Abstract
The contribution made by saltmarsh to the production of estuarine zooplankton was examined through a comparison of inputs and outputs of tidal water at a site on Towra Point, NSW, Australia. Saltmarsh proved to be a net exporter of crab and gastropod larvae, although it functioned as a sink for copepods and amphipods. Further, the highest density of zooplankton in estuarine nearshore habitats (saltmarsh, mangrove, seagrass, and open water) during a high tide event was found in the saltmarsh. The presence of high concentrations of zooplankton, predominantly crab and gastropod larvae, in the saltmarsh and lesser extent in the mangrove represents a source of food for estuarine fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The export of detritus from saltmarshes (Nixon 1980; Dame et al. 1986) and mangrove forests (Boto and Bunt 1981; Flores-Verdugo et al. 1987) has been studied extensively, though mostly in the form of larger litter. The materials exported from these habitats to coastal water by tides are believed assist primary production as well as provide food for many aquatic consumers including fish. However, experimental studies of detritus utilization by macro-consumers have shown that the role of detritus in estuarine food chains is complex (Boesch and Turner 1984). While some studies of saltmarshes in North America stated that a major part of the primary production of a saltmarsh is exported to adjacent waters, this was not found to be the case in detailed studies in the southern hemisphere (Morrisey 1995). Studies of the productivity of various plant communities in Botany Bay (Larkum 1981) suggested that saltmarshes contributed only 6% to primary productivity within the bay, while seagrass, mangrove and phytoplankton were the major contributors.
We investigated the role of saltmarsh in contributing to estuarine productivity through the production and export of saltmarsh derived zooplankton. The study was conducted at Towra Point, Botany Bay, across a range of nearshore habitats and provides a case-study in the concept of trophic relay developed by Kneib (1997). Previous studies at Towra Point indicated that nekton entering the saltmarsh during spring tides (Mazumder et al. 2006) may be consuming larvae and transferring carbon to other estuarine habitats. Our aim was therefore to examine the role saltmarshes might play in contributing to zooplankton availability. This in part can be achieved through the comparison of zooplankton concentrations across shallow habitats of saltmarsh, mangrove, seagrass and open water.
Materials and methods
Study sites
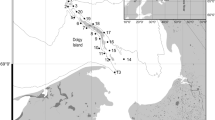
The study investigated zooplankton import and export from a saltmarsh environment in Botany Bay (Fig. 1). An extensive mangrove environment is present at Towra Point with adjacent saltmarsh dominated by a Sarcocornia quinqueflora/Sporobolus virginicus association, and Triglochin striata growing in less well-drained positions. Juncus kraussii and Suaeda australis are also present in higher elevation environments (Clarke and Hannon 1967; Adam et al. 1988). Four species of crab, Heloecius cordiformis, Parasesarma erythrodactyla, Helograpsus haswellianus and Paragrapsus laevis have been found in saltmarsh, and three of these species (H. cordiformis, P. erythrodactyla, and P. laevis) were also found in the adjacent mangrove (Mazumder and Saintilan 2003).
The saltmarsh, mangrove and seagrass communities are distributed around the whole of Towra Point and into Woolooware Bay. Tides are diurnal, with a maximum spring tide inundation of 2.0 m Australian Height Datum. Spring tides of this height would inundate the saltmarsh to mean depth of approximately 20 cm, ranging up to 40 cm in depth at the mangrove fringe. Tidal water is completely emptied from the saltmarsh with the ebbing tide, though may be retained in shallow depressions.
Sampling inputs and outputs of zooplankton
The saltmarsh and mangrove environments on the Woolooware Bay side of Towra Point are separated by a low natural levee, punctured at intervals by tidal creeks. Two of these breaks in the levee were utilized as fixed replicate points for sampling zooplankton inputs and outputs from saltmarsh on monthly basis from March 2001 to August 2002.
As other research suggests that stage 1 zoea larvae of crabs are most abundant in surface water as compared to mid depth and near bottom waters (Epifanio et al. 1984), a behavior also revealed in laboratory studies (Sulkin et al. 1980), we examined only the surficial distribution of larva in this study. One 1.5 m long 350 μm mesh plankton net with 40 cm diameter opening was deployed at each of these two points within the levee breaks during flood and ebb tides. Four replicate samples were taken at each of the fixed points during the flood and ebb, each set being of 2 min duration, to yield a total of 16 samples in each month. Sampling of the incoming tide began when sufficient water was entering the saltmash to inundate the entire net. Sampling of the outgoing tide began immediately following the tidal peak, and continued for 30 min. At maximum high tide the depth of water in the gap was of the order of 50 cm. The volume of water passing through the net was calculated using General Oceanics Flow-meter (model M-2030).
Sampling between habitats on the ebbing tide
At the conclusion of the main sampling exercise, zooplankton was sampled once, in September 2002, along a shore-normal transect extending from the saltmarsh, through mangrove and seagrass habitats, to open water mudflat (Fig. 2). The same plankton nets were used. One net was again fixed to collect samples for 2 min from the gap in the levee at the edge of the saltmarsh. The second (identical) net was hauled for 2 min through each of the other three locations during the ebbing tide. The transect was thus divided into four sectors (saltmarsh, mangrove, seagrass and open water) to examine zooplankton distribution. Collection from the mangrove habitat was at least 50 m seaward of the gap in the levee. Similarly, the seagrass collection site was 50 m from the place where the mangrove samples were taken, and the open water was 50 m from the seagrass location. The latter sites were sampled to determine the rate of dilution of intertidal marsh-sourced zooplankton into the estuarine waters. Four replicate zooplankton samples were taken at 20-min intervals from the gap and the three sectors during the outgoing tide. The volume of water passing through the net was calculated using General Oceanics Flow-meter (model M-2030).
Statistical methods and data analysis
Univariate analysis (ANOVA) was performed to determine differences in abundances of five zooplankton functional groups between incoming and outgoing tides at the levee gaps, and to determine differences in zooplankton abundance along the transect from the saltmarsh to the open water. Data were square root transformed before analysis to remove heterogeneity of variances. In the first ANOVA location and time were fixed factors; location was a fixed factor in the second analysis.
Results
Zooplankton density in flood and ebb tides in saltmarsh
Figure 3 illustrates the monthly variation in abundance of the crab larvae, gastropod larvae, copepods, amphipods, and other zooplankton. Differences were found in the densities between incoming and outgoing tides for all functional groups. Crab and gastropod larvae were more abundant on the outgoing tide, whereas copepods, amphipods and other taxa were more abundant on the incoming tide (Table 1).
Larval abundance along the transect
Along the shore-normal transect the volume of water passing through the net was 14.08 m3 in the saltmarsh, 10.44 m3 in the mangrove, 11.09 m−3 in the seagrass and 12.33 m−3 in the open water, respectively. The mean zooplankton abundance (individuals per cubic meter) was always highest in saltmarsh and then consistently decreased along the transect: saltmarsh—8924.25 m−3, mangrove—3062.25 m−3, seagrass—2516 m−3 and open water—2285.75 m−3, respectively (Fig. 4). ANOVA indicated that crab larval abundance between locations along the transect varied significantly (P < 0.0001).
Discussion
A detailed year round study (Mazumder et al. 2006) examined crab larval abundance between flood and ebb tides at Towra Point and found during the ebb tide crab larvae in numbers several orders of magnitude higher than at flood tide. Current investigation expands on the previous result by assessing the presence of other zooplankton taxa, and found that the difference in zooplankton density between the flood and ebb tides at Towra Point saltmarsh was mainly due to the export of crab and gastropod larvae from the saltmarsh. A recent study at a nearby estuary (P. Freewater, unpublished results) also found that crabs living in the saltmarsh-mangrove complex released significantly higher numbers of larvae in the ebbing tides. Changes in density of zooplankton over the course of the outgoing tide were not considered in his study, though may vary with tidal velocity and the degree of marsh inundation.
The most abundant zooplankton components of the flood tide water onto the marsh were copepods and amphipods (Table 1, Fig. 3), but the Towra Point saltmarsh appeared to act as a sink for these taxa. Whether their loss is due to predation by fish or by other factors is unclear from the present research. If these zooplankters settle or are stranded on the marsh surface a feeding opportunity for grazing omnivores would be created.
Transect data demonstrated that zooplankton densities were substantially higher in the saltmarsh than in other estuarine habitats during the peak of the spring high tide due to the introduction of newly hatched crab and gastropod larvae from saltmarsh. The lesser densities in the mangrove, seagrass and open water habitats may be the result of dilution at the point of sampling (∼1–1.5 m in depth as opposed to 40 cm maximum in saltmarsh), and/or lower production of crab larvae from these habitats. As we examined only the sacrificial distribution of larvae it would be useful in future studies to examine the vertical distribution as was done by Sulkin et al. (1980) and Epifanio et al. (1984).
Very little is known about the distribution of gastropod larvae within southern hemisphere estuaries and their contribution to food webs. Morrisey (1995) suggested that the eggs of estuarine gastropods are laid when the tide is high and that the larvae settle when the tide is high enough to bring them back inshore. Other studies, based on the stomach content analysis of fish (Bell et al. 1984; Kaly 1988; Mazumder et al. 2006) suggest that adult gastropods contribute to the diet of fish. Kaly (1988) reported that snails contributed a small part of the diet (less than 10% of volume) of the toad fish Teractenos hamiltoni and less than 5% to yellow-finned bream Acanthopagrus australis. If, as suggested by Roach (1998) adult fish spit out the indigestible shell component making the soft bodies of gastropods difficult to identify in the stomach, then other approaches are needed to identify the contribution of gastropods to estuarine food webs.
The high concentration of larvae within the saltmarsh, and to a lesser extent mangrove waters (Fig. 4), represents an abundant, if temporary food source for some juvenile and small species of fish. Stomach content analysis of fish leaving temperate saltmarsh habitat during high spring tides has demonstrated that Port Jackson glassfish, Ambassis jacksoniensis, fed predominantly on crab larvae (Mazumder et al. 2006). Another temperate study (Crains 2007) provided further support for this trophic pathway, finding glassfish to be periodically feeding on crab larvae and copepods depending on availability. In a sub tropical Queensland marsh, Hollingsworth and Connolly (2006) similarly reported the consumption of crab larvae by A. jacksoniensis from saltmarsh during high tide. In contrasting these fish with those sampled in alternative estuarine habitats they found the gut fullness index of the former to be higher.
Consumption of crab larvae by glassfish in eastern Australian saltmarsh supports the ‘trophic relay’ concept proposed by Kneib (1997) where he hypothesized nekton as transporters of organic matter across the marsh ecotone to the open estuary through predator prey interactions in the food web. A. jacksoniensis fulfills this role as they transport the energy produced in the saltmarsh to other/deeper parts of the estuary where it is available to larger fish, including A. australis (Mazumder et al., unpublished results).
References
Adam P, Wilson NC, Huntley B (1988) The phytosociology of coastal saltmarsh vegetation in New South Wales. Wetlands (Aust) 7:35–84
Bell JD, Pollard DA, Burchmore JJ, Pease BC, Middleton MJ (1984) Structure of a fish community in a temperate tidal mangrove creek in Botany Bay, New South Wales. Aust J Mar Freshw Res 35:33–46
Bosech DF, Turner ER (1984) Dependency of fishery species on saltmarshes: the role of food and refuge. Estuaries 7:460–468
Boto KG, Bunt JS (1981) Tidal export of particulate organic matter from a northern Australian mangrove system. Estuar Coast Shelf Sci 13:247–255
Clarke LD, Hannon NJ (1967) The mangrove swamp and saltmarsh communities of the Sydney district. I. Vegetation, soil and climate. J Ecol 55:753–771
Crains J (2007) An investigation of the feeding habits and trophic status of glassfish (Ambassis jacksoniensis) in south east Australian estuaries through stomach content and stable isotope analysis. Honours thesis, University of Wollongong, NSW, Australia, 62 pp
Dame R, Chrzanowski T, Bildstein K, Kjerfre B, McKellar H, Nelson D, Spurrier S, Stancyk S, Stevenson H, Vernberg J, Zingmark R (1986) The outwelling hypothesis and North Inlet, South Carolina. Mar Ecol Prog Ser 33:217–229
Epifanio CE, Valenti CC, Pembroke AE (1984) Dispersal and recruitment of blue crab larvae in Delaware Bay, USA. Estuar Coast Shelf Sci 18:1–12
Flores-Verdugo JF, Day JW, Briseno-Duenas R (1987) Structure, litterfall, decomposition and detritus dynamics of mangroves in a Mexican coastal lagoon with an ephemeral inlet. Mar Ecol Prog Ser 35:83–90
Hollingsworth A, Connolly RM (2006) Feeding by fish visiting inundated subtropical saltmarsh. J Exp Mar Biol Ecol 336:88–98
Kaly UL (1988) Distribution, abundance and size in mangrove and saltmarsh gastropods. PhD thesis, University of Sydney
Kneib RT (1997) The role of tidal marshes in the ecology of estuarine nekton. Oceanogr Mar Biol Annu Rev 35:163–220
Larkum AWD (1981) Marine primary productivity. In: Clayton MN, King RJ (eds) Marine botany: an Australian perspective. Longman Cheshire, Melbourne, pp 369–385
Mazumder D, Saintilan N (2003) A comparison of sampling techniques in the assessment of burrowing crab abundance in saltmarsh and mangrove in temperate Australia. Wetlands (Aust) 21:1–15
Mazumder D, Saintilan N, Williams RJ (2006) Trophic relationships between itinerant fish and crab larvae in a temperate Australian saltmarsh. Mar Freshw Res 57:193–199
Morrisey D (1995) Saltmarshes. In: Underwood AJ, Chapman MG (eds) Coastal marine ecology of temperate Australia. University of New South Wales Press
Nixon SW (1980) Between coastal marshes and coastal waters—a review of twenty years of speculation and research on the role of salt marshes in estuarine productivity and water chemistry. In: Hamilton P, MacDonald KB (eds) Marine science, vol II: estuarine and wetland processes. Plenum Press, New York
Roach AC (1998) Effects of predation on the size structure of the gastropod Salinator solida (Martens) populations at Towra Point, NSW, Australia. Mar Freshw Res 49:779–784
Sulkin SD, Van Heukelem W, Kelly P, Van Heukelem L (1980) The behavioural basis of larval recruitment in the crab Callinectes sapidus Rathbun: a laboratory investigation of ontogenetic changes in geotaxis and barokinesis. Biol Bull 159:402–417
Acknowledgements
This research was supported by an Australian Research Council APAI scholarship to Debashish Mazumder and funding by the Department of Infrastructure, Planning and Natural Resources (DIPNR), and Department of Primary Industries (NSW Fisheries). National Parks and Wildlife Service provided access to Towra Point Nature Reserve. Assistance with the collection of samples was provided by Kamal Hossain, Swapan Paul and Sayed Chowdhury. Sarah Imgraben and Cath Hughes assisted with the development of Figs. 2 and 3, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazumder, D., Saintilan, N. & Williams, R.J. Zooplankton inputs and outputs in the saltmarsh at Towra Point, Australia. Wetlands Ecol Manage 17, 225–230 (2009). https://doi.org/10.1007/s11273-008-9102-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-008-9102-x