Abstract
Recently, the scientific community is interested in the synthesis of biodegradable and bioactive packaging to replace oil-based ones. Therefore, the present study aims to elaborate an active and biodegradable material using chitosan (CS-film) combined with pelargonium, tea tree, marjoram, and thyme essential oils (EOs), and then evaluate their different properties and biological activities. The obtained data showed an augmentation in CS-film thickness and opacity following the addition of EOs ranging from 17 ± 3 to 42 ± 2 μm and from 1.53 ± 0.04 to 2.67 ± 0.09, respectively. Furthermore, a significant decrease in the water vapor transmission rate and moisture content parameters was recorded as regards the treated CS-films. On the other hand, the treatment with EOs engenders random modifications in the physicochemical and mechanical characteristics of the material. Concerning the biological activities, the treated CS-films scavenged around 60% of DPPH radical while the control CS-film exhibited a negligible antioxidant activity. Finally, the CS-films containing pelargonium and thyme EOs exhibited the strongest antibiofilm-forming activity against Escherichia coli, Enterococcus hirae, Staphylococcus aureus, and Pseudomonas aeruginosa with values of inhibition greater than 70%. These encouraging results verify the effectiveness of CS-films containing EOs such as pelargonium and thyme EOs as biodegradable and bioactive packaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, food loss represents a worldwide concern. The factors that mainly cause food spoilage are contact with oxygen (Cichello 2015) and water (Tapia et al. 2020), and the proliferation of microorganisms (Abd El-Hack et al. 2022). The latter is responsible for the deterioration of 1.3 billion tons of food, more than a half million sick, and 420.000 dead cases annually (Gustavsson et al. 2011; Fahey et al. 2022). As a result, various procedures such as refrigeration, freezing, and thermal processing were employed to prevent food spoilage and contamination (Huang et al. 2019). However, the freshness and minimally-processing of food are highly required criteria. Thereby, other alternatives are needed. The food industry relies principally on the utilization of physical barriers such as food packaging for food protection and conservation (Gupta et al. 2022). So far, fossil-based plastics e. g, polyethylene, polyvinyl chloride, polyethylene terephthalate, and polystyrene are the most utilized materials in the packaging sector due to their cost-effectiveness, abundance in nature, and great mechanical properties (Asgher et al. 2020). Nevertheless, fossil-based plastics remain ecologically harmful (Jem and Tan 2020). In this context, attention was given to the synthesis of biodegradable, natural, and bioactive packaging (Phothisarattana and Harnkarnsujarit 2022). Among the biopolymers used in packaging elaboration, various researchers have studied chitosan due to its abundance, biological activities, biodegradability, film-forming and mechanical properties, and visual transparency (Shahidi et al. 1999; Dutta et al. 2009; Wang et al. 2011). As regards food packaging, it is necessary to use films that are endowed with a good barrier property to water, UV light, and microorganisms in order to protect food and maintain its quality (Siracusa et al. 2008; Zhang et al. 2021). It was claimed that chitosan exhibits weaker bioactivities when it is used as film (Ouattara et al. 2000). Therefore, the introduction of bioactive agents into CS-film to improve the above-mentioned properties and activities is a research need. Previous investigations have studied the mixture of chitosan and other biopolymers e. g, gelatin and starch (Jovanović et al. 2021; Janik et al. 2022). Other researchers have discussed the impact of introducing silver and Fe3O4 nanoparticles on the CS-film activities and mechanical properties (Li et al. 2022; Yang et al. 2022; Zarandona et al. 2022). Meanwhile, Peng and Li (2014) and Azadbakht et al., (2018) confirmed the optimization of CS-film following EOs addition. EOs containing terpene alcohols as major constituents (pelargonium, tea tree, marjoram, and thyme EOs) are recognized for their great antibacterial activities (Tariq et al. 2019). Furthermore, several works have studied the combination of CS-film with some of these EOs (Sánchez-González et al. 2010; Cerempei et al. 2014; Sedlaříková et al. 2017; Vidács 2022; Mouhoub et al. 2023b). However, the studies were limited to a few properties and activities.
In this paper, we aimed to carry out a global characterization of CS-films incorporated with pelargonium, tea tree, marjoram, and thyme EOs and to highlight the potential application of these films as packaging for food.
Materials and methods
Materials
The tested strains were S. aureus ATCC29213, E. hirae CIP5855, P. aeruginosa ATCC53, and E. coli K-12 MG1655.
Pelargonium asperum (refractive index: 1.468, density: 0.895, optical rotation: − 11.16°), Melaleuca alternifolia (refractive index: 1.487, density: 0.898, optical rotation: + 9.9°), Origanum majorana (refractive index: 1.473, density: 0.895, optical rotation: + 22.5°), and Thymus satureioides EOs (refractive index: 1.483, density: 0.938, optical rotation: − 10.75°) were provided by Nectarome society. Chitosan C3646 (DDA > 75% and Mw = 400 kDa) was purchased from Sigma-Aldrich.
Growth conditions
Muller-Hinton Agar medium (MHA) and lysogeny broth (LB) supplemented with sucrose 1% were used in the agar diffusion and antiadhesion tests, respectively. The bacterial suspensions were adjusted to 108 cells/mL by a spectrophotometer (Jenway 6305, UK).
EOs analysis using GC-FID
The capillary column DB-Wax 127-7023 (20 m × 100 µm) was used for EOs analysis. The operating conditions details were highlighted in our previous study (Mouhoub, Guendouz, et al. 2022a, b). Briefly, carrier gas (Hydrogen) flow rate was 1 mL/min. The oven program was: 60 °C for 2 min, then 12 °C/min up to 248 °C, and finally held for 5 min.
Films synthesis
The film preparation was detailed in our previous work (Mouhoub et al. 2022a). Briefly, a combination of acetic acid 1%, chitosan 2%, glycerol, Tween 80, and EO (2% v/v) was continuously stirred for 6 h using magnetic stirrer, poured onto supports, and then placed in an oven and allowed to dry overnight at 30 ℃. The film without emulsifier and EO was considered as control. The films were collected and then stored for subsequent tests (Fig. 1).
Films’ thickness and opacity
The thickness of the films was measured in 5 different areas by a digital caliper (INGCO HDCD01150, China). The films opacity was measured using an UV–Visible spectrophotometer (Jenway 6305, UK). The adopted blank was the empty cell (Siripatrawan and Harte 2010).
where Abs600 and t refer to the absorbance at 600 nm and film thickness (mm), respectively.
Determination of moisture content (MC), swelling level (SL), and hydrosolubility
The sample (1 × 1 cm) was weighed w1 and then placed into the oven (24 h at 50 °C) to determine the dry weight w2. The dried film was incubated in a beaker containing distilled water for 24 h. The swollen film was dried superficially and then weighed w3. Finally, the sample was dried again for 24 h at 50 °C to define the final dry weight w4. The experiment was performed in triplicate. The MC, SL, and hydrosolubility of the films were measured as follows:
Measurement of the water vapor transmission rate (WVTR)
The WVTR of the CS-films was measured based on He et al. (2021) procedure with minor modifications. Briefly, CS-film samples were firmly fixed over glass cups (3.14 cm2 of transmission area) containing equal amounts of CaCl2. The cups were placed inside a desiccator where the relative humidity and temperature were adjusted to 90% and 25 °C, respectively.
The cups were periodically weighed to the nearest 0.1 mg. The WVTR (g/h.m2) was measured as follows:
where \(\frac{\Delta w}{\Delta t}\) and A represent the slope of the weight vs time and the effective film area, respectively.
Evaluation of surface free energy and hydrophobicity
The physicochemical characteristics of the CS-films surfaces were estimated based on the sessile drop method (Blanco et al. 1997). The contact angles of diiodomethane θd, distilled water θw, and formamide θf were monitored by a goniometer (GBX, France)- computer-camera system (Mouhoub et al. 2022a). The electron acceptor (γ+), and donor (γ−), the Lifshitz-Van der Waals component (γLW), and the free energy of interaction (ΔGiwi) were determined following the Young-Van Oss equation (Oss 1995).
where S refers to the solid surface and L to the liquid phases. The surface free energy (γSTotal) is calculated by the following equation:
The hydrophobicity of films’ surfaces can be predicted qualitatively based on the water contact angle (Lekbach et al. 2019). θw < 65° and θw > 65° refer to hydrophilic and hydrophobic surfaces, respectively (Vogler 1998). Otherwise, the procedure of Van Oss et al., (1988) was utilized to assess the hydrophobicity quantitatively. The surface is considered hydrophilic if ΔGiwi > 0 and conversely for hydrophobic surfaces.
Where:
Films’ mechanical properties
The tensile strength (TS) and elongation at break (EB) parameters of the films were evaluated using INSTRON 3369 electrohydraulic instrument. The films’ strips (20 × 50 mm) were mounted between the grips and the selected cross-head speed was 2 mm/min. Data were treated using the Bluehill program. The experiment was realized in quintuplicate for each sample.
Films’ antioxidant activity
The antioxidant activity of the CS-films was monitored as described by Siripatrawan and Harte, (2010). Two hundred milligrams of the film were submerged in 3 ml of methanol. The extract solution (0.9 mL) was sampled after 24, 48, and 72 h of the reaction and then mixed with 0.3 mL of the methanolic solution of DPPH 1 mM. The extract solution was replaced by methanol in the control. The vortexed mixture was incubated in dark for 30 min and then the absorbance was measured at 517 nm. For each sample, three repetitions were performed. The percentage of DPPH inhibition was calculated as follows:
where A represents the absorbance at 517 nm.
Films’ antibacterial activity
The antibacterial capacity of the CS-films was assessed using the agar diffusion method. The bacterial suspensions (1 mL) previously adjusted to 108 cells/mL were spread on the MHA surface and then the excess was withdrawn. The films’ discs of 6 mm diameter were UV sterilized and then deposited on the medium surface. The diameter of inhibition zones (IZ) was measured after 24 h of incubation at 30 °C. The experiment was performed in triplicate.
Coating of microplate wells using CS-films
One hundred microliters of the solution used for the films’ preparation were introduced in the bottom of 96-well microplate wells and dried at 30 °C overnight. The coated microplate was sterilized by UV light for the antiadhesion test.
Films’ antiadhesion activity
Two hundred microliters of the bacterial suspensions (108 cells/mL) were introduced into each microplate well and then incubated at 30 °C. After 24 h, the wells content was transferred to another microplate and the turbidity was read at 630 nm by Bio-Tek ELx800 microplate reader. The experiment was conducted in triplicate. The coated microplate was rinsed multiple times with distilled water and then dried for 1 h to fix the sessile cells. The wells were dyed by adding 200 μL of crystal violet 0.1%. After 10 min, the microplate was rinsed to remove the dye, and then 200 μL of ethanol 95% were added to each well and allowed to react for 15 min. The wells content was transferred to a new microplate and the absorbance was read at 550 nm. The experiment was performed in triplicate.
Statistical analysis
Data were presented as mean ± standard deviation. The experimental results were subjected to an ANOVA test using SPSS software (version 25.0). The statistical significance was established at P < 0.05.
Results
EOs’ chemical composition
As mentioned in Table 1. The analysis of P. asperum, M. alternifolia, O.majorana, and T. satureioides EOs by GC-FID revealed the presence of 40, 33, 30, and 33 compounds, respectively. Qualitative resemblances accompanied by quantitative disparities were noticed between the four EOs compositions. However, terpene alcohols were the main compounds for all EOs. Indeed, citronellol represented 33.98% of P. asperum EO. Whereas, terpinen-4-ol was the major component of O.majorana (23.37%) and M. alternifolia (40.33%). In contrast, T. satureioides contained mainly α-terpineol and borneol (43.90%).
Physical characteristics of the films
Table 2 presents the Hydrosolubility, SL, MC, opacity, WVTR, and thickness of the prepared films. Data showed high Hydrosolubility values (> 60%) for all tested films. The treatment by EOs increased significantly the opacity and thickness of the films while decreased the MC and WVTR parameters. Except for the film containing M. alternifolia EO, the recorded SL values were considerably high (> 600%).
Physicochemical characteristics of the films
Table 3 compares the physicochemical characteristics of the prepared films. Results revealed a drop in the water contact angle following the treatment by EOs. Whereas, only the film containing M. alternifolia EO was found to be quantitatively hydrophilic (ΔGiwi > 0 mJ). The treatment by EOs reduces the γAB values of the control film and conversely for γLW.
Mechanical characteristics of the films
The mechanical characteristics of the different CS-films are presented in Table 4. Data showed that the treated films had low stretching ability compared to the control film. As regards TS parameter, the treatment with tea tree and pelargonium EOs decreased the film resistance and conversely for marjoram EO treatment. On the hand, no significant modification in TS was recorded between the control film and the one treated with thyme EO (P < 0.05).
Films’ antioxidant activity
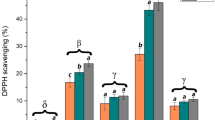
The antioxidant activities of the different CS-films were statistically compared in Fig. 2. Results showed negligible activity in the case of the control. For all treatments, the antioxidant activity was stronger after 72 h of the material-methanolic solution contact (percentage of DPPH inhibition > 55%). The film treated with Thyme EO showed a slightly high activity compared to the other treated films.
Films’ antibacterial activity
The methods of diffusion in liquid and solid mediums were utilized to assess the antibacterial effect of the prepared materials (Table 5). Among all tested films, only the ones treated with pelargonium and thyme EOs generated IZ against the four bacteria. The diameter of IZ variedbetween 8 and 12.67 mm depending on the bacterial strains. The diffusion in the liquid medium test revealed that the control film and the film treated with thyme EO exhibited the weakest and strongest activities, respectively.
Films’ antiadhesion activity
Figure 3 compares statistically the antiadhesion activities of the prepared materials. From the data, three levels of activity can be defined, strong, moderate, and weak antiadhesion activity in which the inhibition percentages are around 20%, 50%, and 80%, respectively. The films containing thyme and pelargonium EOs showed strong activity while the ones containing marjoram and tea tree EOs exhibited moderate activity. On the other hand, the weakest antiadhesion activity was recorded in the case of the control film.
Discussion
The assessment of the physical, physicochemical, and mechanical characteristics of the materials is important to predict their usefulness as packaging. The physical characteristics of the material such as opacity and thickness are involved in product protection. Moreover, the film thickness is correlated to its mechanical parameters (Simsek et al. 2020). The results confirmed that the increase in film thickness following the treatment with EOs modified randomly the tensile strength property and caused a drop in the elongation at break parameter. It was reported that resistant polymers possess high stretching ability (Shaikh et al. 2021). Nevertheless, low film thickness led to a greater amount of dioxygen in the package’s headspace and therefore the product oxidation (Del Nobile et al. 2007). As well as thickness, the reduction in film opacity negatively affects the light barrier parameter against harmful light (Zhang et al. 2021). Numerous studies have demonstrated an increase in the material opacity and thickness following the incorporation of pelargonium, tea tree, marjoram, and thyme EOs (Sedlaříková et al. 2017; Lian et al. 2019; Coyotl-Pérez et al. 2022; Jesser et al. 2022; Song et al. 2022; Wang et al. 2022). The modification of these parameters can be explained by the EOs impact on the material refractive index and microstructure, respectively. In addition to dioxygen and light barrier, a food packaging material should minimize the water-product contact. The evaluation of the WVTR parameter allows to define the capability of the packaging material to protect food in humid environment. The decrease in the WVTR values of the CS-film following the introduction of EOs could be related to the high thickness. Similar results were noticed in previous investigations (Priyadarshi et al. 2018; Akhter et al. 2019). Findings revealed that the treatment by the EOs decreased as well the moisture content of the CS-film 2-folds. Similar results were found in previous investigations (Ojagh et al. 2010; Hafsa et al. 2016). This drop in the moisture content could be associated to the interaction between the free OH groups of the chitosan and the EO components.
Basically, chitosan is known as an antimicrobial agent (Khan et al. 2020), The inhibitory effect against microorganisms is ensured by the presence of the positively charged NH3+ groups located on the carbon C2 of the chitosan molecule. These functional groups interfere with the negatively charged cell surface of the microorganism leading to its disorganization (Riaz Rajoka et al. 2020). It has been claimed that the antimicrobial behavior of the chitosan was higher against fungi than bacteria, with Gram-positive bacteria being less resistant in comparison with Gram-negative bacteria (Orzali et al. 2017). Xing et al., (2015) explained this variation in the antimicrobial activity by the difference in the cell wall constitution and cell structure. Moreover, chitosan can inactivate the replication of viruses and viroids (Kulikov et al. 2006). Nevertheless, numerous studies reported the limited activities of chitosan when applied as film (Wang et al. 2011; Mouhoub et al. 2023a).
In addition to the above-mentioned advantages of the EOs introduction into CS-film. These substances are well known to exhibit strong antioxidant and antimicrobial activities (Tariq et al. 2019). These biological activities essentially depend on the nature and concentration of the bioactive molecules present in the EO. The chemical assessment of the tested EOs indicates that citronellol, terpinen-4-ol, α-terpineol, and borneol which are all terpene alcohols, were the major constituents of the tested EOs. Previous results confirmed these findings (Fachini-Queiroz et al. 2012; Cerempei et al. 2014; Santana et al. 2014; Taoufik et al. 2017; Abbasi-Maleki et al. 2020; Song et al. 2022). In fact, terpene alcohols were defined as a good antioxidant and antimicrobial agents (Park et al. 2012; Ouedrhiri et al. 2018). Among all tested materials, the films containing pelargonium and thyme EOs exhibited the strongest bioactivities. These findings confirm the results of previous studies where the antioxidant and antimicrobial activities of pelargonium, tea tree, marjoram, and thyme EOs were compared (Teixeira et al. 2013; Alibi et al. 2020; Milenković et al. 2021; Mouhoub, Guendouz, et al. 2022a, b). This disparity in biological activities could be attributed to the qualitative and quantitative variation of the EOs' chemical components. The antimicrobial and antioxidant actions of citronellol which is the main constituent of pelargonium EO were previously highlighted (Santos et al. 2019; Sharma et al. 2020). Furthermore, An et al., (2019) demonstrated that terpinen-4-ol and α-terpineol exhibited the greatest antifungal activity against Aspergillus niger with slightly higher activity as regards α-terpineol. These findings could explain the effectiveness of thyme EO in comparison with tea tree and marjoram EOs and confirm that the stronger antimicrobial activity of tea tree EO, when compared to marjoram EO, was related to the high percentage of terpinen-4-ol (40.33%).
In natural conditions, It is well known that microorganisms attach to different surfaces and establish a complex structure called biofilm (Flemming and Wingender 2010). This arrangement of microorganisms enhances their resistance by 2000-folds against antimicrobial agents (Tabak et al. 2009; Wang et al. 2018). Moreover, biofilms engender different diseases including foodborne illnesses (Kulshrestha and Gupta 2022), and cause surfaces’ damage (Lv et al. 2022) which leads to colossal economic losses. The contact angle analysis was realized to determine an eventual correlation between the bacterial adherence rate and the physicochemical characteristics of the CS-films. According to the obtained results, a drop in the qualitative hydrophobicity was recorded for all the treated CS-films. However, the quantitative hydrophobicity of the material was randomly modified following the treatment by EOs. From these findings, It is more accurate to attribute the antiadhesion activity of the treated CS-film to the direct action of the EOs. Numerous investigations highlighted the multiple mode of action of EOs and their components against biofilm formation (Guo et al. 2021). E. g, the anti-quorum sensing and bactericidal activities (Burt et al. 2014; Merghni et al. 2018; Tariq et al. 2019), the inhibition of adhesins and binding proteins production (Marinas et al. 2015; Kot et al. 2019), and the targeting of the extracellular polymeric substrates (Zhao et al. 2018; Kang et al. 2019).
Based on these promising findings, we conclude that the treatment of CS-film containing pelargonium, tea tree, marjoram, and thyme EOs improves the physical properties and enhances the antioxidant and antimicrobial activities.
Conclusion
In summary, pelargonium, tea tree, marjoram, and thyme EOs contain terpene alcohols as major components. The introduction of these EOs into CS-film improved its physical properties by increasing the thickness, opacity and water barrier property, and decreasing the moisture content. Moreover, the biological activities such as the antibacterial, antioxidant, and antibiofilm activities of the CS-film were considerably enhanced by the EOs introduction, especially in the case of pelargonium and thyme treatments. Overall, these promising results emphasize the eventual utilization of CS-film containing pelargonium and thyme EOs as biodegradable food packaging.
Data availability
The authors do not have permission to share data.
References
Abbasi-Maleki S, Kadkhoda Z, Taghizad-Farid R (2020) The antidepressant-like effects of Origanum majorana essential oil on mice through monoaminergic modulation using the forced swimming test. J Tradit Complement Med 10(4):327–335. https://doi.org/10.1016/j.jtcme.2019.01.003
Abd El-Hack ME, El-Shall NA, El-Kasrawy NI, El-Saadony MT, Shafi ME, Zabermawi NM, Alshilawi MS, Alagawany M, Khafaga AF, Bilal RM (2022) The use of black pepper (Piper guineense) as an ecofriendly antimicrobial agent to fight foodborne microorganisms. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-17806-7
Akhter R, Masoodi F, Wani TA, Rather SA (2019) Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int J Biol Macromol 137:1245–1255. https://doi.org/10.1016/j.ijbiomac.2019.06.214
Alibi S, Selma WB, Ramos-Vivas J, Smach MA, Touati R, Boukadida J, Navas J, Mansour HB (2020) Anti-oxidant, antibacterial, anti-biofilm, and anti-quorum sensing activities of four essential oils against multidrug-resistant bacterial clinical isolates. Curr Res Transl Med 68(2):59–66. https://doi.org/10.1016/j.retram.2020.01.001
An P, Yang X, Yu J, Qi J, Ren X, Kong Q (2019) α-terpineol and terpene-4-ol, the critical components of tea tree oil, exert antifungal activities in vitro and in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control 98:42–53. https://doi.org/10.1016/j.foodcont.2018.11.013
Asgher M, Qamar SA, Bilal M, Iqbal HM (2020) Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res Int 137:109625. https://doi.org/10.1016/j.foodres.2020.109625
Azadbakht E, Maghsoudlou Y, Khomiri M, Kashiri M (2018) Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: potential as an antimicrobial carrier for packaging of sliced sausage. Food Packaging Shelf Life 17:65–72. https://doi.org/10.1016/j.fpsl.2018.03.007
Blanco M, Blanco J, Sanchez-Benito R, Perez-Giraldo C, Moran F, Hurtado C, Gomez-Garcia A (1997) Incubation temperatures affect adherence to plastic of Candida albicans by changing the cellular surface hydrophobicity. Microbios 89(358):23–28
Burt SA, Ojo-Fakunle VT, Woertman J, Veldhuizen EJ (2014) The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS One 9(4):e93414. https://doi.org/10.1371/journal.pone.0093414
Cerempei A, Muresan EI, Cimpoesu N (2014) Biomaterials with controlled release of geranium essential oil. J Essent Oil Res 26(4):267–273. https://doi.org/10.1080/10412905.2014.910711
Cichello SA (2015) Oxygen absorbers in food preservation: a review. J Food Sci Technol Health Care 52(4):1889–1895. https://doi.org/10.1007/s13197-014-1265-2
Coyotl-Pérez WA, Rubio-Rosas E, Morales-Rabanales QN, Ramírez-García SA, Pacheco-Hernández Y, Pérez-España VH, Romero-Arenas O, Villa-Ruano N (2022) Improving the shelf life of avocado fruit against clonostachys rosea with chitosan hybrid films containing thyme essential oil. Polymers 14(10):2050. https://doi.org/10.3390/polym14102050
Del Nobile MA, Licciardello F, Scrocco C, Muratore G, Zappa M (2007) Design of plastic packages for minimally processed fruits. J Food Eng 79(1):217–224. https://doi.org/10.1016/j.jfoodeng.2006.01.062
Dutta P, Tripathi S, Mehrotra G, Dutta J (2009) Perspectives for chitosan based antimicrobial films in food applications. Food Chem 114(4):1173–1182. https://doi.org/10.1016/j.foodchem.2008.11.047
Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, Carvalho MDdB, Cunha JM, Grespan R, Bersani-Amado CA, Cuman RKNJE-BC, Medicine A (2012) Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med 2012:10. https://doi.org/10.1155/2012/657026
Fahey JW, Smilovitz Burak J, Evans D (2022) Sprout microbial safety: a reappraisal after a quarter-century. Food Front. https://doi.org/10.1002/fft2.183
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. https://doi.org/10.1038/nrmicro2415
Gustavsson J, Cederberg C, Sonesson U, Otterdijk Rv, Meybeck A (2011) Global food losses and food waste: extent, causes and prevention. https://www.semanticscholar.org/paper/Global-food-losses-and-food-waste%3A-extent%2C-causes-Gustavsson-Cederberg/19c0065b1ad3f83f5ce7b0b16742d137d0f2125e.
Guo N, Bai X, Shen Y, Zhang T (2021) Target-based screening for natural products against Staphylococcus aureus biofilms. Crit Rev Food Sci Nutr Diab. https://doi.org/10.1080/10408398.2021.1972280
Gupta V, Biswas D, Roy S (2022) A comprehensive review of biodegradable polymer-based films and coatings and their food packaging applications. Materials 15(17):5899. https://doi.org/10.3390/ma15175899
Hafsa J, Ali Smach M, Khedher MRB, Charfeddine B, Limem K, Majdoub H, Rouatbi S (2016) Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT-Food Sci Technol Health Care 68:356–364. https://doi.org/10.1016/j.lwt.2015.12.050
He X, Li M, Gong X, Niu B, Li W (2021) Biodegradable and antimicrobial CSC films containing cinnamon essential oil for preservation applications. Food Packaging Shelf Life 29:100697. https://doi.org/10.1016/j.fpsl.2021.100697
Huang T, Qian Y, Wei J, Zhou C (2019) Polymeric antimicrobial food packaging and its applications. Polymers 11(3):560. https://doi.org/10.3390/polym11030560
Janik W, Nowotarski M, Shyntum DY, Banaś A, Krukiewicz K, Kudła S, Dudek G (2022) Antibacterial and biodegradable polysaccharide-based films for food packaging applications: comparative study. Materials 15(9):3236. https://doi.org/10.3390/ma15093236
Jem KJ, Tan B (2020) The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv Indus Eng Polym Res 3(2):60–70. https://doi.org/10.1016/j.aiepr.2020.01.002
Jesser E, Castillo L, Alonso Y, Urrutia R, Murray A, Domini C, Werdin-González J (2022) Development of active biodegradable films based on chitosan and essential oil to prevent infestation of Plodia interpunctella (Lepidoptera: Pyralidae). Food Packaging Shelf Life 34:100999. https://doi.org/10.1016/j.fpsl.2022.100999
Jovanović J, Ćirković J, Radojković A, Mutavdžić D, Tanasijević G, Joksimović K, Bakić G, Branković G, Branković Z (2021) Chitosan and pectin-based films and coatings with active components for application in antimicrobial food packaging. Progr Org Coat 158:106349. https://doi.org/10.1016/j.porgcoat.2021.106349
Kang J, Jin W, Wang J, Sun Y, Wu X, Liu L (2019) Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 101:639–645. https://doi.org/10.1016/j.lwt.2018.11.093
Khan F, Pham DTN, Oloketuyi SF, Manivasagan P, Oh J, Kim Y-MJC, Biointerfaces SB (2020) Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surf B Biointerfaces 185:110627. https://doi.org/10.1016/j.colsurfb.2019.110627
Kot B, Sytykiewicz H, Sprawka I, Witeska M (2019) Effect of trans-cinnamaldehyde on methicillin-resistant Staphylococcus aureus biofilm formation: metabolic activity assessment and analysis of the biofilm-associated genes expression. Int J Mol Sci 21(1):102. https://doi.org/10.3390/ijms21010102
Kulikov S, Chirkov S, Il’Ina A, Lopatin S, Varlamov V. (2006) Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl Biochem Microbiol 42(2):200–203. https://doi.org/10.1134/S0003683806020165
Kulshrestha A, Gupta P (2022) Polymicrobial interaction in biofilm: mechanistic insights. Pathogens Dis 80(1):ftac010. https://doi.org/10.1093/femspd/ftac010
Lekbach Y, Li Z, Xu D, El Abed S, Dong Y, Liu D, Gu T, Koraichi SI, Yang K, Wang F (2019) Salvia officinalis extract mitigates the microbiologically influenced corrosion of 304L stainless steel by Pseudomonas aeruginosa biofilm. Bioelectrochemistry 128:193–203. https://doi.org/10.1016/j.bioelechem.2019.04.006
Li S, Mu B, Zhang H, Kang Y, Wang A (2022) Incorporation of silver nanoparticles/curcumin/clay minerals into chitosan film for enhancing mechanical properties, antioxidant and antibacterial activity. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2022.11.046
Lian H, Peng Y, Shi J, Wang Q (2019) Effect of emulsifier hydrophilic-lipophilic balance (HLB) on the release of thyme essential oil from chitosan films. Food Hydrocoll 97:105213. https://doi.org/10.1016/j.foodhyd.2019.105213
Lv M, Du M, Li Z (2022) Investigation of mixed species biofilm on corrosion of X65 steel in seawater environment. Bioelectrochem 143:107951. https://doi.org/10.1016/j.bioelechem.2021.107951
Marinas IC, Oprea E, Chifiriuc MC, Badea IA, Buleandra M, Lazar V (2015) Chemical composition and antipathogenic activity of Artemisia annua essential oil from Romania. Chem Biodivers Conserv 12(10):1554–1564. https://doi.org/10.1002/cbdv.201400340
Merghni A, Noumi E, Hadded O, Dridi N, Panwar H, Ceylan O, Mastouri M, Snoussi M (2018) Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1, 8-cineole against methicillin-resistant Staphylococcus aureus strains. Microbial Pathog 118:74–80. https://doi.org/10.1016/j.micpath.2018.03.006
Milenković L, Ilić ZS, Šunić L, Tmušić N, Stanojević L, Stanojević J, Cvetković D (2021) Modification of light intensity influence essential oils content, composition and antioxidant activity of thyme, marjoram and oregano. Saudi J Biol Sci 28(11):6532–6543. https://doi.org/10.1016/j.sjbs.2021.07.018
Mouhoub A, Er Raouan S, Guendouz A, El Alaoui-Talibi Z, Koraichi SI, El Abed S, Delattre C, El Modafar C (2022) Antiadhesion effect of the Chitosan-based film incorporated with essential oils against foodborne bacteria. Indus Crops Prod. https://doi.org/10.1016/j.indcrop.2022.115742
Mouhoub A, Guendouz A, Belkamel A, El Alaoui TZ, Ibnsouda Koraichi S, El Modafar C, Delattre C (2022b) Assessment of the antioxidant, antimicrobial and antibiofilm activities of essential oils for potential application of active chitosan films in food preservation. World J Microbiol Biotechnol 38(10):1–16. https://doi.org/10.1007/s11274-022-03363-9
Mouhoub A, Er Raouan S, Guendouz A, Alaoui-Talibi E, Ibnsouda Koraichi S, El Abed S, Delattre C, El Modafar C (2023a) The effect of essential oils mixture on chitosan-based film surface energy and antiadhesion activity against foodborne bacteria. World J Microbiol Biotechnol 39(3):1–11. https://doi.org/10.1007/s11274-023-03520-8
Mouhoub A, Er Raouan S, Guendouz A, Alaoui-Talibi E, Ibnsouda Koraichi S, El Abed S, Delattre C, El Modafar C (2023b) Inhibition of multi-species biofilm formation using chitosan-based film supplemented with essential oils. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2023.111943
Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH (2010) Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem 122(1):161–166. https://doi.org/10.1016/j.foodchem.2010.02.033
Orzali L, Corsi B, Forni C, Riccioni L (2017) Chitosan in agriculture: a new challenge for managing plant disease. Biol Act Appl Mar Polysacch. https://doi.org/10.5772/66840
Ouattara B, Simard RE, Piette G, Bégin A, Holley RA (2000) Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int J Food Microbiol 62(1–2):139–148. https://doi.org/10.1016/S0168-1605(00)00407-4
Ouedrhiri W, Balouiri M, Bouhdid S, Harki EH, Moja S, Greche H (2018) Antioxidant and antibacterial activities of Pelargonium asperum and Ormenis mixta essential oils and their synergistic antibacterial effect. Environ Sci Pollut Res 25(30):29860–29867. https://doi.org/10.1007/s11356-017-9739-1
Park S-N, Lim YK, Freire MO, Cho E, Jin D, Kook J-K (2012) Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 18(3):369–372. https://doi.org/10.1016/j.anaerobe.2012.04.001
Peng Y, Li Y (2014) Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll 36:287–293. https://doi.org/10.1016/j.foodhyd.2013.10.013
Phothisarattana D, Harnkarnsujarit N (2022) Migration, aggregations and thermal degradation behaviors of TiO2 and ZnO incorporated PBAT/TPS nanocomposite blown films. Food Packaging Shelf Life 33:100901. https://doi.org/10.1016/j.fpsl.2022.100901
Priyadarshi R, Kumar B, Deeba F, Kulshreshtha A, Negi YS (2018) Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll 85:158–166. https://doi.org/10.1016/j.foodhyd.2018.07.003
Riaz Rajoka MS, Mehwish HM, Wu Y, Zhao L, Arfat Y, Majeed K, Anwaar SJ (2020) Chitin/chitosan derivatives and their interactions with microorganisms: a comprehensive review and future perspectives. Crit Rev Biotechnol 40(3):365–379. https://doi.org/10.1080/07388551.2020.1713719
Sánchez-González L, González-Martínez C, Chiralt A, Cháfer M (2010) Physical and antimicrobial properties of chitosan–tea tree essential oil composite films. J Food Eng 98(4):443–452. https://doi.org/10.1016/j.jfoodeng.2010.01.026
Santana O, Andres MF, Sanz J, Errahmani N, Abdeslam L, González-Coloma AJNpc (2014) Valorization of essential oils from Moroccan aromatic plants. Nat Prod Commun. https://doi.org/10.1177/1934578X1400900812
Santos PL, Matos JPS, Picot L, Almeida JR, Quintans JS, Quintans-Júnior LJ (2019) Citronellol, a monoterpene alcohol with promising pharmacological activities—a systematic review. Food Chem Toxicol 123:459–469. https://doi.org/10.1016/j.fct.2018.11.030
Sedlaříková J, Doležalová M, Egner P, Pavlačková J, Krejčí J, Rudolf O, Peer P (2017) Effect of oregano and marjoram essential oils on the physical and antimicrobial properties of chitosan based systems. Int J Polym Sci. https://doi.org/10.1155/2017/2593863
Shahidi F, Arachchi JKV, Jeon Y-JJTifs, Technology (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10(2):37–51. https://doi.org/10.1016/S0924-2244(99)00017-5
Shaikh S, Yaqoob M, Aggarwal P (2021) An overview of biodegradable packaging in food industry. Curr Res Food Sci 4:503–520. https://doi.org/10.1016/j.crfs.2021.07.005
Sharma Y, Rastogi SK, Ahmedi S, Manzoor N (2020) Antifungal activity of β-citronellol against two non-albicans Candida species. J Essent Oil Res 32(3):198–208. https://doi.org/10.1080/10412905.2020.1737588
Simsek M, Eke B, Demir H (2020) Characterization of carboxymethyl cellulose-based antimicrobial films incorporated with plant essential oils. Int J Biol Macromol 163:2172–2179. https://doi.org/10.1016/j.ijbiomac.2020.09.075
Siracusa V, Rocculi P, Romani S, Dalla RM (2008) Biodegradable polymers for food packaging: a review. Trends Food Sci Technol Health Care 19(12):634–643. https://doi.org/10.1016/j.tifs.2008.07.003
Siripatrawan U, Harte BR (2010) Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll 24(8):770–775. https://doi.org/10.1016/j.foodhyd.2010.04.003
Song X, Wang L, Liu L, Li J, Wu X (2022) Impact of tea tree essential oil and citric acid/choline chloride on physical, structural and antibacterial properties of chitosan-based films. Food Control 141:109186. https://doi.org/10.1016/j.foodcont.2022.109186
Tabak M, Scher K, Chikindas ML, Yaron S (2009) The synergistic activity of triclosan and ciprofloxacin on biofilms of Salmonella Typhimurium. FEMS Microbiol Lett 301(1):69–76. https://doi.org/10.1111/j.1574-6968.2009.01804.x
Taoufik F, Anejjar A, Asdadi A, Salghi R, Chebli B, El Hadek M, Idrissi Hassani LJJMES (2017) Synergic effect between Argania spinosa cosmetic oil and Thymus satureioides essential oil for the protection of the carbon steel against the corrosion in sulfuric acid medium. J Mater Environ Sci 8:582–593
Tapia MS, Alzamora SM, Chirife J (2020) Effects of water activity (aw) on microbial stability as a hurdle in food preservation. Water Activity Foods Fundam Appl. https://doi.org/10.1002/9781118765982.ch14
Tariq S, Wani S, Rasool W, Shafi K, Bhat MA, Prabhakar A, Shalla AH, Rather MA (2019) A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb Pathog 134:103580. https://doi.org/10.1016/j.micpath.2019.103580
Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JM, Saraiva JA, Nunes ML (2013) Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Indus Crops Prod 43:587–595. https://doi.org/10.1016/j.indcrop.2012.07.069Get
Van Oss C (1995) Hydrophobicity of biosurfaces—origin, quantitative determination and interaction energies. Colloids Surf B: Biointerfaces 5(3–4):91–110. https://doi.org/10.1016/0927-7765(95)01217-7
Van Oss CJ, Chaudhury MK, Good RJ (1988) Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev 88(6):927–941. https://doi.org/10.1021/cr00088a006
Vidács A (2022) Antibacterial effect of edible coatings with essential oil. Analecta Technica Szegedinensia 16(1):71–76. https://doi.org/10.14232/analecta.2022.1.71-76
Vogler EA (1998) Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interface Sci 74(1–3):69–117. https://doi.org/10.1016/S0001-8686(97)00040-7
Wang L, Liu F, Jiang Y, Chai Z, Li P, Cheng Y, Jing H, Leng X (2011) Synergistic antimicrobial activities of natural essential oils with chitosan films. J Agric Food Chem 59(23):12411–12419. https://doi.org/10.1021/jf203165k
Wang H, Cai L, Li Y, Xu X, Zhou G (2018) Biofilm formation by meat-borne Pseudomonas fluorescens on stainless steel and its resistance to disinfectants. Food Control 91:397–403. https://doi.org/10.1016/j.foodcont.2018.04.035
Wang B, Yan S, Qiu L, Gao W, Kang X, Yu B, Liu P, Cui B, Abd El-Aty A (2022) Antimicrobial activity, microstructure, mechanical, and barrier properties of cassava starch composite films supplemented with geranium essential oil. Front Nutr. https://doi.org/10.3389/fnut.2022.882742
Xing K, Zhu X, Peng X, Qin S (2015) Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agron Sustain Dev 35(2):569–588. https://doi.org/10.1007/s13593-014-0252-3
Yang D, Liu Q, Gao Y, Wan S, Meng F, Weng W, Zhang Y (2022) Characterization of silver nanoparticles loaded chitosan/polyvinyl alcohol antibacterial films for food packaging. J Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2022.108305
Zarandona I, Correia DM, Moreira J, Costa CM, Lanceros-Mendez S, Guerrero P, de la Caba K (2022) Magnetically responsive chitosan-pectin films incorporating Fe3O4 nanoparticles with enhanced antimicrobial activity. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2022.11.286
Zhang X, Ismail BB, Cheng H, Jin TZ, Qian M, Arabi SA, Liu D, Guo M (2021) Emerging chitosan-essential oil films and coatings for food preservation—a review of advances and applications. Carbohydr Polym 273:118616. https://doi.org/10.1016/j.carbpol.2021.118616
Zhao X, Liu Z, Liu Z, Meng R, Shi C, Chen X, Bu X, Guo N (2018) Phenotype and RNA-seq-based transcriptome profiling of Staphylococcus aureus biofilms in response to tea tree oil. J Microbial Pathog 123:304–313. https://doi.org/10.1016/j.micpath.2018.07.027
Author information
Authors and Affiliations
Contributions
Conceptualization, AM; SIK; AG; ZET; CD and CEM; methodology, AM; AG; SIK and CEM; software, AM; validation, AM; AG; CD; ZET and CEM; formal analysis, AM; AG; CD; ZET and CEM; resources, SIK and CD; data curation, AM and AG; writing, AM; writing-review, AM and AG and supervision, AG; SIK and CEM. the final version of the manuscript has been approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
There are no conflicts of interest that need to be disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mouhoub, A., Guendouz, A., El Alaoui-Talibi, Z. et al. Elaboration and general evaluation of chitosan-based films containing terpene alcohols-rich essential oils. World J Microbiol Biotechnol 39, 146 (2023). https://doi.org/10.1007/s11274-023-03597-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03597-1