Bower et al. 2014), and delineated provenance regions are even ruled by national legislation (Ying and Yanchuk 2006; Beatty et al. 2015). In Germany, for example, six provenance regions were determined on the basis of broad natural landscape units for 55 woody species as the basis for the selection of planting material in the open landscape (Barsch et al. 2012). It is under debate whether such a general delineation of provenance regions on the basis of merely environmental characteristics is a suitable approach. For example, genetic diversity and genetic differentiation patterns are strongly dependent on the life history of species (e.g. regarding dispersal traits) despite potential selective forces acting on local populations (Hamrick and Godt 1996; Austerlitz et al. 2000; Kremer et al. 2012). Hence, ignoring species-specific population genetic processes might compromise the effort to conserve the adaptive potential if provenance delineation based on environmental characteristics does not match population genetic patterns.
Population genetic patterns might also be shaped by historic processes. The re-immigration history of woody species into Central Europe frequently played a substantial role in interpreting patterns of genetic diversity and differentiation (Petit et al. 2003; Heuertz et al. 2004; Cornille et al. 2013; Havrdová et al. 2015). It is commonly accepted that at least for thermophile woody species, three main refugial areas contributed to postglacial re-immigration into Central Europe, the Iberian and/or Balkan refugia being effective refugia for northern biota whereas the Appenine refugia often being isolated (Taberlet et al. 1998). While a delimitation of refugial lineages sometimes has per se been taken for seed zone delineation (Bucci et al. 2007), particularly at so-called leading edges of recolonization, e.g. in German ranges, admixture between different refugial lineages, e.g. Iberian and Balkan lineages, may happen thereby explaining comparably high levels of genetic diversity (Hampe and Petit 2005; Havrdová et al. 2015). The latter may additionally serve as an important criterion for selecting restoration material. Hence, conservation and restoration measures focussing on the long-term population fitness should consider population genetic patterns and underlying processes potentially influencing genetic diversity (Hufford et al. 2012).
We aim at contributing to the understanding of processes shaping the multiscale population genetic structure of non-wind-pollinated and non-wind-dispersed woody species for an effective delineation of provenance regions to safeguard the sustainability of restoration measures. For a thorough analysis of recent gene flow, we investigated the population genetic structure of the insect-pollinated and bird-dispersed shrub species Frangula alnus using a set of seven microsatellite loci. To unravel patterns of population genetic diversity and differentiation and potential processes behind these patterns, we examined genetic data on three different scales, i.e. the country-wide scale, the regional (between populations within larger geographic regions) and the local scale (within populations). Additionally, we analysed populations for two chloroplast DNA (cpDNA) markers to identify potential underlying structures of postglacial recolonization. From a range wide study at chloroplast marker loci, it was concluded that F. alnus recolonized from a Balkan refugial area into Central Europe (Hampe et al. 2003). While those authors used a PCR-RFLP approach, we aimed at higher resolution analyses by sequencing the marker loci. Working on the sample design of our nuclear SSR study, our cpDNA data cover a much larger and thus more representative German distribution range of the species as compared to Hampe et al. (2003). A remaining question is whether there is a genetic imprint revealing/supporting a single refugial origin (the Balkan) without any admixture of other large refugial gene pools. If so, it could be helpful in creating a more accurate picture of past and recent gene flow as mediated by seed dispersal as one important mechanism behind the spatiotemporal distribution of genetic variation and diversity. Finally, we investigated relations of genetic data with geographic and environmental variables to check if environmental patterns match the genetic ones and thus if the delineation of provenance regions by environmental characteristics is justified.
Materials and methods
Studied species
F. alnus (alder buckthorn) is an insect-pollinated and mainly bird-dispersed deciduous shrub species that is naturally distributed throughout most parts of Europe as well as Western Asia (Hampe et al. 2003). In Central Europe, F. alnus is widespread and occurs as an early to mid-successional species on moist and often acid soils in open woods, forest edges and bogs with certain demands for light hence vanishing in dense forest stands (Hampe et al. 2003; Zecchin et al. 2016). In Germany, though usually no used in forestry, F. alnus is often planted in the open landscape, e.g. in hedgerows or along roadsides.
Sampling and genetic analysis of microsatellites and cpDNA
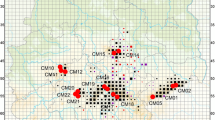
Overall, 30 populations throughout Germany were sampled (Fig. 1) in the years 2014 and 2015 with a total of 10 to 26 randomly selected individuals per population (Table 1). Populations were more or less evenly distributed across Germany to cover all six suggested provenance regions (Fig. 1). Distances between populations ranged from 51 to 730 km with an average of 85 km. Individuals of populations were sampled within a radius of about 15 km following a specific scheme with the closest individuals located at a median minimum distance of about 18 m and the farthest at a median maximum distance of about 8.3 km. The individual geographic position was recorded using GPS. Several leaves of each individual were collected, stored in paper bags and oven dried for 24 h at 36 °C prior to DNA extraction.
From about 30 mg of dried leaf tissue, DNA was extracted according to the protocol of Eimert et al. (2003). All individuals were genotyped at seven microsatellite loci identified by Rigueiro et al. (2009), i.e. FaA110, FaA3, FaA7, FaB106, FaB4, FaB8 and FaB9. PCR was conducted individually for each locus differing in concentrations of PCR chemicals and PCR cycles (for details, see Supplementary material, S1). PCR products were separated by a capillary electrophoresis using a Beckmann Coulter CEQ 8800 (Beckmann, Fullerton, CA, USA) and sizes scored using GenomeLab GeXP (Beckmann, Fullerton, CA, USA). Allele binning was conducted using the R package MsatAllele (Alberto 2009).
Furthermore, two chloropast DNA regions were sequenced using two primer pairs, i.e. trnD/trnT and trnS/trnG (Hampe et al. 2003), for a subsample of each population (Table 1 and Supplementary material Table S2). The Sanger sequencing was conducted by Macrogen Europe (Amsterdam, The Netherlands), and the sequences were aligned using the Muscle algorithm as implemented in CodonCode 6.0.2 (CodonCode Corporation, www.codoncode.com) and corrected manually.
Data analysis
Microsatellite analysis
Microsatellite data were examined regarding the occurrence of null alleles using Micro-Checker 2.2.3 (van Oosterhout et al. 2004). Loci were checked for linkage disequilibrium by Genepop 4.5.1 (Rousset 2008), and significant deviations from Hardy-Weinberg equilibrium (HWE) per locus were tested using FStat 1.2 (Goudet 1995). We computed the observed heterozygosity using GenAlEx 6.5 (Peakall and Smouse 2006). Allelic richness R t with rarefaction on the basis of 10 individuals, Nei’s unbiased gene diversity D and the inbreeding coefficient F IS were calculated with FStat 1.2 (Goudet 1995). Significance of the latter was tested by a permutation procedure with 4200 randomizations.
Population structure based on microsatellite data
To investigate population genetic structures on the country-wide scale, a principal coordinate analysis (PCoA) was performed using population pairwise Nei’s unbiased genetic distances as implemented in GenAlEx 6.5 (Peakall and Smouse 2006). Furthermore, a Bayesian cluster analysis using the program STRUCTURE 2.3.2 (Pritchard et al. 2000) was computed to examine substructuring of populations. Assuming an admixture model and correlated allele frequencies of populations, microsatellite data were tested for 1 to 31 clusters. Computations were run with 50,000 burn-ins followed by 100,000 Monte Carlo Markov chain replications, and runs were repeated eight times. To identify the most probable number of clusters, the approach of Evanno et al. (2005) was employed, and afterwards, CLUMPP was used for cluster alignment across the eight runs for the most likely number of clusters (Jakobsson and Rosenberg 2007). Since there was strong evidence for an underlying gradient related to latitude and longitude regarding the structuring of populations from the STRUCTURE analysis, we tested the relation of cluster affiliation of populations against UTM coordinates using a generalized linear model (GLM) and used a quasibinomial error term for proportion data due to the occurrence of overdispersion.
We tested for isolation by distance (IBD) to examine differentiation patterns in a more regional context. Therefore, a Mantel test correlating genetic distance and log-transformed geographic distance was performed. For this, we initially calculated overall and population pairwise D est values. D est is suggested to be a better measure for population differentiation than other parameters such as G ST when gene diversity is high (Jost 2008). D est as well as the Mantel test itself was calculated using R 3.1.1 (R Development Core Team 2014) and the packages Demetics (Gerlach et al. 2010) and Vegan (Oksanen et al. 2015), respectively. Significance of Pearson correlation was tested by 10,000 permutations. To investigate IBD in more detail, a Mantel test was first run for the whole data set. In a second step, data were divided according to the substructuring found by the STRUCTURE analysis. For this, populations were assigned to the respective cluster with cluster affiliations >0.65. Populations with intermediate values not assignable to a specific cluster were treated as an own group, and accordingly, Mantel tests were employed separately.
Spatial autocorrelation analysis was conducted to test for small-scale spatial genetic structure (SGS) focussing on correlations within populations on a local scale using the software SPAGeDI 1.4 (Hardy and Vekemans 2002). For this, distance classes were predefined emphasizing the SGS of individuals within populations, and relationship of individuals was represented by Moran’s index across loci (Hardy and Vekemans 1999). Significance of autocorrelation was tested by 10,000 permutations.
Analysis of cpDNA data
The cpDNA sequences obtained by the use of the two primer pairs were combined, and assignment of multilocus haplotypes was performed using GenAlEx 6.5 (Peakall and Smouse 2006). Haplotype diversity was computed with the program CONTRIB (Petit et al. 1998). We tested the data set for the presence of a phylogeographic structure by comparing G ST and N ST values according to Pons and Petit (1996) and Burban et al. (1999) using PermutCpSSR 1.2.1. Differentiation between populations was calculated using the allelic frequencies only (G ST) and also considering similarities between the haplotypes (N ST). A permutation procedure with 1000 permutations was conducted to test whether N ST was significantly higher than G ST. To visualize phylogenetic relations between the observed haplotypes, we calculated a reduced median network of haplotypes using Network 5 (fluxus-engineering.com, Bandelt et al. 1995). Geographic distribution of haplotypes within populations was plotted using ArcMap 10.2 (ESRI, Kranzberg, Germany). To test whether diversity parameters of chloroplast und nuclear markers are similarly distributed, we correlated haplotype diversity with gene diversity D of the microsatellites.
Further analyses including spatial and environmental variables
To investigate if population genetic patterns coincide with environmental conditions and spatial structures, we used several variables related to temperature and precipitation, geographic coordinates as well as altitude (Supplementary material, Table S3). Climate variables were extracted from raster data sets with a resolution of 1 km × 1 km (source: CDC, Deutscher Wetterdienst, 2015), and altitude was obtained from a digital elevation model with a resolution of 200 m × 200 m (source: GeoBasis-DE/BKG 2015). These data were extracted on the basis of the populations (i.e. values for individuals subsequently averaged across each population) using ArcGIS (ESRI, Kranzberg, Germany).
To identify the correlation structure of all environmental variables, a principal component analysis (PCA) was conducted with standardized data. It revealed strong multicolinearity of the variables (Supplementary material, Fig. S4). Temperature means displayed the strongest correlations with PCA axis 1, whereas axis 2 was best represented by the continentality index (negatively correlated) as well as latitude (positively correlated). The third axis was best represented by longitude. Hence, we used coordinates, the annual temperature mean, the continentality index, the annual precipitation mean as well as altitude as potential descriptors for further analyses.
These variables were used as explanatory factors in a redundancy analysis (RDA) performed on the microsatellite allele frequencies of populations as the multivariate response data, which were Hellinger transformed before (Legendre and Gallagher 2001). Additionally to the environmental variables, we included the cpDNA haplotypes (with occurrence frequencies >1) as explanatory variables into the RDA analysis to infer relations of ancient and more recent gene flow. The full RDA model including the reduced data set of environmental variables as well as four cpDNA haplotypes still displayed strong correlation structures as indicated by large variance inflation factors. Hence, a stepwise forward selection procedure was performed as implemented in function ordi2step of the Vegan package with 1000 permutation steps to identify those variables actually contributing to the model as indicated by an increased adjusted R 2 (Borcard et al. 2011). Finally, we carried out variation partitioning using partial RDAs to clarify the importance of the different variable groups, i.e. geographic coordinates, environment and haplotypes.
To examine if there is any spatial pattern regarding the distribution of genetic diversity, we tested the gene diversity and the allelic richness in a linear model against the geocoordinates and the altitude.
Results
Microsatellite performance and total variation
Examinations of microsatellite data revealed the occurrence of null alleles at four different loci within three populations, i.e. two populations with one locus each and one population with two loci. Overall, no significant deviations from HWE were detectable for the seven loci. Significant linkage disequilibrium was detected for one loci combination, i.e. FaB4 and FaB8, but a detailed inspection revealed linkage for only 4 of the 30 populations regarding this marker combination. Since the occurrence of null alleles as well as linkage disequilibrium occurred only in a few cases and were not consistently distributed across all populations, all loci were kept for further analyses.
Basic investigations of population genetic diversity measures (Table 1) revealed an allelic richness ranging between 5.4 and 7.2 and a mean of 6.2. Expected heterozygosity was similar across populations averaging 0.758 (SD ± 0.031) with highest as well as lowest values occurring in the eastern populations. Only one population (no. 20 from Dieburg) showed significant deviations from HWE which might be attributable to the abovementioned occurrence of null alleles at two loci in this population.
Genetic structure of populations
The first three PCoA axes explained a substantial amount of genetic variation with almost 60% (axis 1 32.3%, axis 2 15.8% and axis 3 10.7%) and revealed a structuring of populations with axis 1 basically matching a longitudinal gradient from east to west (Fig. 2). Populations of the more westerly areas as well as the Central German populations were stronger clumped along the first two axes compared to the populations of the eastern areas.
Results of the STRUCTURE analysis resembled those of the PCoA yielding two clusters by the delta K approach (Fig. 3a, Supplementary material Figs. S5 and S6). Though the most westerly and the most easterly populations were almost completely assigned to the corresponding clusters, populations of Central Germany showed a gradual pattern of admixture exhibiting parts of both clusters. Similarly, these populations that displayed posterior probabilities of population affiliation of 0.35 to 0.65 were located in the middle of the PCoA plot separating populations with main affiliation to cluster 1 from those primarily assigned to cluster 2. Consequently, the GLM gave evidence for a highly significant effect of the longitude (t = −9.921, p < 0.001) as well as a weak effect of the latitude (t = 2.598, p < 0.05) on cluster affiliation, and the model explained almost 87% of the deviance (Fig. 3b).
Results of STRUCTURE analysis. a Relative proportions of alleles for each population assigned to the two STRUCTURE clusters. b Relation of relative affiliation of populations to STRUCTURE cluster 1 with longitude. Lines display GLM predictions with different values for the latitude. Explained deviance of the GLM with longitude and latitude as explanatory variables is given
Testing isolation by distance on the whole data set indicated a highly significant positive, though moderate correlation of genetic and geographic distance (Mantel r = 0.3732, p < 0.001, Fig. 4) with an overall D est (across loci) of 0.307 (p < 0.001) for all populations. When the data set was split into the eastern and western cluster as well as the intermediate populations, however, only the Mantel test for the western cluster still yielded significant correlations (Mantel r = 0.292, p < 0.01, Fig. 4), whereas the other two Mantel tests for the eastern and the intermediate populations were insignificant (therefore models not shown). Noticeably, allelic differentiation was higher for the eastern cluster populations (overall D est = 0.3224, p < 0.001) compared to the western (overall D est = 0.201, p < 0.001) as well as the intermediate populations (overall D est = 0.232, p < 0.001; Fig. 4).
Pairwise genetic differentiation D est in relation to the geographic distance of populations for the eastern (triangles) and the western (dots) cluster as indicated by the STRUCTURE analysis, as well as the intermediate populations (quadrates). The dashed, grey line displays the significant mantel correlation for the western cluster and the dashed, black line the significant correlation when all populations are included. Note the log scale of the x-axis. For Mantel statistics, see text
Assessment of the spatial genetic structure, i.e. spatial autocorrelation analysis, gave significant indication of genetic relatedness of individuals across the whole distance classes tested (Fig. 5). Especially within short distances of up to less 100 m, Moran’s I values were high, i.e. around 0.2, then dropped to values below 0.15 for up to 1000 m. This level was largely kept for distances up to 10 km, and beyond that distance, values decreased further to low levels of about 0.05 to 0. The last two distance classes showed negative values of Moran’s I, indicating genetic dissimilarity of pairs of individuals across these distances.
Spatial autocorrelogram of Moran’s index as an average across all populations for 15 distance interval classes. Displayed are mean values per distance class ± SE. Dashed lines indicate the 95% confidence interval for random pairs of individuals based on 10,000 permutations. Note the log scale of the x-axis
cpDNA and phylogeographic structure
Overall, nine different haplotypes on the basis of eight mutations (six substitutions and two indels) could be detected. There was one highly abundant haplotype occurring in all but one population; two haplotypes were predominantely distributed either more in the west or in the east; one haplotype was located rather centrally, and there were also five singletons (Table 1, Fig. 6a and Table S7). The reduced median network revealed a linear relationship between the haplotypes with single mutation steps (Fig. 6b). N ST values (0.238 ± 0.0709 se) were not found to be higher than the according G ST values (0.274 ± 0.0611 se) at the p = 0.05 level, pointing to a lack of phylogeographic structure. The correlation analyses of the haplotype diversity and the gene diversity resulted in a significant moderate correlation (r s = 0.381, p = 0.037).
Further analyses including spatial and environmental variables
The stepwise selection procedure of the RDA yielded a final model in which only 3 of the formerly 10 variables were retained, i.e. longitude, altitude and one of the haplotypes (no. 6, Fig. 6). Constrained axes explained about 26% of the total variation of the genetic data. Longitude as well as the haplotype was strongly correlated with axis 1, while altitude reflected axis 2 (Fig. 7). Variation partitioning resulted in discrete explanatory values as indicated by an adjusted R 2 of about 26.3% for longitude, 17.9% for altitude and 9.3% for the haplotype. Comparing results of the full RDA model with a PCA performed on the Hellinger-transformed allele frequencies revealed a similar pattern for the sample and species scores in the diagram indicating that explanatory variables represent variation in the genetic data adequately (data not shown).
Testing the spatial structure of gene diversity and allelic richness by relating these variables to the geocoordinates and altitude yielded no significant relations (data not shown).
Discussion
Our analyses of neutral genetic variation of the shrub species F. alnus revealed spatial genetic structures at the country-wide, the regional as well as the local scale that should be considered with regards to the delineation of provenance regions as a baseline for the selection of planting material.
Considering the country-wide scale, the results revealed a clear spatial genetic pattern with an underlying east-west gradient of populations. The assignment of populations to an eastern and a western cluster showed a quite continuous transition. Though populations showed overall significant differentiation, levels were moderate indicating existing gene flow between populations. Results of the Mantel test with weak implications for isolation by distance underline this conclusion and fit into the dispersal patterns for F. alnus. While the species is insect-pollinated, which might be considered a strong limitation to gene flow in comparison to wind-pollinated species, seed dispersal by birds can counteract such a restriction (Nathan et al. 2008; Lavabre et al. 2016). Especially long-distance dispersal events during bird migration in autumn when fruits of F. alnus ripen might be responsible for these gradual patterns of differentiation (Hampe et al. 2003). The observed pattern is stronger associated to the longitude than to the latitude which is in line with the expectation that the connection of populations is stronger from north to south in contrast to the east-west direction during bird migration (Daneck et al. 2016).
This aspect might also play a role when considering the historic patterns inferred from the chloroplast data. Hampe et al. (2003) already investigated haplotypic diversity of F. alnus on the basis of three cpDNA markers and PCR-RFLPs. For Central Europe, they found only one lineage from the Balkan harbouring the Central European haplotypes and hence concluded that F. alnus must have spread from this origin. Similarly to the results of Hampe et al. (2003) who used the same marker loci at less resolutive power though, no phylogeographic signal could be detected in our Central European sample. Together with the branching characteristics of the reduced median network where all haplotypes derived through mutation steps from the most abundant haplotype, we conclude that the study area has been colonized by one single eastern refugial lineage as described by Hampe et al. (2003). An admixture of any so far “hidden” refugial gene pools can thus be excluded. Furthermore, the distribution of the two haplotypes 6 and 8 restricted to either the eastern or the western parts of Germany, respectively, fit into the southward “secondary haplotype migration” hypothesis of Hampe et al. (2003), which assumes a historical secondary southward expansion of northern haplotypes due to bird migration. Likewise reflected by the microsatellite genetic patterns, the cp genetic eastern and western patterns remained largely separated. Highlighted by the RDA analysis (where the eastern haplotype no. 6 strongly correlated with the first RDA axis along with the longitude), the cpDNA genetic patterns are thus strengthening the findings of also recent gene flow following a north-south direction more distinct than an east-west direction. A first synthesis of the results from both marker types suggests bird dispersal as a main driver for distribution of gene and haplotypic diversity in past and more recent periods. The latter is indicated by the results of our correlation analysis. Consequently, a combination of different marker types appears useful to illuminate the potentially different processes behind population genetic patterns (López-Vinyallonga et al. 2015; Daneck et al. 2016) which, in turn, is an important prerequisite to effectively define provenance regions.
On a regional scale, differences between the populations of the western and the eastern parts regarding population differentiation and structure were identifiable. When populations were divided by their affiliation to STRUCTURE clusters, only for the western cluster the IBD pattern maintained significance indicating gene flow restricted by dispersal distance. Moreover, differentiation was remarkably larger for the eastern cluster populations than for the western cluster populations. These regional differences might actually be attributable to the connectivity of the landscape. The (south-) eastern parts of Germany are characterized by overall longer, more massive mountain ranges (Swabian Alb, Frankish Alb, Ore Mountains) that might act as barriers to gene flow (Ashley et al. 2015). Although the south-western to mid-western parts of Germany also feature several low mountain ranges, these mountains are smaller in extent on one hand, and additionally, the rivers Rhine and Weser cut through this region from south to north. These rivers probably act as a dispersal route (potentially also due to a water-mediated secondary dispersal, Hampe 2004) and thereby enhancing gene flow. Thus, the specific spatial context of the landscape should be considered as a potential driving force for shaping regional patterns.
As a third scale, the local aspect should be included with regards to understanding population genetic patterns and the processes behind. Inbreeding coefficients for populations of F. alnus were overall rather small, and except one population, none showed significant deviations from Hardy-Weinberg equilibrium. This is in line with our expectations considering the life history strategies of F. alnus, i.e. insect pollination and ornithochory (Hamrick and Godt 1996), and the fact that F. alnus is widely distributed. However, SGS analysis at the local scale revealed noticeable significant spatial autocorrelation between individuals within populations with two plateaus of autocorrelation values, the first for 10 to 100 m and the second for 1 to 10 km. This, too, can be assumed to being a consequence of both the insect-dispersed pollen as well as the bird-dispersed seeds. Though long-distance dispersal, especially for the latter, might occur from time to time, the fairly high values of autocorrelation across distances of up to 10 km plausibly cover activity ranges of the dispersing bird species during sedentary periods (Vega Rivera et al. 1999). Separate comparison of SGS structures for the western and the eastern populations did not reveal significant differences (data not shown). We conclude that dispersal processes at the local scale work similarly within the populations and differences between populations are a specific feature of the prevailing local conditions. Importantly, these patterns of genetic similarity should be addressed for the selection of planting material, and extensive sampling of individuals especially across short distances of only 10 to a few hundred meters should be avoided.
A final point relates to the aspect of local selection as an important source for the delineation of provenance regions. Though we investigated neutral genetic variation in our study, we nevertheless tested relations to environmental conditions since correlations occur from time to time pointing to selective forces which, for example, can act when neutral markers “hitchhike” with loci under selection (Michalski and Durka 2012). However, no significant contributions of environmental variables typically known to act as selecting forces, i.e. temperature and precipitation, were found. This could have two reasons. First, our microsatellite markers might actually behave completely neutral which appears to be very likely. However, another possibility could be the secondary importance of selective forces with respect to temperature and precipitation on the studied spatial scale. Recently, De Kort et al. (2015) investigated the population genomic signature of environmental selection of F. alnus on different spatial scales (i.e. a regional, an intermediate and a continental) on the basis of SNPs. They identified substantial numbers of SNPs under selection with the highest amount being associated with the continental scale (reaching from France to Sweden and Italy). Moreover, tests of relations of these adaptive SNPs with environmental factors revealed the strongest association at the continental scale (with temperature). At the regional (populations within Belgium) and intermediate scale (French and Belgian populations), longitude explained more of the genetic variation of these loci under selection than merely environmental factors, similarly to our RDA results. In this context, they stressed the important role of regional population genetic processes to preserve the adaptive potential of regional genetic variation in the face of, e.g. climate change. Consequently, finding no pattern of adaptive variation in our data does not indicate the absence of selective forces, and local adaptation should be addressed before provenance regions are specified. However, classical population genetic approaches are also a vital part of these processes since gene flow and local selection are both part of the adaptive nature of populations leading to the population genetic and landscape genomic patterns.
Conclusion
Summarizing, we could demonstrate that for the insect-pollinated and bird-dispersed species F. alnus, distinct population genetic patterns do exist that should be considered for the delineation of provenance regions. The strongest pattern was an east-west gradient. With regards to the already suggested delineations (Fig. 1), especially the largest zone in the northern lowland areas of Germany should be divided into two zones with respect to this pattern.
Secondly, the low differentiation for populations in the west compared to those in the east suggests from a population genetic point of view that planting material within the western cluster can be collected from more distantly located populations and beyond the borders of the so far delineated provenance regions. Generally, for the selection of the most appropriate planting material, a trade-off has to be made between too local and too foreign to prevent the risk of inbreeding on the one hand and maladaptation and outbreeding depression on the other (McKay et al. 2005). Regarding the western cluster, gene flow acts across distances beyond the delimitation of populations. Since inter-population mixing for the preservation of genetic diversity and of adaptation potential have been emphasized (De Kort et al. 2015; Reiker et al. 2015; Zavodna et al. 2015), selecting non-local planting material appears to be a justified measure for reforestation projects in these areas. Moreover, results from the autocorrelation analysis suggest that sampling across longer distances is beneficial regarding potential inbreeding aspects and distances beyond 1 km should be considered as a compromise between costs and benefits.
Though adaptive variation is proclaimed to being the most important aspect for the selection of suitable planting material (McKay et al. 2005), preserving genetic diversity as the source for the adaptability of species is an important task for the management of their populations. As our study demonstrated, classical population genetic approaches can assist in understanding patterns of genetic diversity and especially the processes behind as one significant part for managing species for conservation and restoration projects.
References
Alberto F (2009) MsatAllele_1.0: an R package to visualize the binning of microsatellite alleles. J Hered 100(3):394–397. doi:10.1093/jhered/esn110
Ashley MV, Abraham ST, Backs JR, Koenig WD (2015) Landscape genetics and population structure in valley oak (Quercus lobata Nee). Am J Bot 102(12):2124–2131. doi:10.3732/ajb.1500182
Austerlitz F, Mariette S, Machon N, Gouyon P, Godelle B (2000) Effects of colonization processes on genetic diversity: difference between annual plants and tree species. Genetics 154:1309–1321
Azpilicueta MM, Gallo LA, van Zonneveld M, Thomas E, Moreno C, Marchelli P (2013) Management of Nothofagus genetic resources: definition of genetic zones based on a combination of nuclear and chloroplast marker data. Forest Ecol Manag 302:414–424. doi:10.1002/ece3.1108
Bandelt HJ, Forster P, Sykes BC, Richards MB (1995) Mitochondrial portraits of human populations using median networks. Genetics 141(2):743–753
Barsch F, Heym A, Nehring S (2012) Leitfaden zur Verwendung gebietseigener Gehölze. https://www.bfn.de/fileadmin/BfN/recht/Dokumente/leitfaden_gehoelze_.pdf. Accessed 25 July 2015
Beatty GE, Montgomery WI, Tosh DG, Provan J (2015) Genetic provenance and best practice woodland management: a case study in native alder (Alnus glutinosa). Tree Genet Genomes 11(5):92. doi:10.1007/s11295-015-0919-1
Booy G, Hendriks RJJ, Smulders MJM, Groenendael JM, Vosman B (2000) Genetic diversity and the survival of populations. Plant Biol 2(4):379–395. doi:10.1055/s-2000-5958
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Bower AD, St. Clair JB, Erickson V (2014) Generalized provisional seed zones for native plants. Ecol Appl 24(5):913–919. doi:10.1890/13-0285.1
Bucci G, Gonzalez-Martinez SC, Le Provost G, Plomion C, Ribeiro MM, Sebastiani F, Alia R, Vendramin GG (2007) Range-wide phylogeography and gene zones in Pinus pinaster Ait. revealed by chloroplast microsatellite markers. Mol Ecol 16(10):2137–2153. doi:10.1111/j.1365-294X.2007.03275.x
Burban, Petit, Carcreff, Jactel (1999) Rangewide variation of the maritime pine bast scale Matsucoccus feytaudi Duc. (Homoptera: Matsucoccidae) in relation to the genetic structure of its host. Mol Ecol 8(10):1593–1602. doi:10.1046/j.1365-294x.1999.00739.x
Cornille A, Giraud T, Bellard C, Tellier A, Le Cam B, Smulders MJM, Kleinschmit J, Roldan-Ruiz I, Gladieux P (2013) Postglacial recolonization history of the European crabapple (Malus sylvestris Mill.), a wild contributor to the domesticated apple. Mol Ecol 22(8):2249–2263. doi:10.1111/mec.12231
Daneck H, Fér T, Marhold Fls K (2016) Glacial survival in northern refugia?: Phylogeography of the temperate shrub Rosa pendulina L. (Rosaceae): AFLP vs. chloroplast DNA variation. Biol J Linn Soc 119(3):704–718. doi:10.1111/bij.12619
De Kort H, Mergeay J, Vander Mijnsbrugge K, Decocq G, Maccherini S, Kehlet Bruun HH, Honnay O, Vandepitte K, Bugmann H (2014) An evaluation of seed zone delineation using phenotypic and population genomic data on black alder Alnus glutinosa. J Appl Ecol 51(5):1218–1227. doi:10.1111/1365-2664.12305
De Kort H, Vandepitte K, Mergeay J, Mijnsbrugge KV, Honnay O (2015) The population genomic signature of environmental selection in the widespread insect-pollinated tree species Frangula alnus at different geographical scales. Heredity 115(5):415–425. doi:10.1038/hdy.2015.41
Eimert K, Reutter G, Strolka B (2003) Fast and reliable detection of doubled-haploids in Asparagus officinalis by stringent RAPD-PCR. J Agr Sci 141(1):73–78. doi:10.1017/S0021859603003447
Ellstrand NC (2014) Is gene flow the most important evolutionary force in plants? Am J Bot 101(5):737–753. doi:10.3732/ajb.1400024
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14(8):2611–2620. doi:10.1111/j.1365-294X.2005.02553.x
GeoBasis-DE/BKG (2015) Digitales geländemodell gitterweite 200m. Bundesamt für Kartographie und Geodäsie. Dataset: http://www.geodatenzentrum.de/geodaten/gdz_rahmen.gdz_div?gdz_spr=deu&gdz_akt_zeile=5&gdz_anz_zeile=1&gdz_unt_zeile=3&gdz_user_id=0
Gerlach G, Jueterbock A, Kraemer P, Deppermann J, Harmand P (2010) Calculations of population differentiation based on GST and D—forget GST but not all of statistics! Mol Ecol 19(18):3845–3852. doi:10.1111/j.1365-294X.2010.04784.x
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486. doi:10.1093/oxfordjournals.jhered.a111627
Hampe A (2004) Extensive hydrochory uncouples spatiotemporal patterns of seedfall and seedling recruitment in a ‘bird-dispersed’ riparian tree. J Ecol 92(5):797–807. doi:10.1111/j.0022-0477.2004.00918.x
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8(5):461–467. doi:10.1111/j.1461-0248.2005.00739.x
Hampe A, Arroyo J, Jordano P, Petit RJ (2003) Rangewide phylogeography of a bird-dispersed Eurasian shrub: contrasting Mediterranean and temperate glacial refugia. Mol Ecol 12(12):3415–3426. doi:10.1046/j.1365-294X.2003.02006.x
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos T Roy Soc B 351(1345):1291–1298. doi:10.1098/rstb.1996.0112
Hardy OJ, Vekemans X (1999) Isolation by distance in a continuous population: reconciliation between spatial autocorrelation analysis and population genetics models. Heredity 83(2):145
Hardy OJ, Vekemans X (2002) Spagedi—a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2(4):618–620. doi:10.1046/j.1471-8286.2002.00305.x
Havrdová A, Douda J, Krak K, Vít P, Hadincová V, Zákravský P, Mandák B (2015) Higher genetic diversity in recolonized areas than in refugia of Alnus glutinosa triggered by continent-wide lineage admixture. Mol Ecol 24(18):4759–4777. doi:10.1111/mec.13348
Heuertz M, Fineschi S, Anzidei M, Pastorelli R, Salvini D, Paule L, Frascaria-Lacoste N, Hardy OJ, Vekemans X, Vendramin GG (2004) Chloroplast DNA variation and postglacial recolonization of common ash (Fraxinus excelsior L.) in Europe. Mol Ecol 13(11):3437–3452. doi:10.1111/j.1365-294X.2004.02333.x
Hufford KM, Krauss SL, Veneklaas EJ (2012) Inbreeding and outbreeding depression in Stylidium hispidum: implications for mixing seed sources for ecological restoration. Ecol Evol 2(9):2262–2273. doi:10.1002/ece3.302
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioninformatics 23(14):1801–1806. doi:10.1093/bioinformatics/btm233
Jost L (2008) GST and its relatives do not measure differentiation. Mol Ecol 17(18):4015–4026. doi:10.1111/j.1365-294X.2008.03887.x
Kremer A, Ronce O, Robledo-Arnuncio JJ, Guillaume F, Bohrer G, Nathan R, Bridle JR, Gomulkiewicz R, Klein EK, Ritland K, Kuparinen A, Gerber S, Schueler S (2012) Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol Lett 15(4):378–392. doi:10.1111/j.1461-0248.2012.01746.x
Lavabre JE, Gilarranz LJ, Fortuna MA, Bascompte J (2016) How does the functional diversity of frugivorous birds shape the spatial pattern of seed dispersal?: a case study in a relict plant species. Phil Trans R Soc B 371(1694):20150280. doi:10.1098/rstb.2015.0280
Legendre P, Gallagher E (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129(2):271–280. doi:10.1007/s004420100716
López-Vinyallonga S, López-Pujol J, Constantinidis T, Susanna A, Garcia-Jacas N (2015) Mountains and refuges: genetic structure and evolutionary history in closely related, endemic Centaurea in continental Greece. Mol Phylogenet Evol 92:243–254. doi:10.1038/srep37818
Malaval S, Lauga B, Regnault-Roger C, Largier G (2010) Combined definition of seed transfer guidelines for ecological restoration in the French Pyrenees. Appl Veg Sci 13(1):113–124. doi:10.1111/j.1654-109X.2009.01055.x
McKay JK, Christian CE, Harrison S, Rice KJ (2005) “How local is local?”—a review of practical and conceptual issues in the genetics of restoration. Restor Ecol 13(3):432–440. doi:10.1111/j.1526-100X.2005.00058.x
Michalski SG, Durka W (2012) Assessment of provenance delineation by genetic differentiation patterns and estimates of gene flow in the common grassland plant Geranium pratense. Conserv Genet 13(2):581–592. doi:10.1111/1365-2664.12636
Mijangos JL, Pacioni C, Spencer PBS, Craig MD (2015) Contribution of genetics to ecological restoration. Mol Ecol 24(1):22–37. doi:10.1111/mec.12995
Miller SA, Bartow A, Gisler M, Ward K, Young AS, Kaye TN (2011) Can an ecoregion serve as a seed transfer zone? Evidence from a common garden study with five native species. Restor Ecol 19(201):268–276. doi:10.1111/j.1526-100X.2010.00702.x
Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A (2008) Mechanisms of long-distance seed dispersal. Trends Ecol Evol 23(11):638–647. doi:10.1016/j.tree.2008.08.003
Oksanen JF, Blanchet G, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) vegan: Community Ecology Package. R package version 2.3–1. http://CRAN.R-project.org/package=vegan. Accessed 12 November 2015
van Oosterhout C, Hutchinson WF, Wills DP, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4(3):535–538. doi:10.1111/j.1471-8286.2004.00684.x
Pautasso M (2009) Geographical genetics and the conservation of forest trees. Perspect Plant Ecol Evol Syst 11(3):157–189. doi:10.1016/j.ppees.2009.01.003
Peakall RO, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6(1):288–295. doi:10.1093/bioinformatics/bts460
Petit RJ, El Mousadik A, Pons O (1998) Identifying populations for conservation on the basis of genetic markers. Conserv Biol 12(4):844–855. doi:10.1111/j.1523-1739.1998.96489.x
Petit R, Aguinagalde I, de Beaulieu JL, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M, Mohanty A, Müller-Starck G, Demesure-Musch B, Palmé A, Martín JP, Rendell S, Vendramin GG (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300(5625):1563–1565. doi:10.1126/science.1083264
Pluess AR, Frank A, Heiri C, Lalagüe H, Vendramin GG, Oddou-Muratorio S (2016) Genome-environment association study suggests local adaptation to climate at the regional scale in Fagus sylvatica. New Phytol 210(2):589–601. doi:10.1111/nph.13809
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144(3):1237–1245
Potter KM, Hargrove WW (2012) Determining suitable locations for seed transfer under climate change—a global quantitative method. New Forest 43(5–6):581–599. doi:10.1007/s11056-012-9322-z
Pritchard JK, Stephens M, Donelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. URL http://www.R-project.org
Reiker J, Schulz B, Wissemann V, Gemeinholzer B (2015) Does origin always matter? Evaluating the influence of nonlocal seed provenances for ecological restoration purposes in a widespread and outcrossing plant species. Ecol Evol 5(23):5642–5651. doi:10.1002/ece3.1817
Rigueiro C, Arroyo JM, Rodríguez R, Hampe A, Jordano P (2009) Isolation and characterization of 16 polymorphic microsatellite loci for Frangula alnus (Rhamnaceae). Mol Ecol Notes 9(3):986–989. doi:10.1111/j.1755-0998.2009.02527.x
Rousset F (2008) genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Notes 8(1):103–106. doi:10.1111/j.1471-8286.2007.01931.x
Taberlet P, Fumagalli L, Wust-Saucy A-G, Cosson J-F (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7(4):453–464
Vega Rivera JH, McShea WJ, Rappole JH, Haas CA (1999) Postbreeding movements and habitat use of adult wood thrushes in Northern Virginia. Auk 116(2):458–466. doi:10.2307/4089379
Ying CC, Yanchuk AD (2006) The development of British Columbia’s tree seed transfer guidelines: purpose, concept, methodology, and implementation. Forest Ecol Manag 227(1–2):1–13. doi:10.1016/j.foreco.2006.02.028
Zavodna M, Abdelkrim J, Pellissier V, Machon N (2015) A long-term genetic study reveals complex population dynamics of multiple-source plant reintroductions. Biol Conserv 192:1–9. doi:10.1016/j.biocon.2015.08.025
Zecchin B, Caudullo G, Rigo DD (2016) Frangula alnus in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European Atlas of Forest Tree Species. Publication Ofice EU, Luxembourg, p e019ee2+ https://w3id.org/mtv/FISE-Comm/v01/e019ee2. Accessed 25.01.2017
Acknowledgements
We are very grateful to all the forest rangers, colleagues and friends who supported this study by collecting leaf material for the genetic analysis throughout Germany. Many thanks go to Christina Mengel and Sascha Liepelt for their support and helpful advices regarding the laboratory work. Moreover, we would like to thank two anonymous reviewers for their helpful comments. This project was funded by the program “Forschung für die Praxis” by the Hesse State Ministry of Higher Education, Research and the Arts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Data archiving statement
Genotyping data of all collected samples for the seven microsatellites as well as cpDNA data of the corresponding number of samples are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.215sn.
Additional information
Communicated by J. Beaulieu
Electronic supplementary material
ESM 1
(DOCX 808 kb)
Rights and permissions
About this article
Cite this article
Mosner, E., Eimert, K., Hüwe, U. et al. Revisiting the provenance delineation of a widespread shrub, Frangula alnus—the role of spatial, temporal and environmental patterns. Tree Genetics & Genomes 13, 63 (2017). https://doi.org/10.1007/s11295-017-1142-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-017-1142-z