Abstract
The role of morpho-anatomical adaptations of six Kyllinga brevifolia populations in successfully invading hyper-saline environments was investigated. Physiological and anatomical characteristics showed a high degree of plasticity indicating its adaptability potential to a variety of environmental conditions. The population from hyper-saline saltmarsh Sahianwala was exposed to physiological drought for a long time and its survival relied on the prevention of water loss attained by decreased stomatal density and area, lignin deposition in the inner and outer cortical region, especially outside vascular tissue. Larger cells of cortical storage parenchyma aided in water storage and wide metaxylem vessels in better conduction of solutes. Higher accumulation of shoot Ca2+ in this habitat protected neutralized the impact of the enhanced shoot and root Na+ ion uptake. Organic osmoprotectants like total free amino acid, proline, soluble proteins, and sugars accumulated in a higher quantity that contributed towards an osmotic adjustment in Sahianwala population. Population from seasonal inundation (Treemu Headworks) showed larger root aerenchyma to supply sufficient oxygen for respiration, broader xylem vessels for better water and nutrient conduction, and greater density of leaf stomata for better transpiration. Maximum shoot and root length, total leaf area, and water potential were observed in the least saline Chinyot population indicating its best growth potential in a slightly saline aquatic environment. Each population showed specific physiological and anatomical modifications to colonize their respective habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Environmental heterogeneity is the distinctive feature of landscape significantly contributing to an increase in richness of biodiversity (Field et al., 2009). The major proportion of the Punjab region comes under the arid and semi-arid zone, however, seasonal inundations during monsoon season and extensive canal systems resulted in the formation of many water bodies like saline waterlogged areas, salt marshes and freshwater ponds (Hanif et al., 2010). These water-bodies support only specific floral composition, which has some specific modifications to colonize aquatic wetlands (Dítě et al., 2019).
Colonizing of plant species to extremely stressed habitats are supported by the development of certain structural adaptations like the development of aerenchyma in root, stem and leaves for the supply of oxygen required for respiration (Takahashi et al., 2014). Gas exchange ability also increases by high stomatal density and size of stomatal complex (Muhlenbock et al., 2007). Bulliform cells development results in leaves rolling for maintenance of moisture content under physiological drought conditions because of high salinities (Alvarez et al., 2008). Ion secretion from glands is a strategy to reduce the salt contents in halophytes of the family Cyperaceae (Grigore & Toma, 2007). Water loss during transpiration is handled by thick and well-developed epidermis (Carignato et al., 2019).
In general, all growth phases of plants are affected by soil type that enables plants to modify morpho-anatomically and physiologically under variable environmental conditions. Most members of family Cyperaceae face severe stresses as they grow in aquatic habitats and salt marshes (Bernhardt & Kropf, 2006). Kyllinga brevifolia is distributed all over the temperate and tropical regions. Like many other species of family Cyperaceae, it is generally inhabitants of saline wetlands (Mishra et al., 2015). This species flourishes mostly in aquatic and cold habitats of the world. It colonizes in the form of the mat and is spread by stolons. This species have a high invasive potential, and invades in rice fields and saline water-bodies (Les, 2020).
No work has previously been reported on Kyllinga regarding the degree of salt tolerance and adaptive components to cope with stressful environments like saline waterlogged areas. It was hypothesized that there must have been flexibility in structural and functional features of this species that enables it to adopt saline aquatic environments. This study was conducted to explore those anatomical and physiological modifications in natural populations of Kyllinga brevifolia, and to investigate the mechanism of adaptation that plays a critical role in this species to successfully colonize in aquatic habitats.
2 Materials and Methods
2.1 Collection Sites
Six populations of Kyllinga brevifolia Rottb. along with soil samples were collected from different aquatic habitats in the Punjab Province varying in their level of soil salinity (Table 1). The collection sites were (from high to low salinity gradient) as: Sahianwala (hypersaline saltmarsh), Treemu Headworks (seasonal inundations), Baloki Headworks (seasonal water body), Changa Manga (artificial forest plantation), Khanki Headworks (waterlogged area), and Chinyot (riparian vegetation). Pictorial view of the collection sites along with coordinates are presented in Fig. 1. Seasonal weather and soil physicochemical characteristics of the collection sites of Kyllinga brevifolia are outlined in Table 1.
2.2 Soil Physicochemical Characteristics
Soil samples were collected from all collection sites of Kyllinga brevifolia to analyze the soil physicochemical attributes. Soil samples were taken at the depth of 15 to 25 cm, at the distance of 50 cm away from the plant roots at all four directions (north, south, east and west) and then average was used for data analysis. To calculate the saturation percentage, ECe and pH, a soil saturation paste was prepared by taking 200 g of dry soil. Soil ECe and pH of soil extract was computed by ECe/pH meter (WTW series Ino LAB pH/Cond 720, USA). Soil sodium (Na+), potassium (K+) and calcium (Ca2+) ions were determined by using a flame photometer (PFP-7, Jenway, UK). Soil Cl− was analyzed by using chloride meter (Model-926, Sherwood Scientific Limited Cambridge, UK).
2.3 Collection of Plant Samples
Five average-sized plants were collected from each population during August 2017. Plants were carefully uprooted using soil auger (15 cm dia.), washed and weighed immediately on a portable digital balance. The samples were then put in plastic zipper bags (24 × 12 cm), sealed carefully and kept in an icebox. The plants were then brought to research laboratory for detailed morpho-anatomical and physiological analysis.
2.4 Morphological Data
Plant height and root length were measured with a scale. The number of leaves and bracts per plant were counted and total leaf area per plant was calculated by the following formula:
The plant samples were then oven-dried for root and shoot dry weight at 60 °C until the constant weight achieved.
2.5 Gas Exchange Parameters
CO2 assimilation rate (A), transpiration rate (E), stomatal conductance (gs), sub-stomatal CO2 concentration (Ci), and water use efficiency (WUE) were measured immediately after uprooting plants from their natural habitats by using LCA-4 ADC portable IRGA (Analytical Development Company, Hoddesdon, England). The third leaf from the top of the plant was selected for data recording. Data for these parameters were recorded from 9:00 a.m. to 11:00 a.m. (chamber temp. 28.4 to 32.4 °C, the molar flow of air per unit leaf area 403.3 mmol m−2 s−1, ambient CO2 conc. 352 μmol mol−1, ambient temp. 22.4 to 27.9 °C, atmospheric pressure 99.9 kPa, the water vapor pressure of chamber 6.0 to 8.9 mbar, PAR 1711 μmol m−2 s−1).
2.6 Water Potential
Portable Scholar-type pressure chamber (1505D, PMS Instrument Company, Albany, USA) was used to determine water potential. Frozen samples (− 20 °C) for one week were used to analyze the solute potential of shoots by vapor pressure osmometer (Wescor 5500, USA). An indirect method was used to calculate turgor potential by deducting the value of solute potential from water potential.
2.7 Plant Ionic Contents
Plant material (both shoot and root) from selected samples (0.1 g) were oven dried and then subjected to grinding. The plant material was digested with concentrated H2SO4 according to Wolf (1982) technique to determine quantity of Na+, K+, and Ca2+ by using a flame photometer (Jenway, PFP-7, UK).
2.8 Osmoprotectants
Moor and Stein (1948) method was used for the quantification of total amino acids. Proline content of samples was determined by following the method of Bates et al. (1973). Soluble proteins were determined by the method of Lowry et al. (1951). Anthrone method was used for the determination of total soluble sugars (Yemm & Willis, 1954).
2.9 Anatomical Parameters
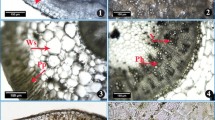
Average-sized stem, the thickest root, largest leaves and bracts selected were rinsed with water and then kept in formalin acetic alcohol solution. Permanent anatomical slides were prepared by the freehand sectioning technique. Thin transverse sections were treated with a series of ethanol percentages for dehydration. Safranin and fast green stains were used to distinguish between lignified and other tissues according to the method designed by Ruzin (1999). Stomata were studied by the epidermis peel method. The epidermis was removed carefully by the double-edge razor blade and placed on glass side, dehydrated by a series of ethanol grades and stained with safranin for improvement of contrast. Photographs of transverse sections were taken by a camera-equipped compound microscope (Nikon 104, Japan). Anatomical data were recorded by ocular micrometer calibrated with a stage micrometer (Fig. 2).
2.10 Statistical Analysis
The data were subjected to analysis of variance (one-way ANOVA) using the completely randomized design by Microsoft Excel (v. 2010) and multivariate redundancy analysis (RDA) done by XLSTAT (v. 2014) statistical software. Means were compared by the least significant difference (LSD) test at a 5% level of significance.
3 Results
3.1 Soil Physicochemical Attributes
Soil samples collected from highly saline habitat Sahianwala surpassed all the habitats regarding soil ECe, saturation percentage, Na+, K+, and Cl− content. The maximum soil pH was recorded at Chinyot while the highest soil Ca2+ at Treemu Headworks. Changa Manga soil possessed the least soil K+ and Ca2+. Soil collected from the Chinyot possessed the minimum ECe, Na+, and Cl− content (Table 2).
3.2 Morphological Characteristics
All the morphological characteristics varied significantly in K. brevifolia populations collected from different habitats of Punjab (Table 2). The Chinyot population surpassed all other habitats regarding plant height (19.2 cm) and root length (11.3 cm). The number of leaves, total leaf and bract area was the maximum in population collected from Chinyot while the minimum was recorded from the population of Sahianwala saltmarsh. Root fresh and dry weights were the maximum in the Chinyot population. The maximum shoot fresh and dry weights were recorded in the Khanki population. Plant height and root length were the minimum in the population collected from Changa Manga. Root fresh weight was the maximum in the Baloki population. Population collected from Sahianwala exhibited the lowest shoot fresh weight, and, shoot and root dry weight.
3.3 Gas Exchange Parameters
Net CO2 assimilation rate was the highest in the Baloki population, while the minimum in the Chinyot population (Table 2). Population collected from Treemu exhibited the highest sub-stomatal CO2 concentration, while least was observed in the Sahianwala population. Transpiration rate and stomata conductance varied non-significantly among the K. brevifolia populations. The Baloki population possessed the maximum water use efficiency and the minimum was observed in the Chinyot population.
3.4 Water Relation Parameters
Population collected from Chinyot had the minimum shoot water potential (1.61 MPa), while the maximum (1.31 MPa) was reported in the Sahianwala population (Table 2). Khanki population depicted the highest shoot osmotic potential and the lowest shoot turgor potential. Baloki population exhibited the minimum shoot turgor potential shoot osmotic potential.
3.5 Plant Ionic Content
Shoot Na+ varied significantly (p < 0.001) among different populations of K. brevifolia (Table 2). The Sahianwala population surpassed all other populations regarding shoot Na+, whereas the least was noted in the Chinyot population. The Sahianwala population accumulated the maximum root Na+ and shoot Ca2+. Root Na+ varied from 14.2 mg g─1 in the Chinyot population to 47.0 mg g─1 in the Sahianwala population. The minimum shoot Ca2+ (13.3 mg g─1) was reported in the Changa Manga population. Variation regarding root Ca2+ were non-significant. The Treemu population depicted the highest concentration of shoot and root K+, while the minimum was observed in the Baloki population.
3.6 Organic Osmolytes
The maximum accumulation of all osmolytes (free amino acids, proline, soluble proteins and soluble sugars) was observed in the Sahianwala population (Table 2). Total free amino acids varied from 1425.7 µg g−1 in the Sahianwala population to 198.5 µg g−1 in the Chinyot population. The highest accumulation of proline was observed in the Sahianwala population and least in the Chinyot population. The maximum accumulation of total soluble proteins was recorded in the population collected from Sahianwala, while the Khanki population possessed the minimum concentration. The concentration of total soluble sugars was the highest in the Sahianwala population and the minimum was observed in the Chinyot population.
3.7 Root Anatomical Characteristics
Root anatomical parameters varied significantly in K. brevifolia populations collected from different vegetation zones in the Punjab (Table 3, Fig. 3). The Treemu population showed higher values for most of the anatomical parameters like root thickness, vascular region thickness, aerenchymatous area and metaxylem area. The Chinyot population showed the maximum cortical region thickness and cellular area, and endodermis thickness.
Population collected from Khanki had the maximum epidermal thickness, whereas cortical cell area, vascular region thickness and aerenchymatous area were also higher than other populations except that collected from Chinyot. The Sahianwala population showed the minimum epidermal thickness, metaxylem area and aerenchymatous area, while Changa Manga population showed minimum root thickness, cortical cell area, endodermal thickness and vascular region thickness.
3.8 Stem Anatomical Characteristics
Significant variation was observed in all populations of K. brevifolia regarding stem anatomical characteristics (Table 3, Fig. 3). The proportion of storage parenchyma was relatively high in the populations collected from Chinyot and Changa Manga. The Treemu population showed the maximum epidermal thickness, sclerenchyma thickness and metaxylem area. The Baloki population showed the highest chlorenchyma thickness and vascular bundle area, while cortical cell and metaxylem areas were greater than all other populations except that of Chinyot population.
The Khanki population showed the maximum cortical cell area, but chlorenchymatous thickness was the minimum in this population. The Sahianwala population had the minimum stem thickness, cortical cell area, metaxylem area, and sclerenchymatous area. Vascular bundle area was the minimum in Changa Manga population, while epidermal and sclerenchyma thicknesses were higher than other populations except for the Treemu population.
3.9 Leaf Anatomical Characteristics
Leaf anatomical characteristics showed significant variation among different populations of K. brevifolia. Sahianwala population showed the maximum leaf lamina thickness, bulliform thickness, and metaxylem area, while stomata density was the minimum in this population (Table 3, Fig. 3). The Treemu population showed the highest leaf anatomical characteristics like epidermal thickness, vascular bundle area, chlorenchymatous thickness, and stomatal area. The Changa Manga population showed high midrib thickness, epidermal thickness, vascular bundle area and stomatal area. Bulliform thickness, metaxylem area and chlorenchyma thickness were the minimum in Changa Manga population.
The Khanki population was characterized by thick epidermis, thick chlorenchymatous region and broad metaxylem vessels, while the stomatal area was the minimum. The Baloki population showed the maximum thickness of leaf midrib, but lamina thickness was the minimum.
3.10 Bract Anatomical Characteristics
Bract anatomical characteristics varied significantly among K. brevifolia populations collected from diverse habitats (Table 3, Fig. 3). Bract midrib thickness, stomatal area, and stomatal density were the maximum in the Sahianwala population. This population showed more bulliform and chlorenchymatous thicknesses. The Changa Manga population had greater vascular bundle area, metaxylem area and chlorenchymatous thickness. Lamina thickness and stomatal density was the minimum in this population. Lamina thickness was the maximum in the Baloki population, which also showed high epidermal thickness, metaxylem area, and stomatal density (Fig. 4).
The Treemu population showed the highest epidermal thickness, whereas midrib thickness, bulliform thickness, and chlorenchymatous thickness were the minimum. The Chinyot population showed the minimum bract anatomical characteristics like epidermal thickness, metaxylem area, stomatal area and stomatal density. Midrib and lamina thicknesses and vascular bundle area were the second higher in this population. Bulliform thickness was the maximum in the Khanki population, while vascular bundle area was the minimum in this population.
3.11 Leaf Sheath Anatomical Characteristics
The Sahianwala population had the highest adaxial and abaxial epidermal thicknesses, vascular bundle area and metaxylem area (Table 3, Fig. 3). Leaf-sheath thickness and cortical cell area was the maximum in the Treemu population, which also showed high abaxial epidermal thickness and aerenchymatous area. Leaf sheath thickness, cortical cell area and vascular bundle area was the second-best in the Chinyot population. The Baloki population showed the minimum cortical cell area, aerenchymatous area and vascular bundle area. Leaf sheath and epidermal thicknesses were the minimum in the Changa Manga population. Aerenchyma development was more prominent in the Khanki population, which also showed the minimum abaxial epidermal thickness and metaxylem area.
3.12 Relationship Between Soil and Plant Morpho-physiological and Anatomical Parameters
Redundancy analysis (RDA) ordination triplot showed the effect of soil physicochemical characteristics of different habitats on morpho-physiological and anatomical parameters of K. brevifolia (Figs. 5 and 6). In the Treemu population, plant height and root length were influenced by soil K+, Ca2+ and Cl−. Stomatal conductance and water potential had a strong effect on Chinyot and Changa Manga populations. Turgor potential and sub-stomatal CO2 concentration were associated with Baloki population. Root Ca2+, shoot Na+, and shoot and root K+ were strongly linked with soil Ca2+, K+ and Cl−. Free amino acids, root Ca2+, root Na+, total soluble sugars and proline were strongly influenced by soil pH and saturation percentage.
RDA ordination triplot showing influence of soil’s physicochemical characteristics (blue labels) of different habitats (red labels) on a morphological, b water relation and gas exchange parameters, c root and shoot ionic content, and d root anatomical characteristics of Kyllinga brevifolia. Abbreviations: Sah, Sahianwala; HT, Treemu Headworks; HB, Baloki Headworks; CM, Changa Manga; HK, Khanki Headworks; Chi, Chinyot; AnR, annual rainfall; PlH, plant height; SFW, shoot fresh weight; RFW, root fresh weight; SDW, shoot dry weight; RDW, root dry weight; A, CO2 assimilation rate; E, transpiration rate; gs, stomatal conductance; Ci, sub-stomatal CO2 conc.; WUE, water use efficiency; WP, shoot water potential; OP, shoot osmotic potential; TP, shoot turgor potential; S-Na, shoot Na + ; R-Na, root Na + ; S-Ca, shoot Ca2 + ; R-Ca, root Ca2 + ; S-K, shoot K + ; R-K, root K + ; AA, total free amino acids; Pro, proline; TSP, total soluble proteins; TSS, total soluble sugars; RtT, root thickness; EpT, epidermal thickness; CT, cortical thickness; CCA, cortical cell area; EnT, endodermal thickness; MVA, metaxylem area; VBT, vascular region thickness; AeA, aerenchymatous area
RDA ordination triplot showing influence of soil’s physicochemical characteristics (blue labels) of different habitats (red labels) on a stem, b leaf, c bract, and d leaf sheath anatomical characteristics of Kyllinga brevifolia. Sah, Sahianwala; HT, Treemu Headworks; HB, Baloki Headworks; CM, Changa Manga; HK, Khanki Headworks; Chi, Chinyot; AnR, annual rainfall; StT, stem thickness; EpT, epidermal thickness; CCA, cortical cell area; VBA, vascular bundle area; MVA, metaxylem area; ScT, sclerenchyma thickness; ChT, chlorenchyma thickness; MdT, leaf midrib thickness; LmT, leaf lamina thickness; BfT, bulliform thickness; StA, stomatal area; StD, stomatal density
Root epidermal thickness depicted a strong relationship with the Changa Manga population. Root vascular bundle thickness and aerenchymatous area were influenced by soil physicochemical properties of Baloki population. Stem cortical cell area was strongly linked with the Chinyot population. Stem vascular bundle area and chlorenchymatous thickness had a strong influence on soil Ca2+ and K+. Leaf midrib thickness possessed a strong association with the Chinyot population. Leaf stomatal density and vascular bundle area were played a key role in adaptability of the Baloki population. Leaf bulliform thickness was strongly influenced by soil Ca2+, K+ and Cl−. Leaf lamina thickness and metaxylem vessel area were strongly affected by soil saturation percentage, EC and Na+.
Bract and lamina thickness, vascular bundle area and metaxylem vessel area possessed a strong influence of soil properties of the Changa Manga and Chinyot populations. Bract midrib thickness, stomatal density and stomatal area of the Sahianwala population showed a strong relationship with soil pH, EC, saturation percentage and Na+ soil. Chlorenchymatous thickness was mainly affected by annual rainfall. Adaxial epidermal thickness, vascular bundle area and metaxylem vessel area of the Sahianwala population were strongly linked to the soil pH, EC, saturation percentage and Na+.
Heatmaps were constructed to show relationship between soil physicochemical attributes of collection sites and morpho-anatomical traits of K. brevifolia (Figs. 7 and 8). Soil K+ and Ca2+ were grouped with root Ca2+ and total soluble proteins. Soil Na+, Cl, ECe and saturation percentage were clustered in a separate group. Soil pH influenced proline and total free amino acids in K. brevifolia. Total soluble salts in soil were associated with root-shoot Na+ and root Ca2+. Annual rainfall influenced total soluble salts, root Na+ and shoot Ca2+. Soil Na+ was associated with the root fresh weight, shoot turgor potential, net CO2 assimilation rate and water use efficiency (Fig. 7). Transpiration rate was associated with sub-stomatal CO2 concentration, root and shoot K+. Plant height, root dry weight, root-shoot fresh weight showed a relationship with stomatal conductance, shoot osmotic potential and water potential.
A heatmap constructed to draw the relationship between soil’s physicochemical characteristics of different habitats and growth, water relations, organic osmolytes, and photosynthetic attributes of Kyllinga brevifolia. Abbreviations: Sah, Sahianwala; HT, Treemu Headworks; HB, Baloki Headworks; CM, Changa Manga; HK, Khanki Headworks; Chi, Chinyot; AnR, annual; So-pH, soil pH; So-ECe, soil ECe, So-SP, soil saturation %; So-Na, soil Na; So-K, soil K; So-Ca, soil Ca; So-Cl, soil Cl; PlH, plant height; SFW, shoot fresh weight; RFW, root fresh weight; SDW, shoot dry weight; RDW, root dry weight; A, CO2 assimilation rate; E, transpiration rate; gs, stomatal conductance; Ci, sub-stomatal CO2 conc.; WUE, water use efficiency; WP, shoot water potential; OP, shoot osmotic potential; TP, shoot turgor potential; S-Na, shoot Na + ; R-Na, root Na + ; S-Ca, shoot Ca2 + ; R-Ca, root Ca2 + ; S-K, shoot K + ; R-K, root K + ; AA, total free amino acids; Pro, proline; TSP, total soluble proteins; TSS, total soluble sugars
A heatmap constructed to draw the relationship between soil’s physicochemical characteristics of different habitats and leaf sheath, leaf blade, stem, and root anatomical attributes of Kyllinga brevifolia. Abbreviations: Sah, Sahianwala; HT, Treemu Headworks; HB, Baloki Headworks; CM, Changa Manga; HK, Khanki Headworks; Chi, Chinyot; AnR, annual; So-pH, soil pH; So-ECe, soil ECe; So-SP, soil saturation %; So-Na, soil Na; So-K, soil K; So-Ca, soil Ca; So-Cl, soil Cl; RtT, root thickness; EpT, epidermal thickness; CT, cortical thickness; CCA, cortical cell area; EnT, endodermal thickness; MVA, metaxylem area; VBT, vascular region thickness; AeA, aerenchymatous area; StT, stem thickness; EpT, epidermal thickness; CCA, cortical cell area; VBA, vascular bundle area; MVA, metaxylem area; ScT, sclerenchyma thickness; ChT, chlorenchyma thickness; MdT, leaf midrib thickness; LmT, leaf lamina thickness; BfT, bulliform thickness; StA, stomatal area; StD, stomatal density (R-, root; S-, stem; L-, leaf; H-, sheath)
Heatmap (Fig. 8) showed a relationship between root, stem, leaf, and bract anatomical characteristics in five major clusters. The first cluster had 4 sub-clusters, where leaf metaxylem area was associated with stomatal density and area of bract. Soil Cl− influenced soil Ca2+ and K+. Soil pH had a relationship with soil saturation percentage and leaf lamina thickness. The last sub-cluster showed an association of soil ECe and Na+ with leaf sheath vascular bundle area and metaxylem area and bract midrib thickness. The second cluster had 3 sub-clusters, where stem thickness, leaf sheath epidermal thickness and leaf stomatal area was closely associated. Leaf stomatal density was related with metaxylem area and chlorenchymatous thickness in bract. Leaf midrib thickness, bract vascular bundle area and stem chlorenchymatous thickness showed a strong association. In the third cluster, root thickness was associated with endodermal thickness, cortical thickness and its cell area, metaxylem area and vascular bundle thickness in root, stem thickness, and bract cortical cell area. In the fourth cluster, leaf epidermal thickness was associated with leaf chlorenchymatous thickness and the root epidermal thickness. Bract epidermal thickness was associated with root aerenchymatous area, stem metaxylem area and sclerenchymatous thickness and leaf vascular bundle area. In the fifth cluster, two sub-clusters were noticed. Leaf sheath thickness was related to leaf sheath adaxial epidermal thickness, stem vascular bundle area, leaf bulliform thickness and bract lamina thickness.
4 Discussion
Members of the family Cyperaceae are among few plant families that can grow in almost every kind of habitat, e.g., cool alpine regions, deserts and semi-deserts, fresh and saltwater marshes, river and canal banks, roadsides and wastelands, and as weeds in agricultural fields (Uddin et al., 2006). The large number of species of family Cyperaceae colonizes moist and aquatic habitats, especially they are well adapted to saline wetlands (Piwpuan et al., 2013). Other than physio-morphological modifications, sedges have modified anatomical features that enable them to grow under saline waterlogged conditions (Abulfatih, 2003). Aerenchyma development in roots and stem is one of striking anatomical features that facilitate oxygen diffusion towards root for respiration (Benz et al., 2007). Modification in endodermis and bundle sheath cells, which surround vascular tissue in root and leaves respectively, significantly contribute towards stress tolerance by preventing radial water loss (Hoque et al., 2018).
In the present study, differently adapted population showed a differential response to the degree of salinity of their respective habitats. The Sahianwala population was collected from a saltmarsh that was highly saline (ECe 27.4 dS m−1). Hyper-saline soils of Sahianwala restricted growth of root and stem in K. brevifolia population, as was previously reported by several authors like Batool et al. (2013) and Younis et al. (2014). Accumulation of K+ ion was observed in this population, which dilutes the toxic effect of Na+ and Cl− accumulation to some extent and contributes towards the continuation of growth (Tavakkoli et al., 2012). This is an important feature of aquatic plants inhabiting saltmarshes (Kafi et al., 2021). Organic osmolytes like total free amino acids, total soluble sugars, total soluble proteins and proline contents increased significantly in this population, and this is the protective mechanism to prevent cell collapse under physiological drought caused by high salinities (Atreya et al., 2009; Hayat et al., 2011).
Leaf and leaf bract are the main photosynthetic organ in K. brevifolia. Any increase in chlorenchymatous thickness (cells containing chloroplast) will regulate the photosynthetic efficiency. This is critical as high salinity slows down many physiological processes (Stoeva & Kaymakanova, 2008). Thick leaf was a characteristic feature if Sahianwala population. Increased succulence in terms of leaf thickness has earlier been reported in halophytic species (Hameed et al., 2009). Additionally, larger bulliform cells in the leaves and bracts ease leaf rolling, which controls water loss due to transpiration and enhance survival under stressful conditions (Alvarez et al., 2008). Leaf anatomical modifications like well-developed bulliform cells, thicker leaves (midrib and lamina) and large stomatal area were observed as the important traits for the growth and survival of the Sahianwala population in highly saline habitats (Grigore et al., 2014; Shimamura et al., 2010). Leaf sheath surrounds the stem base, and hence critically important for stress tolerance, growth, and survival (Kokkonen et al., 2005). An increase of leaf sheath epidermal thickness, vascular bundle area and metaxylem area was noted in the Sahianwala population, which ensured the survival in hyper-saline environments.
The Treemu population was collected from hyper-saline seasonal inundation (ECe 20.2 dS m−1). Soil Ca2+ was the maximum at this site, and this is an important cation that significantly contributed to root dry weight (Bonomelli et al., 2019). Transpiration rate (and sub-stomatal CO2 concentration) was the maximum in this population, which is a general feature of aquatic species like K. brevifolia when sufficient water is available (Hanson et al., 2016). The higher accumulation of K+ and Ca2+ in root and shoot along with high Na+ is critical for salt-tolerant species like K. brevifolia (Ahmad et al., 2014). This neutralizes the toxic effect of Na+ accumulation by maintaining turgor potential under high salinities (Hussain et al., 2009). The notable modifications in the stem was intensive sclerification (particularly outside vascular bundles), which is vital for preventing water loss (Endo et al., 2008; Hameed et al., 2012b). Broader metaxylem vessels in root and stem can facilitate water conduction in the Treemu population (Smith et al., 2013). The stomatal size was exceptionally large in this population, which is certainly advantageous when water availability is sufficient enough (Xu & Zhou, 2008). Leaf sheath was the thickest, primarily due to the high proportion of cortical parenchyma. This increases the storage capacity in leaf sheath, and is a significant modification under highly saline conditions (Hameed et al., 2012a).
Head Baloki is a moderately saline habitat; the population was collected from a temporary water body. Photosynthetic rate and water use efficiency was notably high in this habitat because higher photosynthetic activity leads towards increased water use efficiency (Omamt et al., 2006). Endodermis was relatively thin in this population, which seemed not efficient enough to prevent the influx of solutes. Endodermis is a layer present outside of vascular bundle that acts as a barrier against water and ions influx like Na+ and Cl− (Moller et al., 2009). Stem growth was comparatively better in this population. The Baloki habitat seemed ideal for the development of storage parenchyma that was the main tissue observed in K. brevifolia stem (Grigore & Toma, 2007). This tissue is capable of storing large amount of water, and therefore, critically important when plant faces water deficit conditions (Bell & O’Leary, 2003). Leaf and bract thickness were also higher in this population, and this might be due to less stressful growth conditions (Hameed et al., 2009).
Changa Manga is moderate to low-saline habitat. The population was collected from forest plantations that receive irrigation water occasionally. Root and shoot growth was negatively affected, possibly due to dry salinity and low water availability. Ca2+ content in roots and shoots of this population was the minimum that is the possible reason for retarded growth of this population (Jiang et al., 2013). Root thickness, cortical cells and vascular region thickness decreased in Changa Manga due to inappropriate supply of water for the development of conducting and storage tissues (Younis et al., 2014). Development of stem sclerenchyma might be very beneficial as a plant can survive for much longer periods of water deficit environments by minimizing water loss (Farooq et al., 2009). Population of Changa Manga forest plantation had broader vessels but vascular region thickness decreased, which is directly related to hydraulic conductivity and water conduction efficiency (Smith et al., 2013). Leaves contain dense and smaller stomata that can regulate stomatal opening and closing more efficiently and hence minimizes water loss through transpiration (Bray & Reid, 2002).
Khanki population was collected from the low-saline waterlogged areas near the Headworks. High osmotic potential and maximum shoot fresh and dry weight was recorded in this population. Osmotic potential is directly related to better growth and development (Benzarti et al., 2014). Thicker root in this population was mainly due to larger proportion of cortical cells and extensive aerenchymatous formation. Aerenchyma in the roots enables a plant to maintain their oxygen requirements (Barrett-Lennard, 2003). Large cortical cells generally have larger vacuoles, and this proves beneficial as waterlogged conditions create physiological drought (Nawaz et al., 2012). Smaller-sized stomata as observed on leaves and large bulliform cells in bract ensure water conservation (Camargo & Marenco, 2011; Grigore et al., 2014).
Chinyot population represented better plant height and root growth parameters due to enough availability of river water. Plenty of water and non-saline soil yielded maximum plant growth because morphological characteristics have a negative correlation with salinity as reported by Alam et al (2015) and Qados (2015). Thick stem with extremely thin epidermis provides minimum protection and is an indicator of enough water availability. Desiccation tolerant plants like halophytes and xerophytes are generally equipped with thicker epidermis that is coated with thick waxy layer (Kosma et al., 2009; Liu et al., 2015). Low stomatal density and area in this population can be related to the growth condition as this population might not be best suitable for riverine habitat (Camargo & Marenco, 2011).
5 Conclusion
Kyllinga brevifolia populations showed specific physiological and anatomical modifications at their specific habitats. Growth and development were better in populations from low saline areas. The Sahianwala population collected from hypersaline saltmarsh relied on the high accumulation of Ca2+ and organic osmolytes in shoots for high degree of salinity tolerance. Among anatomical traits, leaves and bracts were thicker and xylem tissue of leaf sheath was larger in this population. The Treemu population collected from saline seasonal inundation accumulated high concentration of K+ and Ca2+ in root and shoot accompanied with thicker roots, broader metaxylem vessels in root and stem, and larger cortical parenchymatous cells. The Baloki population collected from moderately saline temporary water body had the maximum Co2 assimilation rate, water use efficiency, shoot turgor potential, leaf midrib thickness and bract lamina thickness. The Changa Manga population (from artificial forest plantation) showed larger vascular bundle area, metaxylem area and chlorenchymatous area in bracts. The Khanki population showed higher stomatal conductance, stem cortical cell area, root and leaf epidermal thickness, bract bulliform thickness and leaf sheath aerenchymatous area. The Chinyot population from the least saline riparian area showed root modifications like larger cortical region and cell area, endodermal area and vascular region thickness. Stem area was the maximum in this population.
Data Availability
The herbarium samples used for identification of plant species deposited to the Herbarium Collection of the Department of Botany, University of Agriculture Faisalabad.
References
Abulfatih, H. A. (2003). Ecological anatomy of xerophytic leaves from Qatar. Journal of King Saud University, 16, 19–29.
Ahmad, I., & Maathuis, F. J. (2014). Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. Journal of Plant Physiology, 171, 708–714.
Alam, M. A., Juraimi, A. S., Rafii, M. Y., & Hamid, A. A. (2015). Effect of salinity on biomass yield and physiological and stem-root anatomical characteristics of purslane (Portulaca oleracea L.) accessions. BioMed Research Internatinal, 2015, 105695.
Alvarez, J. M., Rocha, J. F., & Machado, S. R. (2008). Bulliform cells in Loudetiopsis chrysothrix (Nees) Conert and Tristachya leiostachya Nees (Poaceae): Structure in relation to function. Brazilian Archives of Biology and Technology, 51, 113–119.
Atreya, A., Vartak, V., & Bhargava, S. (2009). Salt priming improves tolerance to desiccation stress and to extreme salt stress in Bruguiera cylindrica. International Journal of Integrative Biology, 6, 68–73.
Barrett-Lennard, E. G. (2003). The interaction between waterlogging and salinity in higher plants: Causes, consequences and implications. Plant and Soil, 253, 35–54.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39, 205–207.
Batool, R., Hameed, M., & Ashraf, M. (2013). Photosynthetic response of three aquatic species of Schoenoplectus (Reichenb.) Palla under salt stress. Wetlands, 27, 2–11.
Bell, H. L., & O’Leary, J. W. (2003). Effects of salinity on growth and cation accumulation of Sporobolus virginicus (Poaceae). American Journal of Botany, 90, 1416–1424.
Benz, B. R., Rhode, J. M., & Cruzan, M. B. (2007). Aerenchyma development and elevated alcohol dehydrogenase activity as alternative responses to hypoxic soils in the Piriqueta caroliniana complex. American Journal of Botany, 94, 542–550.
Benzarti, M., Rejeb, K. B., Messedi, D., Mna, A. B., Hessini, K., Ksontini, M., Abdelly, C., & Debez, A. (2014). Effect of high salinity on Atriplex portulacoides: Growth, leaf water relations and solute accumulation in relation with osmotic adjustment. South African Journal of Botany, 95, 70–77.
Bernhardt, K. G., & Kropf, M. (2006). Schoenus nigricans (Cyperaceae) xerophytic grasslands on the NE Adriatic islands Cres and Krk (Croatia). Acta Botanica Croatica, 65, 127–136.
Bonomelli, C., Gil, P. M., & Schaffer, B. (2019). Effect of soil type on calcium absorption and partitioning in young avocado (Persea americana Mill.) trees. Agronomy, 9, 837–849.
Bray, S., & Reid, D. M. (2002). The effect of salinity and CO2 enrichment on the growth and anatomy of the second trifoliate leaf of Phaseolus vulgaris. Canadian Journal of Botany, 80, 349–359.
Camargo, M. A. B., & Marenco, R. A. (2011). Density, size and distribution of stomata in 35 rainforest tree species in Central Amazonia. Acta Amazonica, 41, 205–212.
Carignato, A., Piqué, J. V., Tapias, R., Ruiz, F., & Fernández, M. (2019). Variability and plasticity in cuticular transpiration and leaf permeability allow differentiation of Eucalyptus clones at an early age. Forests, 6, 23–31.
Dítě, D., Dítě, Z., Hájková, P., & Šuvada, R. (2019). Vegetation and ecological characteristics of the northernmost salt marshes of the European continent. Nordic Journal of Botany, 3. https://doi.org/10.1111/njb.023347.
Farooq, M., Kobayashi, N., & Fujita, D. (2009). Plant drought stress: Effects, mechanisms and management. Agronomy for Sustainable Development, 29, 185–212.
Field, R., Hawkins, B. A., Cornell, H. V., Currie, D. J., Alexandre, J., Diniz-Filho, F., Guégan, J. F., Dawn Kaufman, M., Kerr, J. T., Mittelbach, G. G., Eileen, T. O., O’Brien, M., & Turner, J. R. G. (2009). Spatial species-richness gradients across scales: A metaanalysis. Journal of Biogeography, 36, 132–147.
Grigore, M. N., & Toma, C. (2007). Histo-anatomical strategies of Chenopodiaceae halophytes: Adaptive, ecological and evolutionary implications. WSEAS Transactions on. Biology and Biomedicine, 4, 204–218.
Grigore, M. N., Ivanescu, L., & Toma, C. (2014). Halophytes. An integrative anatomical study. Springer.
Hameed, M., Ashraf, M., & Naz, N. (2009). Anatomical adaptations to salinity in cogon grass [Imperata cylindrica (L.) Raeuschel] from the Salt Range, Pakistan. Plant and Soil, 322, 229–238.
Hanif, U., Syed, S. H., Ahmad, R., & Malik, K. (2010). Economic impact of climate change on the agricultural sector of Punjab. The Pakistan Development Review, 49, 771–798.
Hanson, D. T., Stutz, S., & Boyer, J. S. (2016). Why small fluxes matter: The case and approaches for improving measurements of photosynthesis and (photo)respiration. Journal of Experimental Botany, 67, 3027–3039.
Hoque, M. I. U., Uddin, M. N., Fakir, M. S. A., & Rasel, M. (2018). Drought and salinity affect leaf and root anatomical structures in three maize genotypes. Journal of Bangladesh Agriculture University, 16, 47–55.
Hayat, S., Hasan, S. A., Fariduddin, Q., & Ahmad, A. (2011). Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. Journal of Plant Interaction, 3, 297–304.
Hussain, K., Majeed, A., Nawaz, K., Khizar, H. B., & Nisar, M. F. (2009). Effect of different levels of salinity on growth and ion contents of black seeds (Nigella sativa L.). Current Research Journal of Biological Sciences, 1, 135–138.
Jiang, Z., Zhu, S., Ye, R., Xue, Y., Chen, A., An, L., & Pei, Z. M. (2013). Relationship between NaCl- and H2O2-induced cytosolic Ca2+ increases in response to stress in Arabidopsis. PLoS ONE, 8, e76130.
Kafi, M., Nabati, J., Ahmadi-Lahijani, M. J., & Oskoueian, A. (2021). Silicon compounds and potassium sulfate improve salinity tolerance of potato plants through instigating the defense mechanisms, cell membrane stability, and accumulation of osmolytes. Communication in Soil Science and Plant Analysis, 52,. https://doi.org/10.1080/00103624.2020.1869768
Kokkonen, M., Jestoi, M., & Rizzo, A. (2005). Determination of selected mycotoxins in mould cheeses with liquid chromatography coupled to tandem with mass spectrometry. Food Additives and Contaminants, 22, 449–456.
Les, D. H. (2020). Aquatic monocotyledons of North America: Ecology, life history, and systematics. CRC Press.
Liu, Y., Li, X., Chen, G., Li, M., Liu, M., & Liu, D. (2015). Epidermal micromorphology and mesophyll structure of Populus euphratica heteromorphic leaves at different development stages. PLoS One, 10, e0141578.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin Phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Mishra, S., Tripathi, A., Tripathi, D. K., & Chauhan, D. K. (2015). Role of sedges (Cyperaceae) in wetlands, environmental cleaning and as food material: Possibilities and future perspectives. Journal of Experimental Botany, 62, 42–53.
Moller, I. S., Gilliham, M., Jha, D., Mayo, G. M., Roy, S. J., Coates, J. C., Haseloff, J., & Tester, M. (2009). Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. The Plant Cell, 21, 2163–2178.
Muhlenbock, P., Plaszczyca, M., Plaszczyca, M., Mellerowicz, E., & Karpinski, S. (2007). Lysigenous aerenchyma formation in Arabidopsis is controlled by lesion simulating disease. The Plant Cell, 19, 3819–3830.
Nawaz, T., Hameed, M., Waqar-U-Nisa, A., & M. S. A., Younis, A., & Kanwal, H. . (2012). Comparative anatomy of root and stem of some native and exotic Asparagus L. species. Pakistan Journal of Botany, 44, 153–158.
Omamt, E. N., Hammes, P. S., & Robbertse, P. J. (2006). Differences in salinity tolerance for growth and water-use efficiency in some amaranth (Amaranthus spp.) genotypes. New Zealand Journal of Crop and Horticultural Science, 34, 11–22.
Piwpuan, N., Zhai, X., & Brix, H. (2013). Nitrogen nutrition of Cyperus laevigatus and Phormium tenax: Effects of ammonium versus nitrate on growth, nitrate reductase activity and N uptake kinetics. Aquatic Botany, 106, 42–51.
Qados, A. M. S. A. (2015). Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). Journal of the Saudi Society of Agricultural Sciences, 1, 7–15.
Ruzin, S. E. (1999). Plant microtechnique and microscopy. Oxford University Press.
Shimamura, S., Yamamoto, R., Nakamura, T., Shimada, S., & Komatsu, S. (2010). Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Annals of Botany, 106, 277–284.
Smith, M. S., Fridley, J. D., Yin, J., & Bauerle, T. L. (2013). Contrasting xylem vessel constraints on hydraulic conductivity between native and non-native woody understory species. Frontiers in Plant Sciences, 4, 1–12.
Stoeva, N., & Kaymakanova, M. (2008). Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). Journal of Central European Agriculture, 9, 385–391.
Takahashi, H., Yamauchi, T., Colmer, T. D., & Nakazono, M. (2014). Aerenchyma formation in plant. In: Low-oxygen stress in plants, oxygen sensing and adaptive responses to hypoxia. van Dongen, J.T., Licausi, F. (Eds.), Plant Cell Monographs (Vol. 21, 247−265). Springer.
Tavakkoli, E., Rengasamy, P., & McDonald, G. K. (2012). High concentrations of Na+ and Cl- ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. Journal of Experimental Botany, 61, 4449–4459.
Uddin, S. J., Mondal, K., Shilpi, J. A., & Rahnan, M. T. (2006). Antidiarrhoeal activity of Cyperus rotundus. Fitoterapia, 77, 134–143.
Wolf, B. (1982). A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Communications in Soil Science and Plant Analysis, 13, 1035–1059.
Xu, Z., & Zhou, G. (2008). Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany, 59, 3317–3325.
Yemm, E. W., & Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal, 57, 508–514.
Younis, A., Riaz, A., Ahmed, I., Siddique, M. I., Tariq, U., Hameed, M., & Nadeem, M. (2014). Anatomical changes induced by NaCl stress in root and stem of Gazania harlequin L. Agricultural Communications, 2, 8–14.
Acknowledgements
This manuscript is a part of the Ph. D. research of Sahar Mumtaz submitted to the Department of Botany, University of Agriculture, Faisalabad.
Author information
Authors and Affiliations
Contributions
Sahar Mumtaz conducted the experiment. Mansoor Hameed and Farooq Ahmad supervised the research work. Muhammad Sajid Aqeel Ahmad analyzed the data statistically and performed the multivariate analysis. Iftikhar Ahmad and Muhammad Hamzah Saleem were involved in the preparation of the manuscript. Muhammad Ashraf proofread is the group leader and finally edited the scientific and English language.
Corresponding author
Ethics declarations
Ethics Approval
The manuscript was submitted solely to Water, Air and Soil Pollution and no part was published or submitted elsewhere. All ethical guidelines set by parent institution(s) were observed during sampling and analysis.
Consent to Participate and Publish
All authors equally participated in the execution of the experiment and unanimously agreed to publish in Environmental Science and Pollution Research.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mumtaz, S., Hameed, M., Ahmad, F. et al. Structural and Functional Determinants of Physiological Pliability in Kyllinga brevifolia Rottb. for Survival in Hyper-Saline Saltmarshes. Water Air Soil Pollut 232, 424 (2021). https://doi.org/10.1007/s11270-021-05391-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05391-x