Abstract
The five major rivers (Ganges, Mahanadi, Godavari, Krishna, and Cauvery) flowing in the north eastern Indian Ocean greatly influence the biogeochemical cycling nearshore waters of the east coast of India. For example, estuaries which are located at the interface between land and ocean are an area of intense recycling of organic matter with a large salinity gradient. The estuarine environment is generally complex, as local circulations and mixing affect the chemistry and deposition of organic matter, thereby controlling the estuarine–coastal nutrient budget. The fluvial inputs are major sources of nutrients to the Bay of Bengal (BoB) which also regulates the phytoplankton dynamics in both estuaries and coastal waters. The annual supply of nutrients by the Ganges and Brahmaputra rivers to the BoB is estimated to be 133 × 109 mol year−1 which is ∼2% of the riverine input to the world ocean. Both river runoff and precipitation are more intense during the southwest monsoon (SWM), resulting in lowering of surface salinity to 3 to 7 units in the Bay of Bengal. This also creates a strong seasonality in phytoplankton primary production in both estuaries and coastal waters. In general, primary production in the nearshore waters of eastern India has been found to covary with reduced suspended material and stability of water column with three seasonal peaks at few locations. Although it was hypothesized that the biological productivity of BoB is low in comparison to its adjacent basin due to various reasons such as a narrow shelf, cloud cover during the summer monsoon, turbidity resulting from sediment influx, etc. However, recent studies suggest the presence of cyclonic eddies can enhance primary production in BoB due to the entrainment of nutrient-rich waters within the oligotrophic mixed layer. All these suggest that factors controlling primary production in BoB remain poorly understood. As growing industrial activities and regulated discharge through dams can influence the riverine nutrient load, it is expected plankton’s primary productivity and estuarine carbon budget may need periodic reassessment in near future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The five major rivers (Ganges, Mahanadi, Godavari, Krishna, and Cauvery) flowing in the north eastern Indian Ocean greatly influence the biogeochemical cycling of the estuarine environment. Estuaries are commonly described as semi-enclosed bodies of water, situated at the interface between land and ocean, where seawater is gradually diluted by the inflow of freshwater. The estuarine environment is generally complex, as local circulations and mixing effects the chemistry and deposition organic matter thereby controlling the estuarine–coastal nutrient budget. Along the east coast of India, the Hugli estuary forms largest delta in the world due to confluence of the distributaries of the Ganges and Brahmaputra (Fig. 11.1). The lower section of the estuary stretches through the rich mangrove forest, known as the Sunderban, covering the Matla River and therefore sometimes referred to as Hugli–Matla estuarine system although hydrologically they are completely different. The inland water connection provides a shipping channel to the major port (Kolkata port trust) through Kolkata and Haldia docks in the upper and lower sections, respectively. While Hugli estuary is shallow, funnel shaped, and considered as a positive estuary that traces its path through the southern region of the West Bengal state of India, several geomorphological changes has been recorded in recent times (Jayappa et al. 2006). The Matla River, on the other hand, no longer receives freshwater influx either from the River Hugli or Bidyadhari and thus becomes an enclosed tidal inlet of the sea with limited wave action and water movements. The region generally becomes filled with seawater during high tide, and most of the water gets drained away toward the sea at low tide, leaving a narrow stream of 0.9–1.2 m water in some places (Sarkar et al. 2004). The Hugli estuary receives a perennial freshwater discharge from the Ganges, and its lower stretches act as an open estuary throughout the year connecting to the coastal Bay of Bengal (BoB). The depth of the Hugli Estuary varies along the channel from ~21 m at Diamond Harbour to ~8 m at the mouth of the estuary (Central Inland Fisheries Research Institute 2012). The seasonality in this region can be described as spring inter-monsoon (February–May), summer monsoon (June–September), fall inter-monsoon (October–November), and northeast monsoon (December–January), respectively. The highest river discharge (3000 ± 1000 m3 s−1) in the Hooghly estuary is observed during the summer monsoon season and reduces to the minimum (1000 ± 80 m3 s−1) during the pre-monsoon season (Mukhopadhyay et al. 2006; Ray et al. 2015). The tidal regime of the estuary is strong and semi-diurnal in nature, with the tide height ranging from 5.2 m during spring to 1.8 m during the neap tide period and a velocity as high as 6 knots with significant influence the currents and the resultant circulation pattern (Sadhuram et al. 2005; Mukhopadhyay et al. 2006). The tidal flow and the opposing freshwater flow bring in a huge volume of organic and inorganic suspended sediments that result in high turbidity of the water column. The fluvial inputs are major sources of nutrients to the BoB; however, most of the nutrients are found to be removed within the estuary itself (Sarma et al. 2009). The annual supply of nutrients by the Ganges and Brahmaputra rivers to the BoB is 133 × 109 mol year−1 accounting for 2% riverine input to the world ocean (Sarin et al. 1989). In addition to these, significant agricultural activities along the banks of rivers due to the availability of freshwater have also been reported (Sarma et al. 2014) Isotopic signatures of δ15N Particulate Organic Matter (POM) in the Indian estuaries from the discharge period suggest strong enrichment in highly polluted estuaries (>10‰) compared to relatively clean estuaries (<6‰) (Sarma et al. 2014). Apart from this Hugli estuary receives approximately 13 m3 sec−1 of industrial effluent and urban wastewater (Sadhuram et al. 2005, and references therein) from densely populated and industrial cities such as Kolkata, Haldia.
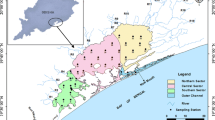
Shows the stations at the river end, mixing zone, and sea end. The seasonal distribution of nutrients and their fluxes at these locations are described along with influence of tidal fluctuations at Station A and Station B offshore. (Reproduced from Das et al. 2017 with permission from Elsevier)
To the south of the Hugli estuary lies the Mahanadi river system, which is the third-largest river of India and the largest river system in the Odisha state. The Mahanadi river basin (19°20′ to 23°35′N and 80°30′–86°50′E) extends over an area of approximately 141,600 km2 (65,628 km2 in Odisha), has a total length of 851 km and a peak discharge of 44,740 m3/s (Konhauser et al. 1997). The main branch of the Mahanadi river meets the BoB at Paradip, and known for its industrial activity (Radhakrishna 2001), while the minor distributaries meet the BoB near Chilika. Mangrove patches are also situated in the estuary (Sundaray et al. 2006). Human influences are more pronounced in the industrial cities, viz. Sambalpur, Cuttack, Bauda, Choudwar, Jagatpur, and Paradip (Sundaray et al. 2006). While Hugli and Brahmaputra can be termed as glacial rivers due to the influence of snow melt, the rivers in the peninsular India are typical monsoonal rivers, as these rivers receive large amount freshwater during the wet period compare to the dry months (Sarma et al. 2014). These include Mahanadi, Godavari, Krishna and Cauvery. Due to heavy discharge during monsoon period, the estuaries turn into freshwater system with no vertical salinity gradient (Vijith et al. 2009; Sridevi 2013; Sridevi et al. 2015). The discharge of these rivers is in unison with the precipitation pattern of the area, and are therefore called “Monsoonal estuaries” and exhibit a non-steady state behavior (Vijith et al. 2009).
The Godavari estuary in peninsular India is located around 16o15′ N and 82o5′ E, covering an area of 330 km with a basin area of 3.1 × 105 km2. Near the town Rajahmundry the river flow is regulated by a century-old dam at Dowleswaram (Sarma et al. 2014). Strong seasonal variability in nutrient distribution is observed here between monsoon and non-monsoon period (Sarma et al. 2010). While Krishna estuary is about 45 km in length and the estuarine system of Krishna river covers an area of about 320 km2 with all its four distributaries, the tidal portion extends up to 39 km upstream near Penumudi in the river. It is essentially a shallow one with a mean width of 1.2 km and an average depth of 5–7 m. The estuary has a well-developed sandy coast that experiences longshore drift, build-up spits, and barriers across river mouths, creating a coastal lagoon (Kumari and Rao 2010). It is a meso-tidal estuary with a tide range of 2–3 m and strong tidal current of 1.2 m sec−1. Dissolved and particulate loads of the Krishna river are derived from a variety of igneous, metamorphic, and sedimentary rocks in their catchment areas (Kumari and Rao 2010). Similarly, the fourth-largest river of southern region, Cauvery, is an easterly flowing river of the peninsular India that runs across three of the southern Indian states, that is, Karnataka, Tamil Nadu, Kerala, and the Union Territory of Puducherry. The river in its 800 km long journey from the Western Ghats traverses through Mysore plateau and finally forms a delta on the eastern coastline of the subcontinent before falling into the Bay of Bengal. The point of origin of Cauvery, Talakaveri, is in the Brahmagiri ranges of the Western Ghats at an elevation of 1341 m (https://www.indiawaterportal.org/). In addition to this, the East India Coastal Currents (EICC) which flows along the coastal BoB also influences the local budget which reverses its direction twice a year. It flows northwards from February to September with a strong peak in March–April and flows southwestward from October to January with strongest flow during January (Schott and McCreary Jr 2001). A study by Bristow et al. (2017) suggests that the amount of nutrients coming from the east coast to India through river discharge can make BoB a dead zone due to increase in primary production and sinking carbon fluxes. All these highlight the complex interaction where anthropogenic perturbation as well as physical process can act together in different time scale thereby influencing the estuarine budget. Thus, Indian estuaries need occasional reassessments of nutrient budget and associated fluxes in order to delineate long-term trends.
11.2 Nutrient Dynamics in and Around East Coast of India
Aquatic dissolved nutrients mainly consist of inorganic nitrogen (nitrate + nitrite), phosphorus, and silica (Si (OH) 4), which generally limit phytoplankton growth in natural waters. Studies on nutrient distribution from the Hugli–Matla estuarine system is limited (Mukhopadhyay et al. 2006; Das et al. 2015, 2017). Further, very few studies highlight the influence of tides on nutrient distribution in this region (Das et al. 2015, 2017). Based on Land-Ocean Interactions in the Coastal Zone (LIOCZ) biogeochemical model, Mukhopadhyay et al. 2006 illustrated that fluxes of inorganic nitrogen were in an order of (65.8 × 10^3 t) followed by phosphorus (12.8 × 10 3 t). Further silicate discharge by Hugli estuary was found to be in the order of (Si (OH)4 (42.8 × 10 3 t) where more than 50% of the load was contributed during the monsoon seasons. They also showed that during the estuarine transport, 7.0% of the silicate was removed within the estuary itself; however, there was addition of inorganic nitrogen (59%) and phosphorus (44%), which they attributed to heterotrophic regeneration within the estuary. The seasonal variations of inorganic nitrogen and silicate at three stations (Station 1: sea end; Station 2: mixing zone; Station 3: riverine end) along the Hugli estuarine system is illustrated in Fig. 11.2.
Monthly variation (averaged over 2 years between 1999 and 2001) of (a) silicate and dissolved inorganic nitrogen (Reproduced from Mukhopadhyay et al. 2006 with permission from Elsevier)
Das et al. 2015 studied biogeochemical characteristics at seven different locations along the Hugli estuarine system during highest high tide (HTT) and lowest low tide (LLT) to understand the influence of the tidal fluxes on nutrient distribution. The data reveal that during the LLT hours, a relative increase of freshwater input in the northern BoB can have elevated the nutrient concentration compared with that observed during the HHT hours. The ratio of nutrient concentration is found to deviate significantly from the Redfield ratio in the Hugli estuarine system. The abundance of dissolved inorganic phosphorous (DIP) is much higher compared with that of dissolved inorganic nitrogen (DIN) and dissolved inorganic silica (DSi). The anthropogenic sources of DIP from the upstream flow (especially the domestic effluent of several metropolises) can be mainly attributed during those observations. Apart from this, weathering of rocks is found to release soluble alkali phosphate and colloidal calcium phosphate to the rivers which is ultimately drained in estuary can also be a potential source (Ramesh et al. 2015). Further, Das et al. (2017) did a follow-up study during the high tide and low tide conditions (daytime) of the spring and neap phases throughout two annual cycles (February 2013 to January 2015) to understand how tidal processes principally regulate the nutrient variability in short-term scale in the Hugli estuarine region. Influence of tidal fluxes on nutrient distribution along the Hugli estuarine system is illustrated in Fig. 11.3. The results suggest that spring–neap and high tide–low tide contrast was most prominent in pre-monsoon and post-monsoon season. Hugli estuarine system is mainly fed by glacial rivers (Ganges and Brahmaputra); however, few of the other rivers along the east coast can be called as monsoonal rivers as there is significant difference in the discharge rates between monsoon and non-monsoon periods (Sarma et al. 2010). Discharge from the Indian monsoonal rivers is limited to a few months only because these rivers are fed by the SWM-induced precipitation over the catchment. However, glacial rivers also show remarkably high discharge (50% of the annual discharge) during SWM (June–September) due to the influence of monsoonal precipitation over the catchments (Ittekkot et al. 1991). The detailed nutrient budget along with the catchment area for the east coast of India is illustrated in Table 11.1. Krishna et al. (2016) showed that among the monsoonal rivers in peninsular India, Godavari transports highest amount of nitrate (0.08 Tg yr. −1), while the Krishna transports highest amounts of phosphate (0.02 Tg yr.−1) and silicate (0.36 Tg yr.−1). These rivers export similar amount of dissolved inorganic N (ammonium + nitrite + nitrate) to the BoB (0.12 ± 0.03 Tg yr.−1) as compared to that of Arabian Sea; however, the phosphate and silicate load during monsoon is much higher in BoB than Arabian Sea. The monsoonal rivers were found to export 1.03 ± 0.25 Tg yr.−1 of silicate to the northern Indian Ocean of which 0.91 ± 0.23 Tg yr.−1 enter the BoB (Krishna et al. 2016). Apart from this contribution from atmospheric deposition and submarine groundwater discharge has also been recently quantified. As atmospheric deposition of DIN and DIP from Indo-Gangetic (IGP) plain to BoB remained poorly understood this study is important and timely. Air mass back trajectories suggest conspicuous downwind transport of chemical constituents from the IGP to the BoB during the late northeast monsoon (January–April). Among the water-soluble nitrogen compounds, ammonium was found to dominate 90% of the observed aerosol, followed by 10% of other nitrogen compounds (Srinivas et al. 2016), whereas the abundance of soluble DIP varied between 0.4 to 4.8 nmol m−3. Studies such as this suggest complex source of nutrient enrichment within the estuary and should be further investigated for understanding the regional budget.
The monthly mean spatial differences (between Station A and Station B) in concentrations of DIP, DIN, and DSi (i.e., ΔDIP, ΔDIN, and ΔDSi) during (a) spring tide and (b) neap tide. The error bars show the standard deviation from mean during each month. Blue line: ΔDIN; green line: ΔDSi; maroon line: ΔDIP. (Reproduced from Das et al. 2017 with permission from Elsevier)
Further, Rengrajan and Sarma (2015) studied the submarine groundwater discharge (SGD) and its contribution to nutrient fluxes in the BoB. A model based on the decay of 224Ra relative to 228Ra was used to determine apparent water ages of various bays within the estuary. These ages ranged from 2.6 to 4.8 days during November 2011. Knowing the water age, the distribution of radium in the estuary and the radium isotopic composition of groundwater enabled them to calculate SGD fluxes to the estuary and determine the magnitude and seasonal variability in the nutrient fluxes to the estuary associated with SGD. These nutrient fluxes (in units of mmol m−2d−1) ranged 1–19 (DIN), 0.6–2.6 (DIP), and 5–40 (DSi) in Gautami–Godavari; 19–40 (DIN), 2.6–5.5 (DIP), and 200 (DSi) in Vasishta Godavari; and 120–140 (DIN), 10 (DIP), and 220 (DSi) in Kakinada bay. It was observed that high SGD fluxes to Kakinada bay contribute significant nutrients in the southern peninsula. It is found that the river-borne nutrients (1.74 ± 0.43 Tg yr.−1) support 17% of total primary production (66 Tg yr.−1; Fernandes et al. 2008) in the BoB (upper 10 m).
11.3 Primary Productivity Dynamics from Estuarine and Coastal Waters of Bay of Bengal
Ocean biogeochemical processes modulate the ocean ecosystem through gross primary production (GPP) in the ocean surface where inorganic carbon is fixed by phytoplankton in ocean through photosynthetic processes. Determination of GPP is an important function of phytoplankton biomass and its physiological status which can be measured through various techniques (e.g., from carbon isotopes to satellite based) to determine the phytoplankton primary production. Several units have been used to express primary production in literature. However, the most common are the mmol C m−2 day−1, mg C m−2 day−1, g C m−2 y−1, where the use of moles makes the comparison of the stoichiometric ratios easier between nutrients and carbon and most of the chemical oceanographer prefer this. During the measurement of primary production, often the respiration rates are also determined which when subtracted from GPP yields the net primary production (NPP), which is defined as the amount of photosynthetically fixed carbon available to the first heterotrophic level in the aquatic ecosystem. The global oceanic annual net primary production is about an order of 48.5 Gt (1 Gt = 1015 grams), which contributes nearly 46.2% of total global annual net primary productivity (marine + terrestrial) (Field et al. 1998). The spatial distribution of NPP is not homogeneous and there are regions of low production such as the central gyres, whereas the high production areas include the estuarine and upwelling regions.
Various methods have been used to estimate primary productivity of oceans. Based on the source of nitrogen, used Primary Production (PP) can be categorized into New Production or Regenerated Production. New Production is defined as the primary production that is based on the nitrate as nitrogen source from diffusion/upwelling from below or from the atmosphere via nitrogen fixation or nitrification whereas Regenerated Production is the production based on ammonium and urea as the source of nitrogen. The ratio of nitrate uptake by the sum of uptake by nitrate and ammonium gives the term “f-ratio,” originally coined by Dugdale and Goering (1967). In other words, if we write P = gross production and R = respiration, then we can also approximate f, as f = (P − R/P), which can also be termed as the ration of NPP to GPP. Some of the common methods used are O2 evolution technique, 14C assimilation, 15NO3 assimilation, 15NH4 assimilation, and remote sensing of ocean color. Some of the above techniques can help in measuring the new and regenerated production and have varying time scales from hours to days. Traditionally, BoB is considered to be less productive than the adjacent basin (Arabian Sea), although located in the similar latitudes (Qasim 1977; Sen Gupta et al. 1977; Kumar et al. 2002). The low biological productivity of BOB has been hypothesized due to various reasons, such as narrow shelf, cloud cover during summer monsoon, turbidity resulting from sediment influx, freshwater-induced stratification, and even sunlight (Qasim 1977; Gomes et al. 2000; Kumar et al. 2010). Earlier reports suggest that PP values can range up to 3.0–8.7 g C m2d−1 from the inshore waters of the east coast of India in June–July (Nair et al. 1973). A study by Gomes et al. (2000) suggests that during March–April (spring inter-monsoon), the poleward flow of EICC brings nutrient-laden cooler waters that enrich the inshore waters leading to increase in PP. They observed highest biomass (Chl a, 53 mg m−2) and productivity (4.5 g C m−2d−1) were located in the region of an eddy-like structure along the coast between 13 and 16°N latitude. This study carefully illustrates the linkages between the physical dynamics and primary productivity. During the summer monsoon of 2001, the PP values along the shallow waters of east coast of India ranged 328–520 mg C m−2 d−1, except for a low 40 mg C m−2 d−1 at 15°N (Madhupratap et al. 2003). A vertical profile of primary productions from coastal and open ocean waters in BoB is shown in Fig. 11.4. PP within the mixed layer was about 54% of the total, while below 80 m it was insignificant (8% in both coastal and open waters). About 26% of the PP was associated with the subsurface chlorophyll maxima. The productivity to chlorophyll a ratio was between 10 and 20, although a few higher values (up to 40) occurred at some coastal stations (Madhupratap et al. 2003). Phytoplankton population during summer monsoon of 2001 was dominated by diatoms both in coastal and open ocean waters. Low production in open ocean in BoB in comparison to the coastal waters in BoB is attributed to nitrate deficit due to arising from strong stratification and weaker mixing in comparison to Arabian Sea (Prassana Kumar et al. 2002). During a revisit, Prasanna Kumar et al. (2010) occupied several stations along the western boundary of the BoB during three (summer, fall, and inter) monsoon to understand the influence of light limitation on PP. Along the western boundary of the BOB, the highest PP of 502.01 mg C m−2 d−1 was in the south at 12°N, and the second-highest value of 433.8 mg C m−2 d−1 was in the north at 19°N during summer monsoon (July–August). Their analysis showed that along the western boundary the highest PP in the northern bay was not associated with summer or in the fall inter-monsoon, but occurred in the spring inter-monsoon. This is due to that fact that riverine flux reduced the downward penetration of solar radiation at both times, acting in conjunction with prevailing cloud cover which possibly affected the PP. Recently the role of eddies in influencing the total and size-fractionated PP in BoB has also been illustrated (Sarma et al. 2020). Two cyclonic (CE), one anticyclonic (ACE), and no-eddy (NE) regions were sampled in the BoB during pre-summer monsoon (June 2019). Sarma et al. (2020) illustrated that photic zone integrated total primary production was higher in the CE and NE than ACE regions associated with higher nutrients in the former than latter region. Due to the availability of nutrients within the CE microphytoplankton was more dominant and contributed significant amount to the size-fractionated PP. Here the PP ranged within CE (164 ± 16 mgC m−2 d−1) than ACE (60 ± 26 mgC m−2 d−1). Higher picophytoplankton production was observed at depth below 10 m from surface (10–80%) than nano- and microphytoplankton (1–30%). This study is important as it suggest cyclonic eddies can enhance PP in BoB otherwise known be less productive traditionally. Further role of eddies and its implications on export fluxes in BoB remains poorly understood.
Vertical profiles of primary production (solid circles) and chlorophyll a (open circles) in (a) oceanic and (b) coastal stations (shown by latitudes and longitudes). Mixed-layer depths are shown as shaded areas. (Adapted from Madhupratap et al. (2003) with permission from Elsevier)
A localized study from estuarine waters of Hugli suggests that maximum productivity occurred during winter when cell counts showed highest values as well (136 cells ml−1). On the contrary, minimum productivity with respect to both carbon equivalents as well as total phytoplankton cell counts was recorded in the monsoon months (cells ml−1). Phytoplankton productivity (GPP) at the estuarine station was maximum in December 2010 (227.77 mg C m−3 h−1) when total phytoplankton cell count was maximum as well (203 cells ml−1) and minimum in August 2010 (58.95 mg C m−3 h−1) (Choudhury and Pal 2012). Further, recent satellite-based study suggests a strong link between tropical hilsa shad (Tenualosa ilisha) in the estuarine region of Hugli and Bangladesh. This study describes spatial and temporal variability of productivity in the Bay of Bengal (BoB) relating to hilsa fishery (Hossain et al. 2020). Satellite-based estimates of NPP were found be in an order of >2000 mg C m−2 day−1 within in the Ganges–Brahmaputra–Meghna region covarying with higher fish catch. Their study suggests variations in seasonal productivity linked with nutrients and phytoplankton abundance are important factors for predicting hilsa habitat and their migration patterns in the deltaic regions and shelf waters of BoB. Historical data suggests that most of the primary production is supported by larger phytoplankton called the diatoms although contribution by other classes is also being recognized recently (Madhupratap et al. 2003; Sarma et al. 2020).
11.4 Phytoplankton Community Structure from Estuarine Waters of East Coast of India
The information on phytoplankton community structure from east coast of India is available from 1942 (Chacko 1942), probably the first report that explain the phytoplankton bloom from east coast of India. It is evident that the information on phytoplankton community have been studied and updated on regular intervals by various researchers (Roy 1955; Shetty et al. 1961; Subba Rao 1969; Gopalakrishnan 1971; Santra et al. 1991; Banerjee and Santra 2001; Mukhopadhyay and Pal 2002; Sarkar and Naskar 2002; Choudhury and Pal 2012; Manna et al. 2010; Akhand et al. 2012). The overall results indicate that the phytoplankton community at east coast of India is dominated by diatom group followed by dinoflagellate, blue-green algae, and chlorophyceae. Diatom dominance holds true with different estuarine complex along the east coast of India, such as Hooghly estuary, where diatom contribute more than 50% of the total phytoplankton taxa with 195 species followed by 82 species of green algae, 59 species of cyanophyceae, and 29 species of dinoflagellates (Roshith et al. 2018). Among the dinoflagellate community, further classification based on their mode of nutrition (autotrophic, mixotrophic, heterotrophic), it was noted that that the heterotrophic dinoflagellate were the dominant forms in Hooghly estuary irrespective of the different seasons (Naik et al. 2011). Mahanadi estuarine regime also exhibited the similar pattern of diatom contributing more than 50% of the total phytoplankton taxa identified, followed by cyanobacteria, chrysophyceae, and dinoflagellates (Mishra et al. 2018). While the phytoplankton communities patter from further south estuarine ecosystems (Krishna and Godavari) along the east coast of India, they were not different than the Hugli and Mahanadi estuarine complex, where the diatom contributed more than 80% to the total phytoplankton biomass followed by dinoflagellates, green algae, cyanobacteria, and silicoflagellates (Baliarsingh et al. 2016). Similarly, Bharathi et al. (2018) studied the phytoplankton community dynamics along the northwest and southwest regions of the Bay of Bengal along the east coast and found the diatom being most abundant group of phytoplankton. Studies carried out based on phytoplankton pigments also indicated the dominance of fucoxanthin (marker pigment for diatom) along the coastal Bay of Bengal (Bandyopadhyay et al. 2017). Studies carried out along the Krishna north, Krishna south, Godavari north, and Godavari south estuarine transects indicated the dominance of diatom, with more than 65% contribution to the total phytoplankton biomass followed by other groups (Bharathi et al. 2018).
11.4.1 Phytoplankton Bloom Dynamics Along the East Coast of India
Among the bloom-forming groups of phytoplankton along the east coast of India, blooms caused by diatoms tops the table followed by dinoflagellate and cyanobacteria (D’Silva et al. 2012). Among the diatom, Asterionella japonica (=Asterionella glacialis) was the most common bloom-forming species; Noctiluca scintillans and Trichodesmium erythraeum were the common bloom-forming species belong to dinoflagellate and cyanobacteria group, respectively. D’Silva et al. (2012) in their comprehensive review on algal blooms from Indian waters found that the occurrence of algal bloom along the east coast is throughout the year with the exception of January and November months. While March to May are favorable for diatom dominance, April to August are better for dinoflagellate and cyanobacterial blooms, mostly during month of March.
11.4.2 Causative Factors for Higher Growth of Phytoplankton
Phytoplankton community structure from the east coast of India, specifically from major estuarine habitats, exhibit the more or less similar pattern with dominance of diatom. However, the total biomass and the secondary dominating groups are varying from one region to another. The causative factors explained such as change in salinity due to high influx of freshwater through the rivers, stratification, local upwelling, and also the storms and cyclones. Diatom blooms of A. japonica were related to high nutrient, low temperature conditions due to local upwelling (Rao 1969), whereas recent studies show that the dominance of diatoms along the estuarine complex was related to higher silicate concentrations (Bharathi et al. 2018). Higher abundance of T. erythraeum during the pre-monsoon period was attributed to the prevalence of stratified condition in the Bay of Bengal (Hegde et al. 2008). Among the higher abundance of dinoflagellates, Noctiluca scintillans was associated with nutrient-enriched water during the plankton succession (D’Silva et al. 2012). Physical mechanisms such as eddies and cyclones and their potential role are also highlighted in bloom dynamics of N. scintillans (Naik et al. 2011).
11.5 Summary
The biogeochemical dynamics at nearshore waters of the east coast of India is complex and dynamic, as local circulations and mixing affect the chemistry and deposition of organic matter thereby controlling the estuarine–coastal nutrient budget. The fluvial inputs are major sources of nutrients to the Bay of Bengal (BoB) which also regulates the phytoplankton dynamics in both estuaries and coastal waters. The river-borne nutrients (~1.74 ± 0.43 Tg yr.−1) support 17% of total PP in the BoB (upper 10 m). Although earlier studies have shown that seasonal dynamics in PP are strongly controlled by riverine inputs and stratification due to freshwater fluxes and low light, however recent analysis suggest t that formation of eddies can be an important source of nutrient enhancement in BoB which remains poorly quantified. Therefore future studies should also address the impact of these mesoscale features on regional productivity and carbon cycling from this region along with quantification of size-fractionated PP budget. This will help us to understand carbon cycling across the phytoplankton groups and its importance across the different oceanic environments.
References
Akhand A, Maity S, Mukhopadhyay A et al (2012) Dinoflagellate Ceratium symmetricum pavillard (Gonyaulacales: ceratiaceae): its occurrence in the Hooghly-Matla estuary and offshore of Indian Sundarban and its significance. J Threatened Taxa 26:2693–2698
Baliarsingh SK, Lotliker AA, Trainer VL et al (2016) Environmental dynamics of red Noctiluca scintillans bloom in tropical coastal waters. Mar Pollut Bull 111:277–286.
Bandyopadhyay D, Biswas H, Sarma VV (2017) Impacts of SW monsoon on phytoplankton community structure along the western coastal BOB: an HPLC approach. Estuar Coasts 40:1066–1081
Banerjee A, Santra SC (2001) Phytoplankton of the rivers of Indian Sundarban mangrove estuary. Indian Biologist 33:67–71
Bharathi MD, Sarma VV, Ramaneswari K et al (2018) Influence of river discharge on abundance and composition of phytoplankton in the western coastal Bay of Bengal during peak discharge period. Mar Pollut Bull 133:671–683
Biswas H, Mukhopadhyay SK, De TK et al (2004) Biogenic controls on the air—water carbon dioxide exchange in the Sundarban mangrove environment, northeast coast of Bay of Bengal, India. Limnol Oceanogr 49:95–101
Bristow LA, Callbeck CM, Larsen M et al (2017) N 2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone. Nat Geosci 10:24–29
Chacko PI (1942) An unusual incidence of mortality of marine fauna. Curr Sci 11:404
Choudhury AK, Pal R (2012) Understanding the seasonal dynamics of primary productivity in relation to phytoplankton populations from the Bhagirathi–Hooghly estuary, eastern Indian coast. J. Algal Biomass Utln 3:80–88
CIFRI (2012) Present status of Hilsa in Hugli-Bhagirathi river, Central Inland Fisheries Research Institute, [Available at www.cifri.ernet.in.179.pdf].
D’Silva MS, Anil AC, Naik RK (2012) Algal blooms: a perspective from the coasts of India. Nat Hazards 63:1225–1253
Das S, Chanda A, Giri S et al (2015) Characterizing the influence of tide on the physico-chemical parameters and nutrient variability in the coastal surface water of the northern Bay of Bengal during the winter season. Acta Oceanol Sin 34:102–111
Das S, Giri S, Das I et al (2017) Nutrient dynamics of northern Bay of Bengal (nBoB)—emphasizing the role of tides. Reg Stud Mar Sci 10:116–134
Dugdale R C, Goering. J J (1967) Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol. Oceanogr. 12:196–206
Fernandes V, Ramaiah N, Paul JT et al (2008) Strong variability in bacterioplankton abundance and production in central and western Bay of Bengal. Mar Biol 153:975–85
Field CB, Behrenfeld MJ, Randerson JT et al (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Gomes HR, Goes JI, Saino T (2000) Influence of physical processes and freshwater discharge on the seasonality of phytoplankton regime in the Bay of Bengal. Conti Shelf Res 20:313–330
Gopalakrishnan V (1971) The biology of the Hooghly-Matlah estuarine system (West Bengal, India) with special reference to its fisheries. J Mar Biol Ass India 13:182–194
Gupta RS, De Sousa SN, Joseph T (1977) On nitrogen and phosphorus in the western Bay of Bengal Indian. J Mar Sci 6:107–110
Hegde S, Anil AC, Patil JS et al (2008) Influence of environmental settings on the prevalence of Trichodesmium spp. in the Bay of Bengal. Marine Ecol Progr Ser 18:93–101
Hossain MS, Sarker S, Sharifuzzaman SM et al (2020) Primary productivity connects Hilsa fishery in the Bay of Bengal. Sci Rep 10:1–6
Ittekkot V, Nair RR, Honjo S et al (1991) Enhanced particle fluxes in Bay of Bengal induced by injection of fresh water. Nature 351:385–387
Jayappa KS, Mitra D, Mishra AK (2006) Coastal geomorphological and land-use and land-cover study of Sagar Island, Bay of Bengal (India) using remotely sensed data. Int J Remote Sens 27:3671–3682
Konhauser KO, Powell MA, Fyfe WS et al (1997) Trace element geochemistry of river sediment, Orissa State, India. J Hydrol 193:258–269
Krishna MS, Prasad MH, Rao DB et al (2016) Export of dissolved inorganic nutrients to the northern Indian Ocean from the Indian monsoonal rivers during discharge period. Geochim Cosmochim Acta 172:430–443
Kumar SP, Muraleedharan PM, Prasad TG et al (2002) Why is the Bay of Bengal less productive during summer monsoon compared to the Arabian Sea? Geophys Res Lett 29:88–81
Kumar SP, Narvekar J, Nuncio M et al (2010) Is the biological productivity in the Bay of Bengal light limited? Curr Sci 25:1331–1339
Kumari VR, Rao IM (2010) Suspended sediment dynamics in Krishna estuary, east coast of India. Indian J Mar Sci 39:248–256
Madhupratap M, Gauns M, Ramaiah N et al (2003) Biogeochemistry of the Bay of Bengal: physical, chemical and primary productivity characteristics of the central and western Bay of Bengal during summer monsoon 2001. Deep Sea Res Part II Topi Stud Oceanograp 50:881–896
Manna S, Chaudhuri K, Bhattacharyya S et al (2010) Dynamics of Sundarban estuarine ecosystem: eutrophication induced threat to mangroves. Saline Syst 6:8
Mukhopadhyay A, Pal R (2002) A report on biodiversity of algae from coastal West Bengal (South & North 24-parganas) and their cultural behaviour in relation to mass cultivation programme. Indian Hydrobio 5:97–107
Mukhopadhyay SK, Biswas HD, De TK et al (2006) Fluxes of nutrients from the tropical river Hooghly at the land–ocean boundary of Sundarbans, NE Coast of Bay of Bengal, India. J Marine Syst 62:9–21
Naik RK, Hegde S, Anil AC (2011) Dinoflagellate community structure from the stratified environment of the Bay of Bengal, with special emphasis on harmful algal bloom species. Environ Monit Assess 182:15–30
Nair, P.V.R., Samuel, S., Joseph, K.J., Balachandran, VK (1973) Primary production and potential fishery resources in the seas around India. In: Proceedings of the symposium on Living Resources of the Seas Around India, 1968, Special publication. Central Marine Fisheries Research Institute, Cochin, pp. 184–198. Google Scholar
Qasim SZ (1977) Biological productivity of the Indian Ocean. Indian J Mar Sci 6:U1·137
Radhakrishna I (2001) Saline fresh water interface structure in Mahanadi delta region, Orissa, India. Environ Geol 40:369–380
Ramesh R, Robin RS, Purvaja R (2015) An inventory on the phosphorus flux of major Indian rivers. Curr Sci 10:1294–1299
Rao DS (1969) Asterionella Japonica bloom and discoloration off Waltair, Bay of Bengal. Limnol Oceanogr 14:632–634
Ray R, Rixen T, Baum A, Malik A et al (2015) Distribution, sources and biogeochemistry of organic matter in a mangrove dominated estuarine system (Indian Sundarbans) during the pre-monsoon. Estuar Coast Shelf Sci 167:404–413
Rengarajan R, Sarma VV (2015) Submarine groundwater discharge and nutrient addition to the coastal zone of the Godavari estuary. Mar Chem 20:57–69
Roshith CM, Meena DK, Manna RK et al (2018) Phytoplankton community structure of the Gangetic (Hooghly-Matla) estuary: status and ecological implications in relation to eco-climatic variability. Flora 240:133–143
Roy HK (1955) Plankton ecology of the river Hooghly at Palta, West Bengal. Ecology 36:169–175
Sadhuram Y, Sarma VV, Murthy TR et al (2005) Seasonal variability of physico-chemical characteristics of the Haldia channel of Hooghly estuary, India. J Earth Syst Sci 114:37–49
Santra SC, Pal UC, Choudhury A (1991) Marine phytoplankton of the mangrove delta region of West Bengal. India J Mar Biol Ass India 33:292–307
Sarin MM, Krishnaswami S, Dilli K et al (1989) Major ion chemistry of the Ganga-Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009
Sarkar SK, Frančišković-Bilinski S, Bhattacharya A et al (2004) Levels of elements in the surficial estuarine sediments of the Hugli River, Northeast India and their environmental implications. Environ Int 30:1089–1098
Sarkar NS, Naskar KR (2002) Taxonomy of the diatoms flora of the Sundarban mangals in West Bengal, India. J Interacad 14:81-108
Sarma VV, Gupta SN, Babu PV et al (2009) Influence of river discharge on plankton metabolic rates in the tropical monsoon driven Godavari estuary, India. Estuar Coast Shelf Sci 85:515–524
Sarma VV, Prasad VR, Kumar BS, et al (2010) Intra-annual variability in nutrients in the Godavari estuary, India. Cont Shelf Res 30:2005–14.
Sarma VV, Krishna MS, Prasad VR et al (2014) Distribution and sources of particulate organic matter in the Indian monsoonal estuaries during monsoon. J Geophys Res Biogeosci 119:2095–2111
Sarma VV, Chopra M, Rao DN et al (2020) Role of eddies on controlling total and size-fractionated primary production in the Bay of Bengal. Conti Shelf Res 24:104186
Schott FA, McCreary JP Jr (2001) The monsoon circulation of the Indian Ocean. Prog Oceanogr 51:1–23
Sridevi B (2013) A comprehensive study on physical processes and their impact on biogeochemistry of Godavari estuary, India; PhD thesis, Andhra University, Visakhapatnam
Sridevi B, Sarma VV, Murty TV et al (2015) Variability in stratification and flushing times of the Gautami–Godavari estuary, India. J Earth Syst Sci 124:993–1003
Srinivas B, Rastogi N, Sarin MM et al (2016) Mass absorption efficiency of light absorbing organic aerosols from source region of paddy-residue burning emissions in the Indo-Gangetic plain. Atmos Environ 125:360–370
Sundaray SK, Panda UC, Nayak BB et al (2006) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of the Mahanadi river–estuarine system (India)–a case study. Environ Geochem Health 28:317–330
Vijith V, Sundar D, Shetye SR (2009) Time-dependence of salinity in monsoonal estuaries. Estuar Coast Shelf Sci 85:601–608
Acknowledgement
RR also acknowledges, Chairman ISRO, Director NRSC, and and Deputy Director ECSA, for their support to carry out these investigations under the umbrella of ISRO–GBP–coastal carbon dynamics project. RN would like to acknowledge Director, NCPOR-MoES for his constant encouragement. PMD acknowledges the Dean, School of Earth, Ocean and Atmospheric Sciences; and the Head, Department of Microbiology, for their support. This is NCPOR contribution number B-12/2020-21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Roy, R., Naik, R.K., D’Costa, P.M., Nagamani, P.V., Choudhury, S.B. (2021). Nutrient Cycling and Seasonal Dynamics of Primary Production in Nearshore Waters of East Coast of India. In: Das, S., Ghosh, T. (eds) Estuarine Biogeochemical Dynamics of the East Coast of India. Springer, Cham. https://doi.org/10.1007/978-3-030-68980-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-68980-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68979-7
Online ISBN: 978-3-030-68980-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)