Abstract
Genus Fimbristylis colonizes salt-affected wetlands and salt marshes of tropical and subtropical areas of the world. Populations of three Fimbristylis spp. (F. complanata, F. dichotoma and F. rigidula) were collected from differently salt-affected wetlands (HT-Treemu Headworks, least saline; JR-Jhumra Road, moderately saline and SH-Sahianwala, highly saline). These populations were evaluated for degree of salinity tolerance in relation to shoot ionic contents, biochemical, gas exchanges and anatomical traits. The JR population showed significantly higher concentration of chlorophyll a and b, and, an increased net assimilation (A) and transpiration (E) rates. Stomatal conductance (gs) and water use efficiency were the maximum in highly saline SH populations of F. complanata and F. dichotoma. Leaf osmotic and water potentials turnout to be more negative in SH population. The JR population maintained fairly constant leaf osmotic and water potentials. All SH population maintained high turgor potential. The SH population of F. complanata accumulated high total soluble proteins (TSP) as compared to counterpart populations, while in case of F. rigidula TSP was maximum in JR population. The SH population accumulated excess amounts of total soluble sugars and free amino acids for better osmotic adjustments in all species of Fimbristylis. Additionally, more accumulation of Na+, but also promoted K+ and Ca2+ uptake among all species of Fimbristylis. All Fimbristylis populations showed specific anatomical modifications at higher salinity levels. At root level, an increase in the epidermal cell area, cortical region (enhance water storage), thickened exodermal layers, efficient conducting tissues (metaxylem, phloem) were observed. Stem adaptations included increased stem radius and vascular bundle thickness, and large metaxylem vessels. Leaf modifications consisted of broad midrib and lamina. In conclusion, all these species showed greater salinity tolerance and survived up to the highest saline levels. Therefore, this study strongly recommends using these species for revegetation and phytoremediation of the salt-affected lands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinization is a substantial barrier to the sustainability of natural ecosystems and put many limitations on native fauna and flora. In recent years, problem of salinity has been substantially increased due to substandard irrigation systems, human activities, soil erosion, inappropriate agricultural practices and poor drainage systems (Kausar et al. 2012). High salt concentrations in soils and water employ negative influences on plant biochemical traits by distracting osmotic and ionic equilibrium in plants (Muchate et al. 2016). This causes serious osmotic imbalance that interrupts many physiological processes, mainly photosynthesis (Roy et al. 2014). Such overload of ions under saline condition also causes oxidative damage and affects the developmental stages of plants by hampering protein synthesis and lipid metabolism (Negrão et al. 2017). Such adverse effects not only affect plant’s physiological processes and also limit diversity and distribution of vegetation growing in these saline areas (Zörb et al. 2019).
Salinity adversely alters the anatomical traits of the plants. Many anatomical traits are considered more responsive to environmental constraints, therefore considered as the most effective tool to evaluate the degree of salt tolerance (Dolatabadian et al. 2011; Naskar and Palit, 2015). Anatomical traits that are related to increased salinity tolerance are cross-sectional area of root and stem, which provide more space for water storage and also ion sequestering (Hameed et al. 2011). Other anatomical traits of significance includes thickened epidermis for minimizing water loss, increased size of xylem tissue and enlarged bulliform cells for efficient leaf rolling. All these modifications ensure water conservation, a vital commodity under physiological drought caused by salt stress (Naz et al. 2016). Halophytic plants also adopt many functional modifications in biochemical pathways to mitigate the adverse effects of salt stress by decreasing net assimilation of CO2 and increase concentration of compatible solutes like free amino acids, total soluble proteins, and sugars for osmotic adjustment (Roy et al. 2014; Muchate et al. 2016; Kumari et al. 2019). Logically, populations having better of these anatomical and physiological adaptations are more adaptive to stressful environments than others.
Fimbristylis spp. grows in diverse habitats like saline and polluted wetlands and arid soil habitats. Most of the species are associated with poor soils containing adequate amount of moisture (Khan and Qaiser 2006). A number of Fimbristylis species are salt-accumulating halophytes and a dominant component of vegetation in hyper-saline saltmarshes and inland wetland (Badri and Hamed 2000; Khan and Qaiser 2006; Vaiga and Joseph 2016). This genus is also known for as a soil binder capacity (Banerjee et al. 2012) and has a strong potential for phytoremediation due to higher heavy metal accumulation (Muhammad et al. 2011; Yamamoto et al. 2018). Palatability is high, and domestic livestock (primarily buffaloes and goats) commonly consume Fimbristylis species. Many species of Fimbristylis are economically used in folk medicines (Roy et al. 2019) and also for cordage fibres. Germination potential of Fimbristylis species is high in salt-affected soil (Singh et al. 2015), and therefore can easily be cultivated in saline area for fodder production and phytoremediation of saline soils.
Fimbristylis dichotoma is the less explored species, while absolutely no literature is available on F. rigidula and F. complanata relating to their salinity tolerance potential. The present research is the first detailed study on the structural and functional modifications in differently adapted populations of Fimbristylis spp. It was hypothesized that each population of these species will respond differently to salinity stress under controlled conditions that might be because of their differential adaptation strategies to variously salt-affected environments. The current work aims to elucidate the degree of salinity tolerance in each population of Fimbristylis spp. We also intend to sort out structural and functional mechanisms for salinity tolerance that enables differently adapted populations of all three species to dominate saline wetlands.
Materials and methods
Study area
The habitats types i.e. (i) least saline seasonal inundations near Trimoo Headworks [HT; ECe: 18.45 dS m−1], (ii) moderately saline waterlogged area along Jhumra road [JR; ECe: 31.45 dS m−1] and (iii) hyper-saline salt marsh at Sahianwala [SH; ECe: 47.49 dS m−1] were selected. These habitats were located in various regions of Punjab, Pakistan. Habitat description, GPS coordinates (Garmin eTrex Venture HC, USA), elevation and soil physicochemical characteristics are presented in Table 1 and Fig. 1.
Soil analysis
For the estimation of soil physiochemical parameters, six soil samples were collected from the selected sites (as outlined in Table 1) of each population (n = 6). Soil analysis was done following the method as defined in Handbook # 60 (US Salinity Laboratory Staff 1954). A soil saturation paste was prepared and used for the determination of ECe, pH and ionic contents. Physiochemical characteristics of collected samples like pH and ECe were analysed by using an EC/conductivity meter (WTW series, Ino LAB pH/Cond 720, USA). The concentration of shoot Na+, K+ and Ca2+ was determined by flame photometers (Jenway, PFP-7, UK). Chloride ions (\({\mathrm{C}\mathrm{l}}^{-}\)) were observed by using chloride meter (Model-926, Sherwood Scientific Limited Cambridge, UK). Mg2+ contents were determined by and atomic absorption spectrophotometer (AAnalyst-300, Perkin-Elmer, Germany). The percentage of organic matters (OMs) was assessed by following the method of Sims and Haby (1971). Soil PO43− was determined according to the method of Yoshida et al. (1971).
Plant materials
Three populations of Fimbristylis [F. complanata (Retz.) Link, F. dichotoma (L.) Vahl, and, F. rigidula Nees) were collected from different wetlands of Punjab varying in their salinity level as outlined in “Study area”. Six plants of each population were collected from each study site (n = 162 for 6 plants of 3 populations and 3 species from 3 sites), immediately placed in zipper bags and stored in ice-box to minimize transpirational losses. The plants were immediately brought to the laboratory for morphological, biochemical and physiological analysis.
Physiological traits
Photosynthetic pigments
Plant leaves samples were homogenized in acetone at − 4 °C to assess Chlorophyll a, b by Arnon (1949) and carotenoids following the method of Davis (1976). Homogenized plant extract was centrifuged at 10,000× g for 5 min. and specific absorption was recorded at 480, 645, 663 nm by using UV visible spectrophotometer (Hitachi-220, Japan). The concentrations of photosynthetic pigments were calculated by appropriate formulae given by above cited authors.
Gas exchange parameters
Net assimilation rate (A), stomatal conductance (gs), and transpiration rate (E) of fully expanded matured leaf were estimated through a portable infrared gas analyzer system (LCA-4, ADC, Hoddesdon, England). All traits were assessed in the early morning under moderate light conditions. The IRGA apparatus was adjusted to molar airflow; 403.3 mmol m−2 s−1, vapor pressure; 6–9 bar, atmospheric pressure was 99.9 kPa; PAR, 1711 µmol m−2 s−1, level of CO2 was 352 µmol mol−1 with relative humidity 65% within the temperature of chamber at 26 °C. Water use efficiency was determined by dividing net assimilation rate (A) over transpiration rate (E).
Leaf water relations
A Scholander type pressure chamber was used to record leaf water potential (Ψw). The same leaf was frozen in an ultra-freezer (for one week at − 20 °C) and leaf osmotic potential (Ψs) was assessed through an osmometer (Wescor-5500). The equation Ψp = Ψw − Ψs was used to determine the leaf turgor potential (Ψp).
Assessment of compatible solutes
Free amino acids
Free amino acids were assessed by method described by Moore and Stein (1948). Leaf sample (0.5 g) was extracted in 5 ml sodium citrate buffer having pH 5.0. Homogenized material was incubated at 24 °C for 60 min. After incubation extract was thoroughly centrifuged at 15,000× g for a 10 min. Later 1 ml of aliquot and 2 ml of acid ninhydrin were reacted (1.25 g of ninhydrin was dissolved in 20 ml 6 M H3PO4 and 30 ml of CH3COOH). After one h, all samples were transferred to a boiling water bath, and thereafter, the reaction was terminated into an ice bath. The mixture was diluted by adding 5 ml of n-propanol and H2O in 1:1 ratio in each glass test tube. Absorption of samples was taken at 570 nm with the help of a UV visible spectrophotometer (Hitachi-220, Japan). Quantity of amount of amino acids was calculated by comparing absorbance with concentration developed from a standard calibrated curve of Leucine.
Total soluble sugars
Amount of soluble sugars was quantified according to the method of Dubois et al. (1951). Plant material (500 mg) were pulverized in the 80% (V/V) ethyl alcohol, and then centrifuged at 4000 rpm for 15 min. Extracted material was separated, and volume was maintained to 10 ml by adding 80% alcohol. The 100 µL of aliquot was transferred in small test tubes and volume was maintained to 1 ml with deionized H2O. It was then reacted with 5 ml of anthrone reagent (prepared by 0.2% anthrone and conc. H2SO4.). Reaction mixture was heated in a water bath at 85 °C for a 30 min. Subsequently, test tubes were kept in a cold ice bath and the reaction was terminated. Absorbance was recorded with the help of a spectrophotometer (Hitachi-220, Japan) at 620 nm. The concentration of total soluble sugars were ascertained by the help of standard curve developed using known concentrations of glucose and represented as mg g−1 FW.
Protein estimation
Proteins were assessed by the method of Lowry et al. (1951). Plant leaf sample (200 mg) was grounded in 5 ml of phosphate buffer (0.2 M with pH 7.0). Homogenized material was centrifuged at 5000× g for 10 min. After that, the material was reacted with 1 ml of alkaline assay solution. The Folin-Phenol reagent (500 µL) was mixed after 30 min and reading was recorded at 620 nm by using a spectrophotometer. The protein quantity was estimated by a standard curve developed from known concentration of BSA (Bovine serum albumin).
Shoot ionic content
The dried shoot material 500 mg was digested by using concentrated H2SO4 in large test tubes as described by Wolf (1982). After incubation for 24 h, test tubes were gradually heated to 350 °C on a hotplate until digestion was completed. After cooling, 30% H2O2 was added until the solution becomes colourless or reheated again if necessary. A flame photometer (Jenway, PFP-7) was used to determine shoot Na+, Ca2+, and K+ ions were using a series of standards.
Anatomical characters
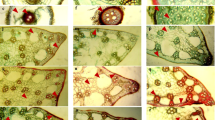
For anatomical characteristics, a fully expanded leaf, a central portion of stem containing inflorescence, and the thickest root from shoot and root junction were separated. Plant material was subsequently kept for 48 h in formalin acetic alcohol (FAA) that contains v/v 5% formaldehyde, 50% ethanol, 10% acetic acid, and 35% distilled H2O. Samples were then transferred to acetic alcohol solution to preserve for the long-term. Freehand sectioning was done by double-edge razor blade then dehydration and staining was done by a standard double staining method following Ruzin (1999). Safranin and fast green were used as a staining agent. The measurements of various tissues were taken by using an ocular micrometre calibrated with a stage micrometer (Fig. 2) with a camera-equipped microscope (Nikon, 104 Japan).
Statistical analysis
Data were subjected to Tukey pairwise comparison test between sites of different species followed by an analysis of variance (ANOVA) at the significance level (P < 0.05) by using Minitab 19. Furthermore, data was also implied to multivariate analysis (PCA) and correlation matrix by using R statistical software (R Core Team, 2019) to assess the relationship between anatomical traits of root, stem, and leaf in all three sites. Response curves of Fimbristylis species to differential saline conditions were drawn by fitting generalized linear model (GLM) in CANOCO version 4.5 for windows.
Results
Morphological characteristics
Leaf area and numbers of the leaves per plant decreased significantly with increase in salinity level among all species of Fimbristylis, and maximum values of these traits were observed in F. rigidula at all three habitats. Plant height, shoot fresh weight, and shoot dry weight of F. rigidula was higher than other two species at all study sites. These traits decreased in all three species with increase in salinity level of the habitat (Table. 1).
Soil physiochemical characteristics
Soil ECe of the Sahianwala (SH) site was the highest (47.49 dS m−1), while that of the Head Trimoo (HT) was the lowest (18.45 dS m−1), and that of the Jhumra road (JR) moderate (31.45 dS m−1). Soil pH of the JR site (8.8) was the maximum (Table.1). Soil Na+ (4557 mg L−1), Mg2+ (0.54 mg L−1), PO43− (7.0 mg L−1), and organic matter % (1.67%) were significantly higher (P ≤ 0.05) in the Sahianwala habitat. Soil K+, Ca2+ and Cl− were the highest in the JR site (Table 1).
Physiological traits
Photosynthetic pigments
Chlorophyll a and b were significantly higher in JR population of each Fimbristylis species. Maximum chlorophyll a (1.92 mg g−1 f. wt.) and Chl b (0.57 mg g−1 f. wt.) was noted in JR population of F. dichotoma. However, no significant (P ≤ 0.05) change in photosynthetic pigments was noticed in the JR and SH populations of F. rigidula. Carotenoids increased significantly in HT populations of all Fimbristylis species, and maximum was observed in F. rigidula (0.06 mg g−1 f. wt.) (Table 2).
Gas exchange traits
Net assimilation rate (A) was substantially higher in JR populations of three Fimbristylis species as 18.0, 8.20 and 17.80 µm m−2 s−1, respectively. The HT populations showed the minimum A compared to its counterpart populations ranging from 1.60 to 13.60 µm m−2 s−1 in all populations of different species. Transpiration rate (E) was significantly high (1.24–2.39 mm m−2 s−1) in the JR populations of all species, while the minimum (0.71–1.01 mm m−2 s−1) in the HT population. The minimum stomatal conductance (gs) was noted in all HT populations (150–200 mm m−2 s−1), and the maximum gs was noticed in SH populations (350–450 mm m−2 s−1) out of all three species. Water use efficiency (WUE) was significantly higher in HT populations of all species, but it decreased significantly in all JR populations. The SH populations showed differential behavior, as WUE was the maximum in F. complanata (22.54 µm CO2 mmol H2O−1) and the minimum (8.97 µm CO2 mmol H2O−1) in F. rigidula (Table 2).
Leaf water relations
Leaf water (Ψw) and osmotic (Ψw) potentials were more negative in the SH populations of all species. The JR populations of two species, F. complanata and F. rigidula showed high osmotic potential (− 0.93 and − 0.99 MPa), respectively. Leaf turgor potential decreased significantly (P ≤ 0.05) with increase in salinity level of the habitat in all Fimbristylis species (ranging from 0.74 to 0.34 MPa). Fimbristylis complanata maintained turgor potential more successfully than its counterparts at all study sites (Table 2).
Compatible solutes
Compatible solutes (total soluble sugars and total free amino acids) were significantly high in SH population of all Fimbristylis species. The SH population of F. rigidula accumulated the maximum total sugars (28.05 mg g−1 f. wt.).The maximum total free amino acids (2400–2865 µg g−1 f. wt.) were recorded in SH populations. All Fimbristylis species responded differently regarding total soluble proteins. The JR populations of F. dichotoma and F. rigidula (1.40–4.80 mg g−1 f. wt.) accumulated significantly high soluble proteins than those collected from SH and HT habitats. The SH population of F. complanata accumulated maximum concentration of soluble proteins, which gradually decreased as salinity level of the habitat increased (Table 2).
Shoot ionic contents
Accumulation of Na+ increased in all Fimbristylis species with increase in salinity level of the habitat. The maximum Na+ content (42.04 mg g−1 d. wt.) was recorded in SH population of F. complanata (Table 2). The SH populations possessed significantly higher shoot K+ (11.10–27.56 mg g−1 d. wt.) and Ca2+ (6.07–9.69 mg g−1 d. wt.) among all species of Fimbristylis (Table 2). Shoot K+ and Ca2+ was the maximum in SH population of F. complanata, while the minimum ions was recorded in SH population of F. rigidula (Table 2).
Anatomical characteristics
Root anatomy
Root cortical thickness was significantly high in SH populations of F. complanata and F. dichotoma (255.29 and 165.36 µm), whereas JR populations of F. dichotoma exhibited the maximum (56.30 µm) of cortical thickness. Cortical region thickness was the minimum in HT population of F. dichotoma (Table 3). Cortical cell area (CCA) was significantly high (730.98 µm) in SH population of F. complanata, while JR populations of F. dichotoma and F. rigidula exhibited the maximum cortical cell area (Table 3). The maximum vascular bundle thickness (192.59 and 268.85 µm) was recorded in JR population of F. complanata and F. dichotoma, while SH population of F. rigidula exhibited the thickest (235.19 µm) vascular bundles (Table. 3). Metaxylem and phloem areas were significantly high in JR population of F. complanata and F. dichotoma. The SH population of F. rigidula showed the highest (540.60 µm) phloem area, while JR population had the maximum (994.84 µm) metaxylem area (Table 3). Epidermal cell area of F. rigidula was significantly high in JR population, but F. complanata and F. dichotoma exhibited a significantly (P ≤ 0.05) high epidermal cell area in all SH populations (Fig. 3, Table 3).
Stem anatomical modification
Stem radii were significantly high in JR populations of all Fimbristylis species. Plants collected from highly saline SH habitats of F. complanata and F. dichotoma exhibited the minimum stem radii (Table 3). Cortical region thickness progressively increased in F. complanata and F. dichotoma as salinity level of the sites increased, being the maximum (509.26–643.14 µm) in SH populations. However, plants of F. rigidula inhabiting moderately saline habitat JR showed the maximum cortical region thickness (Table 3, Fig. 3).
Vascular bundle thickness was significantly high in SH populations of all Fimbristylis species. The maximum vascular bundle thickness was recorded in SH population (234.21 µm) of F. complanata. The JR population of F. complanata showed the maximum (574.72 µm) of metaxylem area, while HT population of F. dichotoma showed the maximum (577.12 µm). Fimbristylis rigidula showed a consistent (P ≤ 0.05) increase in metaxylem area as salinity levels of the habitats increased. Phloem area was the maximum in SH populations of all Fimbristylis species. Phloem tissue was relatively larger (374.08 µm) in SH population of F. dichotoma (Table. 3). The JR populations of F. complanata and F. dichotoma exhibited the highest stem epidermal cell area. The minimum stem epidermal cell area was recorded in SH population of F. complanata. The SH population of F. rigidula exhibited the maximum stem epidermal cell area (Table. 3).
Leaf anatomical modification
Midrib and lamina thicknesses were the maximum in JR populations of all Fimbristylis species. The maximum midrib and lamina thickness were recorded in F. complanata. The SH populations of F. dichotoma and F. complanata showed a significantly thin midrib and lamina. The JR populations of all Fimbristylis species showed significantly larger mesophyll cells (382.61–579.68 µm) as compared to that recorded in other populations. Vascular bundle thickness was significantly higher in JR populations of Fimbristylis species. Metaxylem area of F. dichotoma and F. rigidula were the maximum in JR populations, while SH population of F. complanata possessed the thickest metaxylem. The SH populations of all Fimbristylis species had the thickest phloem area, while the maximum value was recorded in JR populations. The SH populations of all Fimbristylis species possessed significantly higher (536.23–1062.6 µm2) epidermal cell area (Table 3, Fig. 3).
Multivariate analysis and correlation
Physiological traits
Principal component analysis (PCAs) explained variation in responses of physiological traits under different saline habitats of Fimbristylis species (Fig. 4a). The first and second axis of PCAs explained 31.9 and 21.2% (53.1%) variation, respectively, among traits and habitats. Major contributors to the highly saline habitat Sahianwala (SH) included LOP, AA, TSS and gs. Characteristics like LOP, AA, and gs significantly (P ≤ 0.05) increase with positive eigenvalues under higher salinity while TSS sugars reduced at this habitat. No contributor was found in the least saline habitat. Principal components in moderate saline habitat JR were E, Chl a, Chl. b, S-Na+, and, S-K+. The JR population showed a substantial increase in E, Chl a, and b, while a decrease occurred in shoot Na+ and K+ (Fig. 4a). Among studied sites, a significantly (P ≤ 0.05) higher positive correlation was observed among LOP, AA, and g; Chl a, Chl b, LTP and S-Na+; and, S-K+, and A. LOP and Chl b showed a strong negative correlation (Fig. 4b).
PCA biplot showing response of physiological traits of Fimbristylis species collected from salt marshes (a) and Pearson’s correlation among physiological traits (b). WUE water use efficiency, A net assimilation rate, Caro carotenoids, S-Ca2+ shoot calcium ions, LWP leaf water potential, S-K+ shoot K+, AA amino acids, TSS total soluble sugars, gs stomatal conductance, SP soluble proteins, E transpiration rate, Chl, a, b chlorophyll a, b, S-Na+ shoot Na+ and LTP leaf turgor potential
Anatomical characteristics
Principal component analysis (PCAs) demonstrated high variability among studied anatomical characteristics of root stem and leaf in Fimbristylis species collected from salt-affected wetlands (Fig. 5a). The PCAs showed total variability of 57% (PC1 = 31.8%; 25.2%). The principal components of highly saline habitat SH including ECA-R, VBT-S, MtA-S, CRT-R, and ECA-L exhibited negative eigenvalues with a significant decrease. The major contributors to moderately saline habitat VBT-R, VBT-L, SA-S, MtA-L, PhA-L, LTh-L significantly increased under a moderate saline condition with positive values. No contributor was found linked to the least saline site HT. Among studied anatomical traits, MtA-L and PhA-L showed positive correlation with SA-S, LTh-L and MsA-L, while PhA-R, ECA-L, CRT-Shad showed significant correlation with MtA-S. A significant negative correlation was observed between PhA-L and ECA-R (Fig. 5b).
PCA biplot showing response of root, stem, and leaf anatomical traits of Fimbristylis species collected from salt marshes (a), and, Pearson’s correlation among physiological traits (b). R-root, S-stem and L-leaf, PhA phloem area, ECA epidermal cell area, StA stem area, MsA mesophyll area, LTh lamina thickness, VBT vascular bundle thickness, MtA metaxylem area and CRT cortical region thickness
Response curves of Fimbristylis species to differential saline conditions
Differential response of Fimbristylis complanata along increasing salinity gradients was characterized in a generalized linear model (Fig. 6). Chl a showed a strong positive response with an increasing curve along the elevated salinity gradient. The Chl b, Caro, and gas exchange traits (WUE, A, E) showed a negative response as the level of salinity increased (Fig. 6a). Sharp positive responses were observed for AA, TSS, LOP, LWP and S-K, however LTP, SP, S-Na and S-Ca displayed a strong negative response along with increasing saline regimes (Fig. 6b). Root anatomical characters (CCA, CRT, MtA and ECA) showed a strong positive response, while VBT and PhA exhibited strong negative response along with increasing salt levels (Fig. 6c). Stem anatomical traits (CRT, MtA, PhA, VBT, and, StA) showed a sharp increase except for ECA which exhibited a linear response with no significant change along salinity gradients (Fig. 6d). Leaf anatomical traits (LTh and PhA) exhibited an increasing trend with a negative influence of salinity, while all other leaf traits showed a strong positive trend (Fig. 6e).
Generalized linear model showing responses of (a) chlorophyll and gas exchange traits, (b) water relation, organic osmolytes and shoot ions, (c) root anatomical characters, (d) stem anatomical characters, and (e) leaf anatomical characters of Fimbristylis complanata plotted against increasing levels (from 10 dS m−1 at left to 60 dS m−1 at right). WUE water use efficiency, A net assimilation rate, Caro carotenoids, S-Ca2+ shoot calcium ions, LWP leaf water potential, S-K+ shoot K+, AA amino acids, TSS total soluble sugars, gs stomatal conductance, SP soluble proteins, E transpiration rate, Chl, a, b chlorophyll a, b, S-Na+ shoot Na+, LTP leaf turgor potential, PhA phloem area, ECA epidermal cell area, StA stem area, MsA mesophyll area, LTh lamina thickness, VBT vascular bundle thickness, MtA metaxylem area and CRT cortical region thickness
For Fimbristylis dichotoma, Chl a, Chl b and gas exchange traits (A, WUE and gs) represented a slightly negative response, while E showed a linear response (Fig. 7a). Saline conditions showed a positive influence on organic osmolytes (TSS and AA) except for SP which showed a linear response along with salinity levels. A sharp negative response was observed for LTP, S-Na and S-Ca with an increase in salinity levels (Fig. 7b). Root anatomical traits (CRT and MtA) exhibited a negative increase while ECA, VBT and CCA except for PhA showed no change in response of salinity (Fig. 7c). Stem anatomical traits (CRT, ECA, VBT and PhA) responded positively except StA with increasing salinity levels (Fig. 7d). All leaf traits responded negatively, except ECA which showed a positive and increasing pattern along with saline regimes (Fig. 7e).
Generalized linear model showing responses of (a) chlorophyll and gas exchange traits, (b) water relation, organic osmolytes and shoot ions, (c) root anatomical characters, (d) stem anatomical characters, and (e) leaf anatomical characters of Fimbristylis dichotoma plotted against increasing salinity levels (from 10 dS m−1 at left to 60 dS m−1 at right). WUE water use efficiency, A net assimilation rate, Caro carotenoids, S-Ca2+ shoot calcium ions, LWP leaf water potential, S-K+ shoot K+, AA amino acids, TSS total soluble sugars, gs stomatal conductance, SP soluble proteins, E transpiration rate, Chl, a, b chlorophyll a, b, S-Na+ shoot Na+, LTP leaf turgor potential, PhA phloem area, ECA epidermal cell area, StA stem area, MsA mesophyll area, LTh lamina thickness, VBT vascular bundle thickness, MtA metaxylem area and CRT cortical region thickness
The generalized linear model expressed differential response of Fimbristylis rigidula regarding saline conditions (Fig. 7). Chl a and E showed a linear response, while all other traits (Chl b, WUE and A) showed a strong negative curve (Fig. 8a). Organic osmolytes, shoot ions and water relations responded positively except for SP (Fig. 8b). Among root traits, ECA significantly increased under moderate salinity conditions but after that showed a linear response indicating no change. Root VBT and MtA showed a positive response to counterpart traits (Fig. 8c). Stem StA and CRT remained stable, while all other traits showed a strong positive response with increasing salinity gradients (Fig. 8d). In leaf, a strong positive response was noticed for MtA and ECA. In comparison LTh and VBT exhibited a negative increase. Leaf MsA showed a linear trend in response to increasing salt levels (Fig. 8e).
Generalized linear model showing responses of (a) chlorophyll and gas exchange traits, (b) water relation, organic osmolytes and shoot ions, (c) root anatomical characters, (d) stem anatomical characters, and (e) leaf anatomical characters of Fimbristylis rigidula plotted against increasing salinity levels (from 10 dS m−1 at left to 60 dS m−1 at right). WUE water use efficiency, A net assimilation rate, Caro carotenoids, S-Ca2+ shoot calcium ions, LWP leaf water potential, S-K+ shoot K+, AA amino acids, TSS total soluble sugars, gs stomatal conductance, SP soluble proteins, E transpiration rate, Chl, a, b chlorophyll a, b, S-Na+ shoot Na+, LTP leaf turgor potential, PhA phloem area, ECA epidermal cell area, StA stem area, MsA mesophyll area, LTh lamina thickness, VBT vascular bundle thickness, MtA metaxylem area and CRT cortical region thickness
Discussion
Fimbristylis is a genus of halophytic sedges that mostly inhabit saline wetlands, salt marshes, poor nutrient soils, and polluted soils (Khan and Qaiser 2006; Zahoor et al. 2012). Fimbristylis complanata inhabits saline areas like swamps, ditches, river and canal banks, salt marshes and grasslands. Fimbristylis dichotoma is reported as a halophytic plant (Butt et al. 2018), and mostly found in waterlogged areas and moist saline-sodic soils. Fimbristylis rigidula is mostly found on canal banks and along moist roadsides (Zhang et al. 2010).
Photosynthetic pigments enhance photosynthetic efficiency of plants by capturing sunlight energy and are considered a potential indicator to monitor photosynthetic efficiency and chloroplast status under stressful environments (Cannella et al. 2016; Zhang et al. 2019). Halophytic or salt-tolerant genotypes when exposed to salinity stress, increase chlorophyll a & b content, which lead to the enhancement of photosynthetic efficiency (Sghaier et al. 2015; Percey et al. 2016). In the current work, Chl a and b pigments were significantly higher in JR populations collected from moderate salinities. Previous findings suggested higher chlorophyll contents as a biochemical indicator of plant stress tolerance under saline environments (Ashraf and Harris 2013; Stefanov et al. 2016). Chlorophyll pigments significantly decreased in all populations at high salinities. Such a reduction in chlorophyll severely affects photosynthesis, plant growth and development (Akcin and Yalcin 2016). Carotenoids content were significantly higher in HT population from low salinities but generally decreased at higher salinities. In earlier reports, decrease in carotenoids and chlorophyll contents has been linked to the degree of tolerance depending on plant species as well as to the duration of exposure to salt stress (Stefanov et al. 2018).
Salinity imposition ultimately causes a decline in gas exchange parameters that is generally more prominent in salt-susceptible genotypes (Azizian et al. 2015). Leaf gas exchange traits like A, gs and E of salt-sensitive species were strongly inhibited by salinity stress in earlier reports (Reddy et al. 2015). In contrast, few reports are available on improvement of gas exchange characteristics under salinity stress in halophytic species like Poa pratensis (Vaziriyeganeh et al. 2018). Reduction in the rate of transpiration (E) increases the WUE of plants which is considered an imperative strategy for water conservation under salinity induced osmotic stress (Rozentsvet et al.2018). HT populations of all Fimbristylis spp. collected from the least saline habitats showed a significant decline in gas exchange traits. JR populations from moderate salinities showed increased gas exchange parameters as previously been reported in a salt-tolerant plants like Salicornia perennans (Rozentsvet et al. 2018). The WUE was the maximum in SH population of F. complanata and F. dichotoma from high salinities and HT population of F. rigidula from low salinities. Such enhanced WUE in these populations clearly indicated their better photosynthetic efficiency by effectively controlling transpirational losses. Ultimately, enhanced WUE in these populations can be marked as a potential indicator of salinity tolerance in these populations better adapted to hyper-saline areas (Kaleem and Hameed, 2021).
Plant water relation traits, i.e. water potential (Ψw) and solute potential (Ψs) became more negative in the highest saline SH populations, while the least saline population HT showed higher turgor potential (Ψp) in all Fimbristylis species. Salinity induced osmotic stress and an increase in Ψw and Ψs contributes to osmotic adjustments (Negrão et al. 2017). The negative change in a water potential and solute potential is direct consequence of more accumulation of shoot Na+ and soluble sugars (Kumar et al. 2016), as recorded in present findings. Salinity caused a reduction in leaf turgor potential along elevated salinity gradients, as previously documented in Fimbristylis complanata in response to increasing salt regimes (Kaleem and Hameed, 2021).
Plants quickly respond to salinity/alkalinity and accumulate the osmotically active compounds to stabilize the integrity of integral membrane proteins, metabolic complexes, scavenge ROS and regulate cellular osmotic adjustments (Slama et al. 2015; Muchate et al. 2016; Cheng et al. 2018; Wang et al. 2020). Tolerant species and ecotypes accumulate higher amounts of compatible solutes for osmotic adjustments that is directly linked to higher degree of salinity/alkalinity tolerance (Muchate et al.2016; Cheng et al. 2020). Compatible solutes protect the tissues from collapsing under water deficit environments due to higher salinity (Radhakrishnan and Baek, 2017; Escalante-Magana et al. 2019). Level of compatible solutes was the maximum in more saline populations of all Fimbristylis spp. collected from SH habitat. Halophytic plants accumulate plenty of compatible solutes to lower the amount of ROS, maintain structure of integral proteins, and take part actively in membrane stability (Muchate et al. 2016). These compatible osmolytes protect the cell against the chaotropic effect of toxic ions by maintain the cellular osmoticum (Rangani et al. 2016). Higher accumulation of total soluble proteins was assessed among all populations of Fimbristylis, this increase helps to maintain cell turgor and water status as well as improve the activity of transmembrane proteins for ion fluxes (Osakabe et al. 2014; Singh et al. 2015).
Higher accumulation of Na+ and Cl− ions cause morpho-physiological disruptions in plants (Flowers et al. 2015). Ionic imbalance is an immediate response of plants exposed to salinity and is brought about by a higher accumulation of Na+ that interferes with homeostasis of K+, Ca2+ and other cations (Assaha et al. 2017). Halophytic plants better adapt to saline environments by ion sequestration and accumulation in the vacuoles for efficient osmotic adjustments to maintain photosynthetic traits and other metabolic processes (Muchate et al. 2016). Therefore, salt tolerance relies on the ionic homeostasis of Na+ and K+ through the vacuole (Jiang et al.2017) by restricted uptake of Na+ and higher accumulation of K+. Some halophytic species are even reported to utilize excess amount of Na+ in metabolic functions (Shrivastava and Kumar, 2015; Ferchichi et al. 2018). Fimbristylis is a stem succulent. Population from the highest saline habitat showed high concentration of Na+ in shoots that appeared as the possible strategy to either store excess Na+ in vacuole or utilize it in metabolic functions. Potassium (K+) is one of the essential nutrients for many metabolic processes in plants (Kaburagi et al. 2014; Shabala and Pottosin 2014; Shabala 2017). The SH population showed more accumulation of K+ in shoot for osmotic adjustment as was previously been reported in F. dichotoma by some authors (Zahoor et al. 2012). As observed in this tudy, the SH Populations of all Fimbristylis spp. from highly saline habitats accumulated excessive amount of Ca2+ and K+ along with high Na+ concentration for their survival in saline environments. Such higher accumulation of Ca+ and K+ neutralizes the adverse effects of salinity stress and is involved in pH homeostasis as well as in maintaining cell osmotic potential (Nieves-Cordones et al. 2016; Rahman et al. 2016).
Anatomical modifications are commonly more sensitive to environmental fluctuations, and hence show a strong response to biotic and abiotic constraints (Naskar and Palit 2015). Most commonly altered anatomical characteristics under salinity stress include thickened lower and upper epidermis, parenchyma, and mesophyll cells (dos Santos Moura et al. 2019). In roots, an increase in epidermis cell area is considered as a critical check for evaporative loss of water and enhanced water storage capacity under salinity induced osmotic stress (Akcin et al. 2015). Increased root cell area and cortical thickness are effective mechanisms to impede salinity induced osmotic stresses and enhance water storage capacity (Naz et al. 2016). In current study, epidermal cell area, cortical cell area and cortical thickness were relatively higher in saline salt marsh SH populations of F. complanata and F. dichotoma. An increase in protective exodermal tissues in this case plays a key role under extreme salinities for better survival and alters pathways of ion transport in the halophyte populations (Byrt et al. 2018). An increase in vascular bundle thickness in roots is considered as a more striking feature for better conduction of water and solutes (Batool and Hameed 2013). The salt-tolerant species equipped with larger metaxylem vessels are vibrant for enhanced conduction of water (Naz et al. 2015; Naseer et al. 2017). Salt tolerant plants enhance phloem area for better distribution of photo-assimilates that contributes to better growth (Lemoine et al. 2013). In the present work, metaxylem and phloem area and vascular bundle thickness were higher in moderate saline populations of F. complanata, F. dichotoma, and saline salt marsh SH population of F. rigidula. Such an increase in conducting tissue (metaxylem and phloem) under saline condition in halophytic species contributes to better translocation of solutes and photosynthates across plant body in stressful environments to confer salinity tolerance (Jacobsen et al. 2005).
At stem level, population JR of all populations exposed to moderate salinities resulted in an increased stem radii in all Fimbristylis spp. Increase in stem radius generally occurs due to more storage parenchyma, that increase water storage capacity of the succulent stems (Corrêa et al. 2017). Large epidermal cell area in the stem of JR population of F. complanata and F. dichotoma, and SH population of F. rigidula is a preventive strategy against water loss from the exterior plant surface (Liu et al. 2015; Corrêa et al. 2017). The increase in the stem cortical region thickness as previously reported in Sporobolus ioclados and Lasiurus scindicus is crucial for better water storage and account to fitness in salt-induced osmotic stress (Naz et al. 2015, 2016), as observed in SH population of F. complanata. In present work, metaxylem area and thickened vascular bundels were observed in SH population of F. rigidula. The increase in metaxylem size and vascular bundle thickness is positively correlated with conduction efficiency (Smith et al. 2013), as well as improved photosynthetic efficacy of plants (Akhtar et al. 2016). The increase in cortical region thickness occurred along the elevated salinity gradients among all species of Fimbristylis. Large cortical cells contain larger vacuoles to increase water storing capacity (Tahira et al. 2012), and this also facilitate ion sequestring by dumping off toxic ions (Rahat et al. 2019).
Leaf architecture is more responsive to environmental constraints and an important tool to evaluate the changes in stressful environments (Naskar and Palit, 2015). Increased leaf succulence in terms of leaf thickness (midrib and lamina) directly confers salinity tolerance and enhanced storage capacity of water (Naz et al. 2016). Therefore, it is a reliable source to monitor the leaf water status and hence of the entire plant body (Afzal et al. 2017). Leaf thickness of Fimbristylis species was higher in population JR from moderate salinities. Increase in the mesophyll area as previously reported by Naz et al. (2016) in Sporobolus ioclados, Naskar and Palit (2015) in mangroves and Naz et al. (2015) in Lasiurus scindicus ensures enhanced photosynthetic activity. Salt tolerant species generally possess thicker epidermal layer (Corrêa et al. 2017), because this layer directly anticipated with salinity tolerance and lower down water loss (Liu et al. 2015; Mansoor et al. 2019). The moderately saline JR populations of all Fimbristylis species exhibited thicker epidermal layer and large mesophyll area. Salt tolerant plants possess thickened epidermis to minimize water loss in arid climates (Akcin et al. 2015). Palisade spongy mesophylls directly anticipate photosynthesis due to large numbers of chloroplast (Paradiso et al. 2017).
Conclusion
In conclusion, all three populations of genus Fimbristylis accomplished various structural and functional modifications to thrive under saline conditions. All species of Fimbristylis exhibited a reduction in plant biomass, plant height, leaf area and numbers of leaves along the elevated salt gradients. This reduction was identified as an adaptive strategy to reduce the energy expenditures in above-ground parts under salt extremities and to direct more energy to underground roots. In Fimbristylis complanata and Fimbristylis dichotoma, population SH exhibited a higher water use efficiency (WUE) and stomatal conductance, but reduced transpiration rate was observed that was helpful to in osmotic homeostasis under salinity stress. This population also showed more accumulation of soluble sugars, proteins and amino acids, K+ and Ca2+, organic osmolytes that played a protective role by counteracting chaotropic effects of high Na+. The SH population in both species sequestered Na+ in stem that indicated a strong phytoremediation potential. This population had wider metaxylem for better transport of water and enlarged phloem for translocation of photoassimilates. In SH population of Fimbristylis rigidula, more accumulation of carotenoids, higher organic osmolytes, and thickened epidermis was observed. In moderately saline JR population, increased stem radius, leaf succulence (midrib and lamina thickness), mesophyll area, stem cortical area and vascular bundle area was noted. The JR population F. rigidula had higher concentrations of Chl a and Chl b that seemed to be a direct association with photosynthetic efficiency as recorded in this population. In conclusion, these populations of Fimbristylis reflect a greater potential to rehabilitate the salt effect soils and wetlands and can also be used for effectively remediate saline soils.
Data availability
Authors confirm that all relevant data are included in the article and would be ready to share the raw data upon request.
Code availability
R was used for all analyses. This is a free open source software. https://www.r-project.org/.
References
Afzal A, Duiker SW, Watson JE (2017) Leaf thickness to predict plant water status. Biosyst Eng 156:148–156. https://doi.org/10.1016/j.biosystemseng.2017.01.011
Akcin A, Yalcin E (2016) Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata Pall. and Suaeda prostrata Pall. subsp. prostrata (Amaranthaceae). Brazil J Bot 39:101–106. https://doi.org/10.1007/s40415-015-0218-y
Akcin TA, Akcin A, Yalcin E (2015) Anatomical adaptations to salinity in Spergularia marina (Caryophyllaceae) from Turkey. Proc Natl Acad Sci India B 85:625–634. https://doi.org/10.1007/s40011-014-0386-8
Akhtar N, Hameed M, Ahmad R (2016) Structural and functional aspects of ionic relation in roots of Typha domingensis pers. ecotypes under salt stress. Pak J Bot 48:2195–2203
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta Vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190. https://doi.org/10.1007/s11099-013-0021-6
Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509. https://doi.org/10.3389/fphys.2017.00509
Azizian A, Sepaskhah AR, Zand-Parsa S (2015) Modification of a maize simulation model under different water, nitrogen and salinity levels. Int J Plant Prod 9:609–632
Badri MA, Hamed AI (2000) Nutrient value of plants in an extremely arid environment (Wadi Allaqi biosphere reserve, Egypt). J Arid Environ 44:347–356
Banerjee S, Kar D, Banerjee A, Palit D (2012) Utilization of some aquatic macrophytes in Borobandh-a lentic water body in Durgapur, West Bengal, India: Implications for socio-economic upliftment of local stakeholder. Indian J Appl Pure Biol 27:83–92
Batool R, Hameed M (2013) Root structural modifications in three Schoenoplectus (Reichenb.) Palla species for salt tolerance. Pak J Bot 45:1969–1974
Butt MA, Zafar M, Ahmad M, Sultana S, Ullah F, Jan G, Irfan A, Naqvi SAZ (2018) Morpho-palynological study of cyperaceae from wetlands of Azad Jammu and Kashmir using SEM and LM. Microsc Res Tech 81:458–468. https://doi.org/10.1002/jemt.22999
Byrt CS, Munns R, Burton RA, Gilliham M, Wege S (2018) Root cell wall solutions for crop plants in saline soils. Plant Sci 269:47–55. https://doi.org/10.1016/j.plantsci.2017.12.012
Cannella D, Möllers KB, Frigaard NU, Jensen PE, Bjerrum MJ, Johanse KS, Felby C (2016) Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme. Nat Commun 7:1–8. https://doi.org/10.1038/ncomms11134
Cheng B, Li Z, Liang L, Cao Y, Zeng W, Zhang X, Ma X, Huang L, Nie G, Liu W, Peng Y (2018) The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int J Mol Sci 19:2520
Cheng R, Zhu H, Cheng X, Shutes B, Yan B (2020) Saline and Alkaline tolerance of wetland plants—what are the most representative evaluation indicators? Sustainability 12:1913
Corrêa FF, Pereira MP, Madail RH, Santos BR, Barbosa S, Castro EM, Pereira FJ (2017) Anatomical traits related to stress in high density populations of Typha angustifolia L. (Typhaceae). Braz J Biol 77:52–59. https://doi.org/10.1590/1519-6984.09715
Davis BH (1976) Carotenoids. In: Goodwin TW (ed) Chemistry and biochemistry of plant pigments, 2nd edn. Academic Press Inc, London, pp 38–165
Dolatabadian A, Sanavy SAMM, Ghanati F (2011) Effect of salinity on growth, xylem structure and anatomical characteristics of soybean. Not Sci Biol 3:41–45. https://doi.org/10.15835/nsb315627
dos Santos MR, Soares TL, Lima LKS, Gheyi HR, Jesus ON, Coelho Filho MA (2019) Salinity-induced changes in biometric, physiological and anatomical parameters of Passiflora edulis Sims plants propagated by different methods. Arch Agron Soil Sci 5:1–5. https://doi.org/10.1080/03650340.2019.1688789
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168:167. https://doi.org/10.1038/168167a0
Escalante-Magana C, Aguilar-Caamal LF, Echevarría-Machado I, Medina-Lara F, Cach LS, Martínez-Estévez M (2019) Contribution of glycine betaine and proline to water deficit tolerance in pepper plants. HortScience 54:1044–1054. https://doi.org/10.21273/HORTSCI13955-19
Ferchichi S, Hessini K, Dell’Aversana E, D’Amelia L, Woodrow P, Ciarmiello LF, Fuggi A, Carillo P (2018) Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct Plant Biol 45:1096–1109. https://doi.org/10.1071/FP18046
Flowers TJ, Munns R, Colmer TD (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot 115:419–431. https://doi.org/10.1093/aob/mcu217
Hameed M, Ashraf M, Naz N (2011) Anatomical and physiological characteristics relating to ionic relations in some salt tolerant grasses from the salt range. Pakistan Acta Physiol Plant 33:1399–1409. https://doi.org/10.1007/s11738-010-0674-8
Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, Davis SD (2005) Do xylem fibers affect vessel cavitation resistance? Plant Physiol 139:546–556
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Kaburagi E, Morikawa Y, Yamada M, Fujiyama H (2014) Sodium enhances nitrate uptake in Swiss chard (Beta vulgaris var. cicla L.). Soil Sci Plant Nutr 60:651–658
Kaleem M, Hameed M (2021) Functional traits for salinity tolerance in differently adapted populations of Fimbristylis complanata (Retz.). Int J Phytoremed. https://doi.org/10.1080/15226514.2021.1895718
Kausar A, Ashraf MY, Ali I, Niaz M, Abbass Q (2012) Evaluation of sorghum varieties/lines for salt tolerance using physiological indices as screening tool. Pak J Bot 44:47–52
Khan MA, Qaiser M (2006) Halophytes of Pakistan: characteristics, distribution and potential economic usages. Sabkha Ecosystems. Springer, Dordrecht, pp 129–153. https://doi.org/10.1007/978-1-4020-5072-5_11
Kumar A, Kumar A, Lata C, Kumar S (2016) Eco-physiological responses of Aeluropus lagopoides (grass halophyte) and Suaeda nudiflora (non-grass halophyte) under individual and interactive sodic and salt stress. S Afr J Bot 105:36–44
Kumari R, Kumar P, Meghawal D, Sharma V, Kumar H (2019) Salt-tolerance mechanisms in plants. Recent trends in tropical plant research. AkiNik Publications, New Delhi
Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos-Thévenot P, Maurousset L (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4:272. https://doi.org/10.3389/fpls.2013.00272
Liu Y, Li X, Chen G, Li M, Liu M, Liu D (2015) Epidermal micromorphology and mesophyll structure of Populus euphratica heteromorphic leaves at different development stages. PLoS ONE 10:e0137701. https://doi.org/10.1371/journal.pone.0137701
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mansoor U, Fatima S, Hameed M, Naseer M, Ahmad MSA, Ashraf M, Ahmad F, Waseem M (2019) Structural modifications for drought tolerance in stem and leaves of Cenchrus ciliaris L. ecotypes from the Cholistan Desert. Flora 261:151485. https://doi.org/10.1016/j.flora.2019.151485
Mohammed AS, Kapri A, Goel R (2011) Heavy metal pollution: source, impact, and remedies. Biomanagement of metal-contaminated soils. Springer, Dordrecht, pp 1–28
Moore S, Stein WH (1948) Photometric nin-hydrin method for use in the ehromatography of amino acids. J Biol Chem 176:367–388
Muchate NS, Nikalje GC, Rajurkar NS, Suprasanna P, Nikam TD (2016) Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot Rev 82:371–406. https://doi.org/10.1007/s12229-016-9173-y
Naseer M, Hameed M, Zahoor A, Ahmad F, Fatima S, Ahmad MSA, Ahmad KS, Iftikhar M (2017) Photosynthetic response in buttonwood (Conocarpus erectus L.) to salt stress. Pak J Bot 49:847–856
Naskar S, Palit PK (2015) Anatomical and physiological adaptations of mangroves. Wetl Ecol Manag 23:357–370. https://doi.org/10.1007/s11273-014-9385-z
Naz N, Batool R, Fatima S, Hameed M, Ashraf M, Ahmad F, Ahmad MSA (2015) Adaptive components of tolerance to salinity in a saline desert grass Lasiurus scindicus Henrard. Ecol Res 30:429–438. https://doi.org/10.1007/s11284-014-1236-0
Naz N, Fatima S, Hameed M, Naseer M, Batool R, Ashraf M, Ahmad F, Ahmad MSA, Zahoor A, Ahmad KS (2016) Adaptations for salinity tolerance in Sporobolus ioclados (Nees ex Trin.) Nees from saline desert. Flora 223:46–55. https://doi.org/10.1016/j.flora.2016.04.013
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11. https://doi.org/10.1093/aob/mcw191
Nieves-Cordones M, Al Shiblawi FR, Sentenac H (2016) Roles and transport of sodium and potassium in plants. The alkali metal ions: their role for life. Springer, Cham, pp 291–324. https://doi.org/10.1007/978-3-319-21756-7_9
Osakabe Y, Osakabe K, Shinozaki K, Tran L-SP (2014) Response of plants to water stress. Front Plant Sci 5:86. https://doi.org/10.3389/fpls.2014.00086
Paradiso R, Arena C, De Micco V, Giordano M, Aronne G, De Pascale S (2017) Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front Plant Sci 8:674. https://doi.org/10.3389/fpls.2017.00674
Percey WJ, Shabala L, Wu Q, Su N, Breadmore MC, Guijt RM, Bose J, Shabala S (2016) Potassium retention in leaf mesophyll as an element of salinity tissue tolerance in halophytes. Plant Physiol Biochem 109:346–354. https://doi.org/10.1016/j.plaphy.2016.10.011
R Core Team (2019) R foundation for statistical computing. R: a language and environment for statistical computing. R Core Team, Vienna
Radhakrishnan R, Baek KH (2017) Physiological and biochemical perspectives of non-salt tolerant plants during bacterial interaction against soil salinity. Plant Physiol Biochem 116:116–126. https://doi.org/10.1016/j.plaphy.2017.05.009
Rahat QUA, Hameed M, Ahmad MSA (2019) Contribution of root structural and functional features towards salinity tolerance in Diplachne fusca (L.) P. Beauv. ex Roem. & Schult. subsp. fusca. Pak J Bot 51:773–779
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci 7:609. https://doi.org/10.3389/fpls.2016.00609
Rangani J, Parida AK, Panda A, Kumari A (2016) Coordinated changes in antioxidative enzymes protect the photosynthetic machinery from salinity induced oxidative damage and confer salt tolerance in an extreme halophyte Salvadora persica L. Front Plant Sci 7:50. https://doi.org/10.3389/fpls.2016.00050
Reddy PS, Jogeswar G, Rasineni GK, Maheswari M, Reddy AR, Varshney RK, Kishor PBK (2015) Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol Biochem 94:104–113. https://doi.org/10.1016/j.plaphy.2015.05.014
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26:115–124. https://doi.org/10.1016/j.copbio.2013.12.004
Roy R, Daula ASU, Akter A, Sultana S, Barek MA, Liya IJ, Basher MA (2019) Antipyretic and anti-nociceptive effects of methanol extract of leaves of Fimbristylis miliacea in mice model. J Ethnopharmacol 243:112080. https://doi.org/10.1016/j.jep.2019.112080
Rozentsvet O, Nesterov V, Bogdanova E, Kosobryukhov A, Subova S, Semenova G (2018) Structural and molecular strategy of photosynthetic apparatus organisation of wild flora halophytes. Plant Physiol Biochem 129:213–220. https://doi.org/10.1016/j.plaphy.2018.06.006
Ruzin SE (1999) Plant microtechnique and microscopy, vol 198. Oxford University Press, New York
Sghaier DB, Duarte B, Bankaji I, Caçador I, Sleimi N (2015) Growth, chlorophyll fluorescence and mineral nutrition in the halophyte Tamarix gallica cultivated in combined stress conditions: arsenic and NaCl. J Photochem Photobio B Biol 149:204–214. https://doi.org/10.1016/j.jphotobiol.2015.06.003
Shabala S (2017) Signalling by potassium: another second messenger to add to the list? J Exp Bot 68:4003–4007
Shabala S, Pottosin I (2014) Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant 151:257–279
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Sims JR, Haby VA (1971) Simplified colorimetric determination of soil organic matter. Soil Sci 112:137–141. https://doi.org/10.1097/00010694-197108000-00007
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotechnol 14:407–426
Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:433–447. https://doi.org/10.1093/aob/mcu239
Smith MS, Fridley JD, Yin J, Bauerle TL (2013) Contrasting xylem vessel constraints on hydraulic conductivity between native and non-native woody understory species. Front Plant Sci 4:486. https://doi.org/10.3389/fpls.2013.00486
Stefanov M, Yotsova E, Rashkov G, Ivanova K, Markovska Y, Apostolova EL (2016) Effects of salinity on the photosynthetic apparatus of two Paulownia lines. Plant Physiol Biochem 101:54–59. https://doi.org/10.1016/j.plaphy.2016.01.017
Stefanov M, Yotsova E, Markovska Y, Apostolova EL (2018) Effect of high light intensity on the photosynthetic apparatus of two hybrid lines of Paulownia grown on soils with different salinity. Photosynthetica 56:832–840. https://doi.org/10.1007/s11099-017-0735-y
Tahira N, Mansoor H, Ahmad MSA, Adnan Y, Hina K (2012) Comparative anatomy of root and stem of some native and exotic Asparagus L. species. Pak J Bot 44:153–158
U.S. Salinity Laboratory Staff (1954) Diagnosis and improvement of saline and alkali soils. USDA agricultural handbook No 60. USDA, Washington DC, p 160
Vaiga M, Joseph S (2016) Identification of mangrove and mangrove associates in Kannur district of Kerala including their economic–ecological linkages. Int J Bot Stud 1:22–31
Vaziriyeganeh M, Lee SH, Zwiazek JJ (2018) Water transport properties of root cells contribute to salt tolerance in halophytic grasses Poa juncifolia and Puccinellia nuttalliana. Plant Sci 276:54–62
Wang X, Cheng R, Zhu H, Cheng X, Shutes B, Yan B (2020) Seed germination and early seedling growth of six wetland plant species in saline-alkaline environment. Int J Phytoremed 22:1185–1194
Wolf B (1982) An improved universal extracting solution and its use for diagnosing soil fertility. Commun Soil Sci Plant Anal 13:1005–1033. https://doi.org/10.1080/00103628209367331
Yamamoto K, Hashimoto Y, Kang J, Kobayashi K (2018) Speciation of phosphorus zinc and copper in soil and water-dispersible colloid affected by a long-term application of swine manure compost. Environ Sci Technol 52:13270–13278
Yoshida S, Forno DA, Cock JH (1971) Laboratory manual for physiological studies of rice. IRRI, Los Banos
Zahoor I, Ahmad MSA, Hameed M, Nawaz T, Tarteel A (2012) Comparative salinity tolerance of Fimbristylis dichotoma (L.) Vahl and Schoenoplectus juncoides (Roxb.) Palla, the candidate sedges for rehabilitation of saline wetlands. Pak J Bot 44:1–6
Zhang S, Songyun L, Koyama T and Simpson DA (2010) Fimbristylis Vahl, In: Cyperaceae. Flora of China. 23, 200–218
Zhang Z, He K, Zhang T, Tang D, Li R, Jia S (2019) Physiological responses of Goji berry (Lycium barbarum L.) to saline-alkaline soil from Qinghai region. China Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-48514-5
Zörb C, Geilfus CM, Dietz KJ (2019) Salinity and crop yield. Plant Biol 21:31–38. https://doi.org/10.1111/plb.12884
Funding
The project is not funded by any organization.
Author information
Authors and Affiliations
Contributions
This work is part of a Ph.D. dissertation of MK under the supervision of MH.
Corresponding author
Ethics declarations
Conflict of interest
Authors declared that they have no conflict of interest/competing interest.
Ethical approval
The matter given in this manuscript is in compliance with ethical standards. The research work given in this paper does not involve human participants or animals.
Consent to participate
The consent to participate in this study was obtained by the authors from the authorities concerned.
Consent to publish
We consent to have this research published in this journal.
Research involving animal participants
Animals are not involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaleem, M., Hameed, M. Structural and functional modifications in Fimbristylis Vahl for ecological fitness in hyper-saline wetlands. Wetlands Ecol Manage 29, 843–865 (2021). https://doi.org/10.1007/s11273-021-09814-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-021-09814-8