Abstract
Polar shallow marginal seas are of high importance as they are the most productive regions of the Arctic Ocean and serve as filters for terrestrial runoff. Salinity and turbidity gradients create diverse habitats for planktonic organisms in coastal areas. In the present study we aimed at assessing the degree to which environmental gradients influence the abundance and community structure of the zooplankton in a shallow Arctic sea affected by terrestrial runoff. Zooplankton distribution was studied in a coastal zone in the southeastern part of the Barents Sea (Pechora Sea) in July 2014 and September 2016 along the archipelago that stretches from the continent towards the open sea. The ecosystem was in a spring state in July (2014) and in a summer state in September (2016). A clear positive gradient of salinity and a negative cline of turbidity were revealed, directed from the coast towards the open sea. A horizontal salinity gradient was detected in both seasons. The turbidity gradient was most pronounced during summer. Distribution of several species of marine zooplankton (e.g. Pseudocalanus spp., Temora longicornis, Microsetella norvegica) was associated with the salinity gradient. Parameters of community structure (species richness, diversity, evenness, total zooplankton abundance) correlated with turbidity while only diversity and evenness were influenced by salinity. A gradient was observed from a more diverse and less abundant zooplankton community in areas with high turbidity and low salinity towards a less diverse and more abundant community in the open sea. This heterogeneity influences higher trophic levels including commercial fishes and reflects how marginal filters function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zones where fresh and marine waters mix are important barriers as suspended and dissolved matter, including pollutants, moves from land to sea. A specific term “marginal filters” has been introduced to define such areas (Lisitzin 1994, 1999). In these intermixing zones, geochemical transformations and biological processes effectively remove most suspended and dissolved matter from riverine waters. This process includes several successive stages as freshwater flows into the sea. It begins with gravitational sedimentation followed by coagulation and sorption, and later by bacterial and algal assimilation of a major part of the dissolved matter, including biogenic elements. Further planktonic and benthic filter feeders consume microalgae as well as organic and inorganic detritus, and excrete undigested material as fecal pellets, which settle on the bottom. Particulate and dissolved matter removal from terrestrial runoff reaches 95% and 20–40% efficiency, respectively (Lisitzin 1999). Thus zooplankton plays a key role in biosedimentation processes occurring in estuaries and other mixing zones.
Knowledge on quantitative and qualitative changes in zooplankton communities under the influence of freshwater and suspended matter brought to the sea by rivers is important for understanding how marginal filters function. The abundance and production of phytoplankton are significantly reduced in areas with high turbidity despite nutrient sufficiency (Cloern 1987; Northcote et al. 2005). This is due not only to mechanical influence (e.g. by decrease of light penetration into water column), but also to nutrients’ absorption into suspended particles (Shi et al. 2017). Environmental heterogeneity, characteristic for estuaries, influences planktonic organisms, horizontal and vertical distributions of which are determined mainly by environmental gradients (Laprise and Dodson 1994; Plourde et al. 2002). Although zooplankton variability in areas where marine and fresh waters mix has drawn the attention of many researchers (Laprise and Dodson 1994; Prudkovsky 2003; Díaz-Gil et al. 2014; Helenius et al. 2017), its distribution in mixing zones in the open sea, away from narrow estuaries is poorly known (Espinasse et al. 2014).
Besides serving as filters for (and partly owing to) terrestrial runoff, the continental shelf is the most productive zone of the oceans. Zooplankton comprises the basic trophic levels of these ecosystems, so knowledge of its abundance, species composition, quantitative and qualitative distribution and functioning is crucial for understanding processes in the whole system (Raymont 1983). Mesozooplankton and copepods may remove up to 50% of primary production in the coastal zone in the Southern Ocean (Atkinson et al. 2012) and in the White Sea (Berger 2007). Zooplankton is food source of commercially important fish such as herring, Arctic cod and capelin (Podrazhanskaya 1995; Karamushko et al. 1996). Planktivorous fishes account for about 25% of total ichthyofauna in the Barents Sea (Karamushko 2013).
We studied zooplankton abundance and distribution in the shallow coastal area of the Barents Sea—called the Pechora Sea—during two expeditions in 2014 (July) and 2016 (September). The study area is located in a mixing zone strongly influenced by terrestrial runoff.
Russian and American Arctic seas, where many great rivers discharge, are examples of the mixing zones described above. The southernmost parts of these seas, in immediate proximity to river mouths, form marginal filters of great scale. The Pechora Sea, in the southeastern part of the Barents Sea, is one of such systems. This shallow region (Fig. 1) with depths less than 50 m on most of its area experiences substantial continental runoff. In fact, it may serve as a model of the northern marginal seas of Eurasia and America, such as the Kara Sea, the Laptev Sea, the East Siberian Sea, the Chukchi Sea, and the Beaufort Sea, which receive massive river discharge. Besides the runoff itself, the complicated system of local currents significantly influence the hydrological conditions in the Pechora Sea (Byshev et al. 2003; Danilov et al. 2004). Therefore understanding zooplankton interactions with environmental gradients in this area gives insight into the biogeochemical processes in the coastal zone of the Arctic Ocean.
The main currents influencing coastal waters are directed mostly from West to East (Byshev et al. 2003; Danilov et al. 2004) (Fig. 2). The Pechora River and the White Sea waters occupy nearshore areas, while the Kaninskoye and Kolguevo-Pechorskoye currents flow farther from the shore, bringing relatively warm and saline water of Atlantic origin into the Pechora Sea (Byshev et al. 2003). The latter is transformed here through the mixing with the Pechora and White Sea waters. The main source of fresh water in the region is the Pechora River, discharge of which is 130–160 km3 a year, or about 90% of all freshwater inflow into the Pechora Sea (Matishov et al. 1996; Nikiforov and Dunayev 2003). Thus the area of the Pechora Sea affected by the discharge of the Pechora River is very large because of the shallowness of this sea; the influence of Pechora waters was traced all the way up to Vaygach Island (Byshev et al. 2003). The large freshwater inflow supports the existence of strong vertical and horizontal gradients of abiotic parameters, such as salinity, temperature, suspended and dissolved organic matter, etc. (Matishov et al. 1996). According to rather scarce publications, a wide (100–150 km) mixing zone exists in this area between local coastal waters and those flowing out of the White Sea (Danilov et al. 2004). During the warm period of the year, this zone is characterized by a thermo- and halocline at 10–20 m depth, this depth increases further from the continent towards the open sea. The temperature changes from 4 to 9 °C above the pycnocline to below 0 °C under it; salinity varies from 26–30 to 32–34, respectively. The area of influence of Pechora River waters declines during the summer period, shifting the zone of mixing with White Sea waters towards the coast and forming complicated spatial patterns of mixing (Musaeva and Suntsov 2001). Seasonal changes in environment may influence zooplankton community, and thus need special investigations.
Zooplanktonic communities in the Pechora Sea are formed mainly by boreal, boreal-Arctic and Arctic species (Zelikman 1966; Musaeva and Suntsov 2001). The average biomass varies from 9 to 180 mg wet weight m−3 in summer (August–September) to 400–500 mg wet weight m−3 in spring (July) and autumn (October) (Timofeev and Shirokolobova 1996; Troshkov and Gnetneva 2000; Musaeva and Suntsov 2003). Copepods dominate zooplankton throughout the entire ice-free period, although Hydrozoa and larvae of benthic animals (meroplankton) are observed in high densities during July–August (Troshkov and Gnetneva 2000; Musaeva and Suntsov 2003).
The Pechora Sea is situated outside of the main commercial fishing areas in the western part of the Barents Sea, which made it relatively understudied until recent decades. However, after finding oil and gas fields in this area in 1989, interest to local fauna and flora increased significantly because of the possible and actual anthropogenic impact on local ecosystems (Plotitsyna and Kilezhenko 1993; Zelenkov and Miskevitch 2000; Novoselov and Studenov 2008). Moreover, an archipelago of small islands in the Pechora Sea and the adjacent sea area, where we worked, belong to the Nenetsky State Nature Reserve and are considered an Important Bird Area (Nikolaeva et al. 2006). They provide habitats for the Pechora Sea population of Atlantic walrus (Born et al. 1995; Boltunov et al. 2010; Semenova et al. in revision), numerous pinnipeds and small whales (Kondakov 1996) and serve as nesting and feeding grounds for many waterfowl as well as stopovers for water birds migrating along the East-Atlantic flyway (Anufriev 2006; Krasnov et al. 2006; Nikolaeva et al. 2006). Increased anthropogenic pressure along with the necessity to protect important populations of marine birds and mammals in the Pechora Sea has led to a rise in scientific interest in this region (e.g., Druzhkov et al. 1997; Dahle et al. 1998; van der Graaf et al. 2004; Denisenko et al. 2007). The main object of our study, zooplankton, is a key link in the trophic chain of the oceanic ecosystems (Raymont 1983). It is a food source for local planktivorous fishes, e.g. herring (Clupea harengus pallasi n.suworowi) and Arctic cod (Boreogadus saida) (Karamushko et al. 1996), which are important food objects for higher trophic levels, especially marine mammals (Kondakov 1996) and birds (Lønne and Gabrielsen 1992; Borkin 2012). Understanding the mechanisms affecting spatial and temporal distribution and zooplankton community structure parameters is necessary in order to predict possible changes in the Pechora Sea pelagic ecosystems.
Our study aimed at assessing the effect of abiotic factors on zooplankton distribution and its temporal (seasonal) variation in the continent–sea boundary area in the SE part of the Pechora Sea. We hypothesize that hydrological gradients and fields forming in this mixing zone affect zooplankton community structure and quantitative distribution. We also tested whether local or Pechora River runoff exerted more influence on the zooplankton communities in the study area.
Materials and methods
Sampling
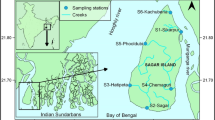
We conducted zooplankton sampling and hydrological measurements during two cruises, 13–17 July 2014 and 1–7 September 2016, within 15-m isobaths (except two stations—St. 5 and 1–30) around Dolgy Island and adjacent islands in the southeastern part of the Pechora Sea (Fig. 1). The number of stations in 2014 and 2016 differed; only those stations where zooplankton was taken were used in the analysis (Table 1). However all stations are presented in the table to show the spatial distribution of abiotic parameters. The distance between the southernmost and northernmost stations was 97 km. Depth varied from 9 (St. 16) to 31 m (St. 1–30). Data on two upper water layers (above thermocline and the layer of thermocline, which was present at those stations) was used in the analysis at St. 5 and St. 1–30, because they were deeper than others (19 and 31 m respectively) (see Table 1).
Hydrological parameters
Vertical profiles of temperature (Temp, °C) and salinity (S, PSU) were obtained using a MIDAS CTD + oceanographic probe (Valeport Ltd., UK). Profiles of concentrations of chlorophyll a (Chl, µg L−1), coloured dissolved organic matter (CDOM, RFU—Relative Fluorescence Units) and turbidity (FTU, Formazin Turbidity Units) were measured with a Cyclops-6 multi-sensor platform (Turner Designs, USA). The average values of these parameters in the upper water layer, corresponding to the zooplankton sampling layer at shallow stations (depth (Z) ≤ 13 m), were calculated. At deep stations (Z > 13 m), zooplankton was collected according to pycnocline position (see below). Tidal height (Tide_H, m) was recorded at the time of sampling at each station. Negative and positive values were assigned to tidal height if sampling occurred during ebb and flood, respectively.
Zooplankton
A Juday net (37 cm mouth diameter and 100 µm mesh size) was used for zooplankton sampling. Vertical hauls were made from the bottom to the surface, or above and below pycnocline: at St. 2 (0–5, 5–13 m) and St. 5 (0–5, 5–10 and 10–19 m) in 2014; at St. 1–30 (0–6, 6–11 and 10–29 m) in 2016. At other stations the total water column was sampled (Table 1). The samples were preserved by formaldehyde addition to the final concentration of 4%. All Copepoda, except rare Harpacticoida, were identified both at the species level and by developmental stage. Other holoplankton organisms were also identified at the species level where possible. Meroplankton organisms—larvae of benthic and nektonic animals—were identified to the highest taxonomic level. Each sample was concentrated to 100 or 200 mL, depending on the abundance of organisms. Abundant species (> 10 ind. per subsample) were counted in 1-mL subsamples, while rare ones were counted in the total sample in Bogorov chamber. We counted three subsamples per station in order to account for possible splitting errors. Zooplankton abundance (ind. m−3) in 0–10 m layer or total sampled depth, if the latter was less than 15 m, was used in the analyses. Zoogeographical attribution (Arctic, boreal-Arctic, cosmopolitan) and ecological traits (cold- and warm-water, marine and brackish-water) were assigned to all species (Musaeva and Suntsov 2001, 2003) (Table 3).
Data analysis
Environmental data
The analysis of water mass structure aimed to accomplish three main tasks: (1) to reveal seasonal changes, (2) to search correlation between hydrological parameters, (3) to reveal spatial structure of water masses. Hydrological parameters (Temp, S, Chl, CDOM, Turbidity, Tide_H) were combined in one matrix, centered to a zero mean and standardized to unit variance, which was analyzed for redundancy (analysis of redundancy, RDA; Legendre and Legendre 2012). Cruises were planned to capture typical spring (late spring) and summer conditions. The season of sampling was used as a constraining factor “Season”, with two gradations: “spring” (July 2014) and “summer”(September 2016). The seasons were determined according to previous studies (Zelikman 1961; Fomin 1985; Matishov et al. 1996; Troshkov and Gnetneva 2000). One constrained axis (RDA1) and five unconstrained axes (PC1–PC5) were extracted. Unconstrained axes characterize variation of water mass parameters after exclusion of the factor “Season”. PC1 and PC2 were the most informative unconstrained axes and thus were chosen for further analysis.
The influence of two main sources of the fresh water was studied: The Pechora River and Khaypudyr Bay. We hypothesized that the influence of the Pechora River, the main source of fresh water and sediments in the Pechora Sea, would lead to differences of hydrological parameters between stations located on different sides of the archipelago. Two regression models of the same type were constructed to examine the influence of geographical position of a station on the variation of unconstrained axes values. PC1 and PC2 were used as the dependent variables. In both models, distance from the southernmost station, St. 13 (reflecting distance from Khaypudyr Bay) and a discrete variable “Group” were used as predictors. The variable “Group” characterized the position of each station relative to the flow from the Pechora River: whether the station is open to it or is shielded by the islands. “East” group included stations 14–19, while others were attributed to the “West” group. The baseline for the variable “Group” was defined as “East”. Therefore, a negative value for this variable means a negative change compared to the values observed to the east of the archipelago (stations 14–19), and the positive sign means positive change.
Zooplankton
Before analysis, we divided zooplankton into two constituents: larvae of benthic animals (meroplankton) and true planktonic organisms (holoplankton), which were studied separately. The effect of environmental parameters on the distribution of zooplankton communities was assessed in the following two ways: (1) using multidimensional data—hydrological parameters and abundance of each taxon, and (2) only for holoplankton, using “one-dimensional” variables, which characterize holoplankton community structure—total holoplankton abundance (N), number of taxa (Sp), Shannon–Wiener diversity index (H) and evenness index (E). The Shannon–Wiener index was calculated based on holoplankton abundance as follows:
where Ni is abundance of ith species (ind. m−3) and N is abundance of all species (ind. m−3). The evenness (E) was calculated as ratio of the actual Shannon–Wiener index to the highest possible value based on the number of species (Pielou 1966):
where H—Shannon–Wiener index; Hmax—highest possible Shannon–Wiener index value for taxa number Sp.
For the multidimensional analysis we reduced taxa number, leaving out those with low abundance. To find the threshold abundance we calculated average abundance values for each of the 35 taxa using data of both cruises (in 2014 and 2016), and built a diagram depicting frequency distribution of abundance values (not shown). Those taxa which formed a peak in the area of small values (ln(N + 1) < 2) were excluded from analysis. After this reduction 16 taxa were used in the analysis. To diminish dispersion between more abundant and less abundant taxa all abundance values were [ln(N + 1)]-transformed.
Redundancy analysis (RDA) was used for constrained ordination (Legendre and Legendre 2012). As with the hydrological parameters, a matrix of standardized (scaled to zero mean and unit variance) values were used in analysis, so that the latter was based on the matrix of correlations. The analysis was conducted in two steps. For the first step, we estimated ordination in constrained axes, based on the model, including all studied predictors, some of which are redundant. At the second step, we selected an optimal model of ordination, for which an algorithm of forward stepwise selection was used to select only meaningful predictors (Borcard et al. 2011). This procedure allowed us to only include those predictors which had a significant effect on ordination of stations and taxa in the model. Significance evaluation of the multidimensional model as a whole, and significance of individual ordination axes was accomplished by permutation method (Legendre and Legendre 2012) with 9999 permutations. In all analyses, the season of sampling was included as a discrete predictor.
In the “one-dimensional” analysis, all taxa of holoplankton were used. Multiple regression analysis was applied to study the connection of holoplankton community structure (H, E, N, Sp) to environmental parameters. Evaluation of multicollinearity of predictors (not presented) has shown that CDOM, Temp and Season demonstrated high values of the variance inflation factor. This violation of regression analysis applicability forced us to include in the models only one of the three correlated factors—Season. The baseline value for this factor was defined as spring; the sign before respective coefficient denotes a change relative to the values in spring: “ + ” means increase, “−”—decrease in summer. Thus, following predictors were used in the models: Season, S, Turbidity, Chl, Tide_H. Control of conditions of regression analysis applicability was accomplished by visual evaluation of residual diagrams.
Because the studied abiotic and biological parameters had spatial gradients, we analyzed spatial variograms for the control of existence of spatial autocorrelations (Zuur et al. 2009). In cases of significant spatial autocorrelations, we introduced a Gaussian correlation function, which models spatial relationships.
One- and multidimensional analyses were conducted with the use of statistical functions in the R software package (R Core Team 2017). For multidimensional analysis, functions of vegan package were used (Oksanen et al. 2018): for the model, which includes all studied predictors, function rda() was used; for optimal model selection function ordisep() was used. “One-dimensional” analysis was accomplished with the use of functions of nlme package (Pinheiro et al. 2017), for model construction we used function gls().
Comparisons of different abiotic parameters, zooplankton abundance and community structure variables in different seasons were made with nonparametric Mann–Whitney U statistics. Analysis was conducted in statistical software Statistica (StatSoft Inc.).
In all analyses, statistical significance was evaluated at 0.05 significance level (p < 0.05).
Results
Spatial and temporal variation of hydrological parameters
Average values of temperature, salinity, chlorophyll a concentration and CDOM in the studied area differed significantly in spring (July 2014) and summer (September 2016) (Table 1, Mann–Whitney U test). In July 2014 temperature in the 0–10 m water layer varied between 1.2 and 6.1 °C and was much lower than in September 2016, when it was between 11.4 and 12.2 °C at all stations. Salinity showed a similar pattern with more brackish water at stations 8–16 in spring, and much less variation in summer. On the contrary, CDOM was about 2.5 times higher in spring than in summer, while the difference between seasons in chlorophyll a concentration was less, albeit significant (Mann–Whitney U test, p = 0.04). Overall, Chla varied in the studied area from 0.8 to 2.2 μg L−1. Turbidity was the only parameter that did not differ significantly in spring and summer, partly because the variation between stations was high (Table 1).
Results of the multidimensional analysis confirmed the existence of seasonal differences. The only constrained axis, RDA1 (permutational F = 12.6, p < 0.0001), associated with the season of sampling, described 41% of total variability, while the unconstrained five axes together described 59%. All stations were divided into two groups, distributed along RDA1 axis according to the season (Fig. 3a). Water temperature (Temp) and salinity (S) demonstrated the highest negative and CDOM the highest positive loadings on the RDA1 axis, which indicates that these three parameters demonstrated the strongest seasonal changes. The association of chlorophyll concentration with RDA1 (i.e. seasonal variation) was somewhat weaker (Table 1).
Ordination of stations (small dark circles and triangles) and water parameters (large grey circles) in the plane of: a constrained RDA1 and unconstrained PC1 and b unconstrained PC1 and PC2 axes. Daggers mark the centroids’ position. Temp temperature, S salinity, Chl chlorophyll a concentration, CDOM coloured dissolved organic matter, Tide_H tidal height
Ordination of stations and water parameters in unconstrained axes PC1 and PC2 (Fig. 3b) demonstrated variation excluding the influence of RDA1, i.e. without the influence of factor “Season”. These two unconstrained axes explained 45% of total variability: 25% (PC1) and 20% (PC2), meaning that two more or less equal horizontal gradients existed in the study area. CDOM and temperature were not connected explicitly to PC1 or PC2, which demonstrates that the variability of these factors was almost exclusively explained by seasonal changes.
We cannot unambiguously interpret PC1 and PC2 as gradients of some definite factors. However, PC1 can be linked to the gradient of Turbidity and Chl as they had maximal negative loadings on this axis. Nevertheless nonzero loadings of Tide_H and S on PC1 did not allow separating input of these parameters from that of Turbidity and Chl. Similarly, maximal positive loadings on PC2 belonged to S and Tide_H. Nonzero negative loadings of Turbidity and Chl allow us to consider PC2 as the gradient between high values of S and Tide_H on the one side and high values of Turbidity and Chl on the other.
Analysis of spatial structure of the two gradients showed a significant latitudinal component (Distance) in the variability of PC2 values (Table 2). PC2 values increased with the distance from Khaypudyr Bay. At the same time no significant connections of both PC1 and PC2 with variable Group (West vs. East side of archipelago) were found, which means, that there was no influence of station position relative to flow from the Pechora River on the measured hydrological parameters at the stations.
Distribution of environmental parameters, which had the highest loadings on the unconstrained axes PC1 and PC2, is shown on Fig. 3b. No gradients of temperature from the continental coast to the sea, along the chain of islands were observed in spring. Salinity increased in that direction as shown by the linear regression model (Table 2). In summer no gradients of Temp or S were detected. A gradient of Turbidity was observed in both seasons, being stronger in summer. A Chlorophyll a gradient was also detected in both seasons, but it was in opposite directions in spring and summer (Fig. 4).
Spatial and temporal variation of zooplankton communities
During two cruises, in July 2014 and September 2016, we identified a total of 43 taxa (Table 3). Species richness in the two sampling periods differed significantly: 42 taxa were found in spring and only 23—in summer. Only one species found in summer, the copepod Oithona similis, was absent in spring. Dominance structure also differed substantially. More than 50% of zooplankton abundance in spring was represented by Bivalvia larvae (46.5%) and Pseudocalanus spp. (26.3%). Oithona similis absolutely dominated zooplankton in summer (78.4%); the second most abundant species, Pseudocalanus spp., accounted for only 5.3%. The other taxa accounted for no more than 5% each. Planktonic Hydrozoa demonstrated the most dramatic difference of taxonomic composition with eight species of this class in spring and only two in summer. Subclass Copepoda was represented by 14 and 9 taxa in spring and summer, respectively (15 taxa in total). Copepods made up 33.9% of total zooplankton abundance in spring and 90% in summer. Meroplankton composition did not differ substantially between the two seasons: larvae of 6 taxa of benthic animals were observed in spring and larvae of 5 taxa were observed in summer, when Nemertea larvae were absent.
Average total zooplankton abundance was (mean ± SE) 20871 ± 2091 ind. m−3, n = 12 in spring and 24030 ± 4686 ind. m−3, n = 9 in summer and did not differ significantly (Mann–Whitney U statistic = 45.0, p = 0.52). However, the ratio of ecological groups in community changed substantially (Table 3). The percentage of cold-water organisms contributing to the overall abundance was much higher in spring: 33.5% versus 5.1% in summer. However, the contribution of warm-water species also decreased in summer: it became 8% compared to 17.1% in spring. Such a dramatic change in these two group’s relative abundance was due to the mass appearance of the eurythermal ubiquitous Oithona similis, which made up 78.4% of community abundance in summer. Marine species constituted the absolute majority of zooplankton organisms in both seasons. Brackish-water species made up no more than 1% of total abundance.
Meroplankton in spring represented 49% of total zooplankton abundance numbering 10,300 ind. m−3, while in summer it represented only 3.6% (870 ind. m−3). The difference was due to higher abundance of Bivalvia larvae and, to a lesser extent, higher abundance of Polychaeta larvae in spring. Bivalvia larvae accounted for 97% of the meroplankton in spring (10,000 ind. m−3).
Analysis of holoplankton association with all studied predictors (RDA, Fig. 5a) showed that many environmental parameters were highly correlated with the first constrained axis RDA1. This axis, as in the case of analysis of hydrologic variables, was determined, first of all, by differences between seasons. The only exception was the height of tidal wave (Tide_H), which did not demonstrate an association with RDA1. As a result of stepwise optimal model selection, the final model of constrained ordination consisted of only three predictors: Season, Salinity and Turbidity (Fig. 5a). This model was statistically significant (Table 4) and explained 75.4% of total variability in terms of total Inertia. All three constrained axes were significant. Ordination of stations in axes RDA2 and RDA3, without the influence of predictor Season, did not show segregation of stations taken in spring and summer (Fig. 5b). RDA2 was associated with salinity (S), and RDA3 demonstrated association with turbidity.
Ordination of taxa in the space of constrained axes (Fig. 6) allowed isolating groups of organisms preferring different environmental conditions. First of all, two distinct groups were isolated by the position of species along axis RDA1 corresponding to their abundance in different seasons (Fig. 6a, Table 3). The most abundant species in July 2014 were: Acartia bifilosa, A. longiremis, Acartia juveniles, Cyclopina sp., Ectinosoma neglectum, Tintinnopsis tubulosa. In September 2016 Centropages hamatus, Evadne nordmanni, Oithona similis and Podon leuckarti were the most numerous.
Ordination of taxa in the plane of constrained axes: a in RDA1 and RDA2; b in RDA2 and RDA3 relative to season, turbidity and salinity. Codes for species: 1. Acartia bifilosa; 2. A. longiremis; 3. Acanthostomella norvegica; 4. Acartia juveniles; 5. Centropages hamatus; 6. Cyclopina sp.; 7. Ectinosoma neglectum; 8. Evadne nordmanni; 9. Fritillaria borealis; 10. Microsetella norvegica; 11. Oithona similis; 12. Podon leuckarti; 13. Pseudocalanus spp.; 14. Rotifera; 15. Temora longicornis; 16. Tintinnopsis tubulosa
The next diagram (Fig. 6b) depicts the relationships of species with environmental parameters, excluding the influence of seasonal variation. Acanthostomella norvegica and Rotifera preferred less saline water (high negative values of RDA2), while Pseudocalanus spp., Microsetella norvegica and Temora longicornis were found in water with higher salinity (high positive values of RDA2). More transparent waters (negative loadings on RDA3) were preferred by Fritillaria borealis; on the contrary, Acartia bifilosa was linked to more turbid habitats (positive loadings on RDA3). Ubiquitous Oithona similis was positioned closest to the center of both axes (Fig. 6b).
Spatial distribution of the species, most closely connected to major environmental parameters (having maximal loadings on RDA2 and RDA3), is presented on Fig. 7. Rotifera was more abundant at the southernmost part of archipelago, closer to the source of fresh water, but they were not numerous in the immediate proximity to Khaypudyr Bay (Fig. 7a). Pseudocalanus spp. preferred the northern part of archipelago with more saline waters. Fritillaria borealis was found in spring only at five northernmost stations (St. 1, 2, 5, 6, 20): notably, at St 1 it was two orders of magnitude more abundant than at other stations. This station differed substantially from other stations in that season by chlorophyll a concentration (Table 1; Fig. 3). In summer F. borealis was most numerous at central stations southwest of the archipelago (Fig. 7b). According to the multidimensional analysis, Acartia bifilosa preferred more turbid waters, which were more pronounced in summer.
Holoplankton community structure was similar in the two seasons in terms of species diversity (Shannon–Wiener index) and evenness (Table 5). However total holoplankton abundance and especially species number differed significantly from season to season.
Linear models for structure variables (H, E, N, Sp) match well with the results of the multidimensional analysis, indicating that variables from the optimal model RDA (Fig. 3) are the most important in this model as well (Table 6). In all cases, significant links of turbidity were found with structural variables: positive with H and E and negative with N and Sp. Diversity (H) and evenness (E) declined with rising salinity (Table 6). Evenness was higher and taxa number was lower in summer compared to spring according to linear model.
Thus, spatial (horizontal) heterogeneity in this shallow nearshore region was expressed in a clear gradient from less salty, more turbid and phytoplankton rich waters near the coast to more transparent and salty waters with less phytoplankton in the open sea. Turbidity and chlorophyll a gradients were much stronger in the summer than in the spring. Only the salinity gradient was similarly pronounced during the whole warm period. True marine species was the only ecological group of the zooplankton which demonstrated a clear relationship with the described horizontal gradient of salinity. It means that salinity was one of the main factors determining the distribution of zooplankton in this zone, which is subject to the continental runoff influence. Community structure was determined mostly by the turbidity—salinity axis. The gradient is observed from more diverse community with evenly represented species in areas with relatively high turbidity and low salinity towards a less diverse community at offshore stations. In places with high turbidity zooplankton also tended to be less abundant.
Discussion
Hydrological gradients
Environmental gradients in coastal areas may have different spatial organization. These are either estuaries, where gradients are mostly two-dimensional (Laprise and Dodson 1994; Prudkovsky 2003; Díaz-Gil et al. 2014; Helenius et al. 2017), or open sea near sources of fresh water, where gradients are usually three-dimensional (Espinasse et al. 2014; Bojanić Varezić et al. 2015). In the latter case winds and currents may break the linearity of changes of hydrological parameters (Espinasse et al. 2014). Distribution of environmental parameters near Dolgy Island and adjacent islands resembles this latter type, and patterns of constant currents and gradients are complicated by periodic (tidal) water movements (Byshev et al. 2001). Nevertheless, we detected pronounced horizontal gradients of some environmental parameters.
We worked in the southeastern part of the Pechora Sea, which is subject to the influence of the most intensive freshwater inflow in the Barents Sea. Almost 90% of fresh water, received by the Pechora Sea, is brought by the Pechora River (Nikiforov and Dunayev 2003). Although the mouth of it is located about 140 km westward of Dolgy and other islands of the archipelago, almost all water discharged from Pechora River flows towards the east—north-east (Fig. 2).We expected an influence of this water on the hydrological characteristics and therefore plankton community around the islands, which were believed to serve as barrier on the way of the flow of Pechora River water. However no significant differences of hydrological parameters were found between the West and East sides of archipelago. The other source of fresh water, Khaypudyr Bay, is located in the immediate proximity to the archipelago (Fig. 1). Khaypudyr Bay receives water from numerous small rivers and streams, and this water heads to the sea through the relatively narrow mouth of the bay. The highest turbidity and the lowest salinity were observed at the southernmost station St. 13, the closest to Khaypudyr Bay. Gradients of increasing salinity and decreasing turbidity were detected, oriented from the continent to the open sea along the chain of islands, suggesting that the flow from Khaypudyr Bay and small streams nearby dominate over the Pechora River’s influence in the studied area. Indeed, detailed analysis of thermohaline structure of the Pechora Sea water masses revealed the mainstream of the flow from Pechora River to the north of Dolgy Island (Byshev et al. 2003).
Turbidity increased towards the south in both seasons, demonstrating a more pronounced gradient in September 2016. Maximum river discharge in the Pechora Sea is normally observed in June and decreases by September (Nikiforov and Dunayev 2003). Therefore, by the end of summer suspended matter is likely transported closer to the shore than in the spring, and a turbidity gradient can be observed at a relatively short distance from the source. A tight connection between chlorophyll a and turbidity distribution may be explained by the fact that phytoplankton is part of the suspended matter. Intensive phytoplankton blooms often follow peaks of river discharge, which is an important source of suspended matter (Guadayol et al. 2009; Bojanić Varezić et al. 2015). Besides that, river discharge causes intensive mixing at coastal shallows, which, in turn, increases turbidity and returns nutrients into photic zone (Guadayol et al. 2009; Espinasse et al. 2014).
Tidal and constant currents are at their highest intensity in the coastal zone of the Pechora Sea and influence horizontal distribution of hydrological parameters (Timofeev and Shirokolobova 1996; Byshev et al. 2001; Musaeva and Suntsov 2001). Coastal waters in the Pechora Sea are formed by the White Sea current and Pechora River discharge, the latter spreading in the surface layer for a distance of 100-150 km from the shore during the warm part of year (Danilov et al. 2004). The strongest vertical gradients of temperature and salinity were detected in the 0–10 m water layer, however, by the end of summer, because of wind mixing and warming of the deeper layers the distribution becomes more even throughout the water column (Danilov et al. 2004). We also observed almost total vertical homogeneity of temperature and salinity in summer 2016. More off-shore waters and circulation pattern in the Pechora Sea is formed under the influence of the waters of Atlantic origin—Kaninskoye and Kolguyevo-Pechorskoe currents (Byshev et al. 2001). Some authors (Musaeva and Suntsov 2001) believe that the mixing zone of the coastal and Atlantic waters begins much closer to the shore, less than 20 km from the coast, implying that Dolgy Island and the other islands of the archipelago lie entirely within this zone. In this mixing zone clear gradients are eroded because of complicated pattern of currents (Musaeva and Suntsov 2001), which supports our observations.
The connection of salinity to the tide height (Tide_H) may be interpreted as partially functional: more saline waters from the open sea flow towards the coast during flood, while less saline waters flow from the coast during ebb tide.
Thus, water mass variability in the study area has two sources. The first one is connected with saline water inflow from the open sea and fresh (and turbid) water outflow from Khaypudyr Bay and nearby rivers which influence the salinity and turbidity. The second source, according to high loadings of Tide_H variable on the PC2 axis, includes tidal currents directed along the chain of islands, which redistribute marine and fresh waters.
By the end of summer, salinity in the coastal zone of the Pechora Sea increases due to intensive wind-mixing and decrease of freshwater runoff (Danilov et al. 2004). This is what we observed in September 2016 compared to July 2014. The difference in absolute temperatures between the two cruises was observed as well in accordance with the seasonality.
Spatio-temporal variation of zooplankton
The documented number of zooplankton species in the Pechora Sea is variable, although the lists of taxa in all studies largely overlap. We found 43 zooplanktonic taxa. This number is close to the minimum observed in this region since 1950s—33 taxa in 1992 (Timofeev and Shirokolobova, 1996). However, our expeditions covered only a relatively small part of the region studied previously. During the first large-scale investigations in the Pechora Sea in 1958–1959, 72 taxa were recorded (Zelikman 1961, 1966). Notably, in those studies no representatives of meroplankton were presented, except for Cirripedia larvae. In the expeditions of 1992, 1998 and 2001 33, 57 and 66 zooplankton taxa were documented, respectively (Timofeev and Shirokolobova 1996; Musaeva and Suntsov 2001; Dvoretsky and Dvoretsky 2009a). The reason for such fluctuations may be in shifts of timing of hydrological and biological seasons, common in high latitudes (Usov et al. 2013)—in different years studies may be conducted in the same time, however the timing of phenological events is not the same from year to year. Number of stations also may influence the completeness of taxonomic list, because of different geographic coverage of cruises.
Distribution of species in hydrological gradients is shaped by their ecological preferences (Fig. 6). Microsetella norvegica and Temora longicornis are typical marine species (Krause et al. 1995; Razouls et al. 2015), and they preferred saline waters. The abundance of boreal-Arctic marine Pseudocalanus spp. tended to rise towards the northern (marine) end of the archipelago both in spring and summer. Some increase in the abundance of cold-water species towards the north was observed earlier in this region (Musaeva and Suntsov 2001), which was explained by increasing depth and average salinity. Such increase of large cold-water copepods’ biomass towards the open sea is typical for the Arctic estuarine systems. A similar pattern was also documented along the estuary of Yenisei River from its mouth to the Kara Sea in summer (Kosobokova and Hirche 2016). Appendicularian Fritillaria borealis, like all representatives of this taxonomic group, is a marine organism (van der Land 2001). Therefore, it tends to be more abundant in low turbidity habitats which are also more saline. Synchaeta tamara, the most numerous Rotifera species in the studied area, is indicated as a brackish water species in some publications (Musaeva and Suntsov 2003), but as true marine in others (Segers 2007). This species is characteristic for ice fauna in the Barents Sea and tolerates a wide range of salinities, from 13 to 32 (Friedrich and De Smet 2000). Unexpectedly, Acanthostomella norvegica being a typically marine species (Davis 1985; Scott and Marchant 2005) demonstrated a pronounced negative link to salinity (Fig. 6b). On the other hand, this species showed rather high correlation with the axis RDA1, connected to the season of sampling. Indeed, A. norvegica was absent in summer (Table 3). Besides that, this species in spring was distributed very unevenly, being presented only at stations 8-18, around the whole archipelago except its North-Western end, and absent at the “most marine” stations. Tidal phase could be a factor influencing distribution of A. norvegica: at all stations, where this species appeared, except one, we worked during the ebb tide. Absence of A. norvegica in September could be explained by its preference for cold waters – it is distributed mostly in high latitudes (Berge et al. 2012; Encyclopedia of Life 2018). We found that turbidity is one of the most important factors for Acartia bifilosa. In the nearby White Sea A. bifilosa inhabits mostly estuaries (Prudkovsky 2003), which explains its preference of less saline and more turbid waters. Some authors consider this species brakishwater-marine and also document its presence in estuaries (Collins and Williams 1982; Villate 1991).
All parameters of community structure in the study area were associated with turbidity. In places with high turbidity, zooplankton community contained fewer species, was less abundant, but was more diverse and even in terms of species’ abundance (dominance was less pronounced). These results indicate that high turbidity may influence zooplankton in following ways: (1) in turbid waters, mineral particles depress zooplankton development interfering with food particles (Kirk 1991; Jönsson et al. 2011), thus decreasing abundance of animals. (2) In such extreme conditions, not all species typical for this region can survive, so we see a decrease in species number. (3) The zone of high turbidity forms an ecotone—a transitional zone from freshwater to marine habitats. High diversity and lack of dominance are characteristic for such zones (Odum 1971; Naumov 1991).
One of the goals of the present study was to reveal specific seasonal states of the zooplankton community. Low temperature and salinity near the water surface as well as the shallow thermo- and haloclines found in July 2014 are typical for spring in this region (Matishov et al. 1996; Danilov et al. 2004). In addition, in July 2014 the expedition took place soon after ice melt, which normally is observed in the region by the end of June (Danilov et al. 2004). Moreover, floating ice around Dolgy Island was seen in the first half of July during our expedition. According to previous work, spring zooplankton development starts in the Pechora Sea in June–July (Fomin 1985), which confirms that in July 2014 the zooplankton community was in the spring state. Higher temperature and salinity, and deeper thermo- and haloclines in September compared to July indicated a summer state of the environment (Matishov et al. 1996). Not only did species diversity change from season to season: the ratio of main ecological groups also changed substantially. Cold-water species dominated in July among holoplanktonic species (Pseudocalanus spp., 26.3%), which is typical for spring. With that, almost a half of the whole community in July consisted of the larvae of bottom animals, primarily bivalve mollusks (46.5%). This situation was also observed in July 1997, when Bivalvia larvae made up 20–30% of total zooplankton biomass (Troshkov and Gnetneva 2000). Analysis of species composition of the Bivalvia larvae group has shown that almost all belonged to boreal-Arctic Macoma calcarea and Chlamys islandica (Flyachinskaya LP, pers. communication). In September 2016 almost 80% of the zooplankton consisted of the ubiquitous Oithona similis. Abundance of this species was proved to attain seasonal maxima in Arctic and sub-Arctic coastal areas during the warm period of the year. The seasonal abundance peak of O. similis in the Arctic Kola Bay was observed in late summer, in September (Dvoretsky and Dvoretsky 2009b); in the White Sea this species also dominates during summer, from July to September (Prygunkova 1974). O. similis was found in the Pechora Sea in the middle of July 2001 at more than 75% of stations and was positively correlated with salinity and depth (Dvoretsky and Dvoretsky 2009a). Hence, the fact that this species was totally absent in spring 2014 leaves a question about existence of year-to-year environmental fluctuations open.
Thus, during the ice-free (warm) season in the coastal zone of the Pechora Sea two communities succeed each other. One develops in the spring and consists of cold-water Arctic and boreal-Arctic species and meroplankton. The other one replaces it in the summer and is dominated by warm-water boreal and ubiquitous (Oithona similis) species. Comparing our results to previous investigations in this region, we can conclude, that hydrological conditions and community state are typical for these times of year.
The zooplankton is one of the most important components of the marginal filters (Lisitzin 1994, 1999). All of the mesozooplankton organisms, feeding on the suspended particles (live or dead), add to the clearance of water. The role of the zooplankton is not as important during the first stages of marginal filter functioning, such as gravitational sedimentation and coagulation sorption, as it is later. Water purification is accomplished at this stage mostly due to physical and chemical processes. In the study area these stages probably correspond to the zone with maximal turbidity. Notably, in this very zone the negative impact of the environment on zooplankton is the greatest, judging by its lowest abundance. In places, where the mechanical influence of environment is not as strong (unlike the zone of high turbidity mentioned above) abundance and accordingly the role of zooplankton increase. These places appear to correspond to the third, and final, stage of a marginal filter—biological filtration (Lisitzin 1994, 1999). At this stage the rest of allochthonous suspended matter is precipitated. These waters contained the least suspended material in our case. Thus, the analysis of turbidity and zooplankton distributions may contribute to understanding how marginal filters function.
Conclusion
Gradients of environmental factors, primarily turbidity and salinity, shape the zooplankton communities in the zones where fresh and marine waters mix. Both abiotic gradients and community structure change from year to year and with changing climate. These changes may also affect higher trophic levels: fishes, marine mammals and birds, numerous in such areas. High latitudes experience intensive climatic changes (Wassmann et al. 2011), the consequences of which are glacier melting and increased precipitation (IPCC 2014). As most models demonstrate, this trend will persist into the next decades (IPCC 2014). These processes lead to an increase in terrestrial runoff and thus an expansion of high turbidity/low salinity areas near the mouths of Arctic rivers is expected. Storminess and coastal erosion also increase, reinforcing these results in high Arctic coastal regions (Węsławski et al. 2011). The ranges of marine zooplankton will therefore also change, affecting distribution and abundance of planktivorous fishes. For example, our investigation has shown that marine species such as Temora longicornis and Pseudocalanus spp. prefer more saline and clear waters. These organisms are among the most important food sources of marine fishes such as herring, Arctic cod and capelin (Huse and Toresen 1996; Karamushko et al. 1996). Thus, if tendencies of climate change persist, we may see a corresponding, significant shift in these fishes’ ranges. The effectiveness of marginal filters may also influence the turbidity gradient. This effectiveness, itself may be affected by the interannual fluctuations and climate change-induced shifts of the system; hence the efficiency of terrestrial runoff purification may change. This latter effect is of great importance to the whole Arctic Ocean, taking into account increasing human impact on the basins of great rivers flowing into the Arctic Ocean. Thus, further, more detailed studies of the whole ecosystem in this boundary zone are of great importance. Extension of the study area along the gradient from the freshwater sources to the open sea also would contribute to our understanding of the abiotic and biological processes shaping this gradient.
References
Anufriev VV (2006) Bird fauna of the islands of the Pechora Sea. Bulletin of the Pomor University. Ser Nat Exact Sci 1:70–79 (in Russian)
Atkinson A, Ward P, Hunt BPV, Pakhomov EA, Hosie GW (2012) An overview of Southern Ocean zooplankton data: abundance, biomass, feeding and functional relationships. CCAMLR Sci 19:171–218
Berge J, Batnes AS, Johnsen G, Blackwell SM, Moline MA (2012) Bioluminescence in the high Arctic during the polar night. Mar Biol 159:231–237. https://doi.org/10.1007/s00227-011-1798-0
Berger VJa (2007) Production potential of the White Sea. Explorations of fauna of the seas 60. Zoological Institute RAS, Saint-Petersburg (in Russian)
Bojanić Varezić D, Vidjak O, Kraus R, Precali R (2015) Regulating mechanisms of calanoid copepods variability in the northern Adriatic Sea: testing the roles of west-east salinity and phytoplankton gradients. Estuar Coast Shelf Sci 164:288–300. https://doi.org/10.1016/j.ecss.2015.07.026
Boltunov AN, Belikov SE, Gorbunov JuA, Menis DT, Semenova VS (2010) The Atlantic walrus of the southeastern Barents Sea and adjacent regions: review of the present-day status. WWF-Russia, Moscow (in Russian)
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Borkin IV (2012) On the importance of the Arctic cod in the feeding of the most abundant marine birds of the Barents Sea. Bulletin of the Baltic Federal University. Ser Nat Med Sci 1:107–117 (in Russian)
Born EW, Gjertz I, Reeves RR (1995) Population assessment of Atlantic walrus (Odobenus rosmarus rosmarus L.). Meddelelser 138, Oslo
Byshev VI, Galerkin LI, Grotov AS (2001) Hydrological conditions in the southeastern part of the Barents Sea in August 1998. In: Romankevitch EA, Lisitzin AP (eds) An experience of system oceanological investigations in Arctic. Nauka, Moscow, pp 85–92 (in Russian)
Byshev VI, Galerkin LI, Galerkina NL, Shcherbinin AD (2003) Dynamics and structure of waters. In: Romankevitch EA, Lisitzin AP, Vinogradov ME (eds) The Pechora Sea. System investigations. More, Moscow, pp 93–116 (in Russian)
Cloern JE (1987) Turbidity as a control on phytoplankton biomass and productivity in estuaries. Cont Shelf Res 7:1367–1381. https://doi.org/10.1016/0278-4343(87)90042-2
Collins NR, Williams R (1982) Zooplankton communities in the Bristol Channel and Severn Estuary. Mar Ecol Prog Ser 9:1–11
Dahle S, Denisenko SG, Denisenko NV, Cochrane SJ (1998) Benthic fauna in the Pechora Sea. Sarsia 83:183–210. https://doi.org/10.1080/00364827.1998.10413681
Danilov AI, Mironov EU, Spichkin VA (eds) (2004) Variability of natural conditions in the shelf zone of the Barents and Kara Seas. Arctic and Antarctic Research Institute, Saint-Petersburg (in Russian)
Davis CC (1985) Acanthostomella norvegica (Daday) in Insular Newfoundland Waters, Canada (Protozoa: Tintinnina). Int Revue Ges Hydrobiol Hydrogr 70:21–26. https://doi.org/10.1002/iroh.19850700103
Denisenko NV, Denisenko SG, Lehtonen KK, Andersin A-B, Sandler HR (2007) Zoobenthos of the Cheshskaya Bay (southeastern Barents Sea): spatial distribution and community structure in relation to environmental factors. Polar Biol 30:735–746. https://doi.org/10.1007/s00300-006-0232-4
Diaz-Gil C, Werner M, Lövgren O, Kaljuste O, Grzy A, Margonski P, Casini M (2014) Spatio-temporal composition and dynamics of zooplankton in the Kalmar Sound (Western Baltic Sea) in 2009–2010. Boreal Environ Res 19:323–336
Druzhkov N, Gronlund L, Kuznetsov L (1997) The zooplankton of the Pechora Sea, the Pechora Bay and the Cheshskaya Bay. Pechora Sea ecological studies in 1992–1995. Finnish-Russian offshore technology working group. Rep B 13:69–91
Dvoretsky VG, Dvoretsky AG (2009a) Summer mesozooplankton structure in the Pechora Sea (south-eastern Barents Sea). Estuar Coast Shelf Sci 84:11–20. https://doi.org/10.1016/j.ecss.2009.05.020
Dvoretsky VG, Dvoretsky AG (2009b) Life cycle of Oithona similis (Copepoda: Cyclopoida) in Kola Bay (Barents Sea). Mar Biol 156:1433–1446. https://doi.org/10.1007/s00227-009-1183-4
Encyclopedia of life. http://www.eol.org. Accessed 13 July 2018
Espinasse B, Carlotti F, Zhou M, Devenon JL (2014) Defining zooplankton habitats in the Gulf of Lion (NW Mediterranean Sea) using size structure and environmental conditions. Mar Ecol Prog Ser 506:31–46. https://doi.org/10.3354/meps10803
Fomin OK (1985) Taxonomic composition of zooplankton. Seasonal cycle of zooplankton. Vertical distribution of zooplankton. In: Matishov GG (ed) Life and environment in the pelagic zone of the Barents Sea. Kola department of the USSR Academy of Sciences, Apatity, pp 128–149 (in Russian)
Friedrich C, De Smet WH (2000) The rotifer fauna of Arctic sea ice from the Barents Sea, Laptev Sea and Greenland Sea. Hydrobiologia 432:73–89. https://doi.org/10.1023/A:1004069903507
Guadayol Ò, Marrasé C, Peters F, Berdalet E, Roldán C, Sabata A (2009) Responses of coastal osmotrophic planktonic communities to simulated events of turbulence and nutrient load throughout a year. J Plankton Res 31:583–600. https://doi.org/10.1093/plankt/fbp019
Helenius LK, Leskinen E, Lehtonen H, Nurminen L (2017) Spatial patterns of littoral zooplankton assemblages along a salinity gradient in a brackish sea: a functional diversity perspective. Estuar Coast Shelf Sci 198:400–412. https://doi.org/10.1016/j.ecss.2016.08.031
Huse G, Toresen R (1996) A comparative study of the feeding habits of herring (Clupea harengus, Clupeidae, L.) and capelin (Mallotus villosus, Osmeridae, Müller) in the Barents Sea. Sarsia 81:143–153
IPCC (2014) Climate Change 2014: synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Core Writing Team, Pachauri RK, Meyer LA (eds). IPCC, Geneva, Switzerland
Jönsson M, Ranåker L, Nicolle A et al (2011) Glacial clay affects foraging performance in a Patagonian fish and cladoceran. Hydrobiologia 663:101–108. https://doi.org/10.1007/s10750-010-0557-4
Karamushko OV (2013) Diversity and structure of ichthyofauna in the northern seas of Russia. Transactions of Kola Science Center. Series 1. Oceanology 1:127–135 (in Russian)
Karamushko LI, Chernitsky AG, Karamushko OV (1996) Ichthyofauna. In: Matishov GG (ed) Ecosystems, bio-resources and anthropogenic pollution of the Pechora Sea. Apatity, pp 72–79. (in Russian)
Kirk KL (1991) Inorganic particles alter competition in grazing plankton: the role of selective feeding. Ecology 72:915–923. https://doi.org/10.2307/1940593
Kondakov AA (1996) Marine mammals. In: Matishov GG (ed) Ecosystems, bio-resources and anthropogenic pollution of the Pechora Sea. Apatity, pp 89–98. (in Russian)
Kosobokova KN, Hirche H-J (2016) A seasonal comparison of zooplankton communities in the Kara Sea—with special emphasis on overwintering traits. Estuar Coast Shelf Sci 175:146–156. https://doi.org/10.1016/j.ecss.2016.03.030
Krasnov Yu, Gavrilo M, Nikolaeva N, Goryaev Yu, Strøm H (2006) East-Atlantic flyway populations of seaducks in the Barents Sea region. In: Boere GC, Galbraith CA, Stroud DA (eds) Waterbirds around the world. The Stationery Office, Edinburgh, pp 512–513
Krause M, Dippner JW, Beil J (1995) A review of hydrographic controls on the distribution of zooplankton biomass and species in the North Sea with particular reference to a survey in January–March 1987. Prog Oceanogr 35:81–125. https://doi.org/10.1016/0079-6611(95)00006-3
Laprise R, Dodson JJ (1994) Environmental variability as a factor controlling spatial patterns in distribution and species diversity of zooplankton in the St. Lawrence Estuary. Mar Ecol Prog Ser 107:67–81
Legendre P, Legendre LF (2012) Numerical ecology, vol 24. Elsevier, Amsterdam
Lisitzin AP (1994) Marginal filter of oceans. Oceanology 34:735–747
Lisitzin AP (1999) The continental-ocean boundary as a marginal filter in the world oceans. In: Gray JS, Ambrose W Jr, Szaniawska A (eds) Biogeochemical cycling and sediment ecology. Springer, Netherlands
Lønne OJ, Gabrielsen GW (1992) Summer diet of seabirds feeding in sea-ice-covered waters near Svalbard. Polar Biol 12:685–692. https://doi.org/10.1007/BF00238868
Matishov GG, Ilyin GV, Matishov DG (1996) Common regularities of the oceanological regime and oceanological conditions of the ice-free period. In: Matishov GG (ed) Ecosystems, bio-resources and anthropogenic pollution of the Pechora Sea. Apatity, pp 25–40. (in Russian)
Musaeva EI, Suntsov AV (2001) On the distribution of the Pechora Sea zooplankton (on the materials collected in August 1998). Oceanology 41:508–516
Musaeva EI, Suntsov AV (2003) Zooplankton. In: Romankevitch EA, Lisitzin AP, Vinogradov ME (eds) The Pechora Sea. System investigations. More Publ, Moscow, pp 207–216 (in Russian)
Naumov AD (1991) Towards the problem of the study of the macrobenthos biocenoses of the White Sea. Proc. of the Zoological Institute of the Academy of Sciences USSR 233:127–147 (in Russian)
Nikiforov SL, Dunayev NN (2003) River discharge. In: Romankevitch EA, Lisitzin AP, Vinogradov ME (eds) The Pechora Sea. System investigations. More Publ, Moscow, pp 36–38 (in Russian)
Nikolaeva NG, Spiridonov VA, Krasnov YV (2006) Existing and proposed marine protected areas and their relevance for seabird conservation: a case study in the Barents Sea region. In: Boere GC, Galbraith CA, Stroud DA (eds) Waterbirds around the world. The Stationery Office, Edinburgh, pp 743–749
Northcote TG, Pick FR, Fillion DB, Salter SP (2005) Interaction of nutrients and turbidity in the control of phytoplankton in a large Western Canadian lake prior to major watershed impoundments. Lake Reserv Manag 21:261–276. https://doi.org/10.1080/07438140509354434
Novoselov AP, Studenov II (2008) Monitoring of water organisms and assessment of anthropogenic impact on the water ecosystems of the Pechora region. Ecological condition of the Pechora Sea region. EcoPechora 2008: International scientific-practical conference (Narian-Mar, 12–15 May 2008). Narian-Mar, pp 7–8. (in Russian)
Odum EP (1971) Fundamentals of ecology. W.B. Saunders Company, Philadelphia-London-Toronto
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2018). Vegan: community ecology package. R package version 2.4-6. https://CRAN.R-project.org/package=vegan
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2017). nlme: Linear and nonlinear mixed effects models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme
Plotitsyna NF, Kilezhenko VP (1993) Ecological conditions of the of construction area of the oil-transfer terminal in the Pechora Sea. Accounting session on the results of PINRO work in 1992. PINRO publishing, Murmansk, pp 279–287 (in Russian)
Plourde S, Dodson JJ, Runge JA, Therriault J-C (2002) Spatial and temporal variations in copepod community structure in the lower St. Lawrence Estuary, Canada. Mar Ecol Prog Ser 230:211–224. https://doi.org/10.3354/meps230211
Podrazhanskaya SG (1995) Feeding and feeding relationships of the White Sea fishes. The White Sea. Biological resources and issues of their rational exploitation. Part 2. Zoological Institute RAS, Saint Petersburg, pp 103–114 (in Russian)
Prudkovsky AA (2003) Life-cycle of Acartia bifilosa (Copepoda, Calanoida) in the White Sea (Chernorechenskaya Inlet, Kandalaksha Bay). Trans White Sea Biol Station Moscow State Univ 9:164–168 (in Russian)
Prygunkova RV (1974) Some peculiarities of seasonal development of zooplankton in Chupa inlet of the White Sea. Explorations of the Fauna of the Seas 13. Zoological Institute AN SSSR, Leningrad, pp 4–55 (in Russian)
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raymont JEG (1983) Plankton and productivity in the oceans. vol 2, Zooplankton. Pergamon Press, Oxford
Razouls C, de Bovée F, Kouwenberg J, Desreumaux N (2015) Diversity and geographic distribution of marine planktonic copepods. Sorbonne Université, CNRS. http://copepodes.obs-banyuls.fr/en
Scott FJ, Marchant HJ (eds) (2005) Antarctic marine protists. Australian Biological Resources Study, Canberra
Segers H (2007) Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564
Semenova V, Boltunov A, Nikiforov V (in preparation) Key habitat areas of walruses of the Pechora Sea in the ice-free period studied by satellite telemetry. Polar Biol (in revision)
Shi Z, Xu J, Huang X, Zhang X, Jiang Z, Ye F, Liang X (2017) Relationship between nutrients and plankton biomass in the turbidity maximum zone of the Pearl River Estuary. J Environ Sci (China) 57:72–84. https://doi.org/10.1016/j.jes.2016.11.013
Timofeev SF, Shirokolobova OV (1996) Zooplankton and its importance in the system of ecological monitoring. In: Matishov GG (ed) Ecosystems, bio-resources and anthropogenic pollution of the Pechora Sea. Apatity, pp 54–60. (in Russian)
Troshkov VA, Gnetneva LV (2000) Zooplankton of the southeastern part of the Barents Sea. Biological resources of the coastal zone of Russian Arctic. Moscow, pp 143–150. (in Russian)
Usov N, Kutcheva I, Primakov I, Martynova D (2013) Every species is good in its season: do the shifts in the annual temperature dynamics affect the phenology of the zooplankton species in the White Sea? Hydrobiologia 7061:11–33. https://doi.org/10.1007/s10750-012-1435-z
van der Graaf AJ, Lavrinenko OV, Elsakov V, van Eerden MR, Stahl J (2004) Habitat use of barnacle geese at a sub-Arctic salt marsh in the Kolokolkova Bay, Russia. Polar Biol 27:651–660. https://doi.org/10.1007/s00300-004-0623-3
van der Land J (2001) Appendicularia. In: Costello MJ (ed) European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, vol 50. Muséum national d’Histoire naturelle, Paris, pp 512–513
Village F (1991) Zooplankton assemblages in the shallow tidal estuary of Mundaka (Bay of Biscay). Cah Biol Mar 32:105–119
Wassmann P, Duarte CM, Agusti S, Sejr MK (2011) Footprints of climate change in the Arctic marine ecosystem. Glob Change Biol 17:1235–1249. https://doi.org/10.1111/j.1365-2486.2010.02311.x
Węsławski J, Kendall M, Włodarska-Kowalczuk M, Iken K, Kędra M, Legezynska J, Sejr MK (2011) Climate change effects on Arctic fjord and coastal macrobenthic diversity—observations and predictions. Mar Biodivers 41:71–85. https://doi.org/10.1007/s12526-010-0073-9
Zelenkov VM, Miskevitch IV (2000) Assessment of possible impact of oil production on marine Arctic ecosystems on the example of Prirazlomnoe oilfield in the Pechora Sea. In: Protection of water bio-resources under conditions of intensive oil and gas development on the shelf and internal water bodies of Russian Federation. International seminar proceedings. Economy and Informatics, Moscow, pp 48–59 (in Russian)
Zelikman EA (1961) Planktonic characteristic of the southeastern part of the Barents Sea (according to the data of August 1958). Hydrological and biological peculiarities of the coastal waters of the Murman. Murmansk, pp 39–58. (in Russian)
Zelikman EA (1966) Notes on the composition and distribution of the zooplankton in the southeastern part of the Barents Sea in August-October 1959. Composition and distribution of the zooplankton and benthos in the southern part of the Barents Sea. Trans MMBI 11:34–49 (in Russian)
Zuur A, Ieno E, Walker N, Saveliev A, Smith G (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We are grateful to the crew of the RV “Professor Vladimir Kuznetsov” for transportation and technical assistance during sampling. This research was supported by the ongoing Program of the Russian Academy of Sciences “Functioning and dynamics of sub-Arctic and Arctic marine ecosystems” (No AAAA-A17-117021300220-3) and by the Programs of Russian Academy of Sciences “Fundamental Research to the Development of Arctic” and “Bio-resources”. An expedition in 2014 has been partly financed by Gazpromneft-Sakhalin (contract GPNS-98). Special thanks to Alexander Myers for thorough language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article belongs to the special issue on the “Ecology of the Pechora Sea”, coordinated by Alexey A. Sukhotin.
Rights and permissions
About this article
Cite this article
Usov, N., Khaitov, V., Smirnov, V. et al. Spatial and temporal variation of hydrological characteristics and zooplankton community composition influenced by freshwater runoff in the shallow Pechora Sea. Polar Biol 42, 1647–1665 (2019). https://doi.org/10.1007/s00300-018-2407-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2407-1