Abstract
Atrazine degradation in soil microbial fuel cells (MFCs) under different anode depths and initial concentrations is investigated for different redox soil conditions, and the microbial communities in the anode and different layers are evaluated. Atrazine degradation is fastest in the upper layer (aerobiotic), followed by the lower layer (anaerobic). A removal efficiency and a half-life of 91.69% and 40 days, respectively, are reported for an anode depth of 4 cm. The degradation rate is found to be dependent on current generation in the soil MFCs rather than on electrode spacing. Furthermore, the degradation rate is inhibited when the initial atrazine concentration is increased from 100 to 750 mg/kg. Meanwhile, the exoelectrogenic bacteria, Deltaproteobacteria and Geobacter, are enriched on the anode and the lower layer in the soil MFCs, while atrazine-degrading Pseudomonas is only observed in very low proportions. In particular, the relative abundances of Deltaproteobacteria and Geobacter are higher for lower initial atrazine concentrations. These results demonstrate that the mechanism of atrazine degradation in soil MFCs is dependent on bioelectrochemistry rather than on microbial degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atrazine is one of the most widely used herbicides (Chan-Cupul et al. 2016) and is a priority control herbicide in China. Atrazine contamination is ubiquitous in aquatic and soil environments (Chung et al. 1995a; Delwiche et al. 2014). Atrazine is considered as an endocrine disruptor and is potentially carcinogenic (Wilhelms et al. 2006). There are some significantly negative impacts on plant photosynthesis (Elsheekh et al. 1994), reproductive growth of batrachian (Coady et al. 2005), and even human breast cancer (Wüst and Hock 1992). In general, atrazine has low spontaneous biodegradability (Kumar and Singh 2016), and increasing concern about atrazine pollution has led to a number of research studies. Literature reports give a half-life (t 1/2) of atrazine in a range from 5 to 731 days (Accinelli et al. 2001; Vanderheyden et al. 1997), and atrazine degradation has been found to depend on soil redox conditions (Chung et al. 1995b; Ro and Chung 1995), depth (Accinelli et al. 2001), nutrient conditions (Chung et al. 1995b), and soil microbial communities (Chan-Cupul et al. 2016).
Atrazine degradation in soil progresses through a number of microbial and chemical processes (Assaf and Turco 2010; Blumhorst and Weber 2010). The major biotransformation pathways of atrazine are dechlorination, dealkylation, and deamination (Kumar and Singh 2016), and the corresponding degradation products are hydroxyatrazine, deethylatrazine, and deisopropylatrazine (Sparling et al. 1998). However, low moisture and soil water potential have been considered as the limited factors influencing microbial activity and then inhibiting atrazine degradation (Bastos and Magan 2009; Chan-Cupul et al. 2016). Neutral pH seems to be beneficial to microbe growth and to realize maximum atrazine degradation, while acidic pH inhibits atrazine degradation (Chan-Cupul et al. 2016; Kumar and Singh 2016). Furthermore, degradation tendencies differ significantly between surface and subsurface soils; atrazine degradation progresses at a much slower rate and over a longer time in subsurface soil than in surface soil (Accinelli et al. 2001; Vanderheyden et al. 1997). Therefore, acceleration of atrazine degradation in subsurface soil is a crucial topic for further study.
Soil microbial fuel cells (MFCs) are a novel method of degrading organic pollutants (Hamdan et al. 2017; Huang et al. 2011; Wang et al. 2012), but few studies have focused on atrazine degradation in subsoil using soil MFCs. External resistance, electrode spacing, substrate type, etc., can influence the performance of MFCs (An et al. 2013; Chae et al. 2009; Rismani-Yazdi et al. 2011), and in particular, embedding the anode at depths where O2, NO3 −, and NO2 − exist causes a partial loss of organics by microorganisms that prefer O2, NO3 −, and NO2 − as thermodynamically more favorable electron acceptors in the sediment (Dichristina 1992; Nealson and Myers 1992), resulting in a decline in anode performance. Therefore, to enhance anode performance, embedding the anode at an optimal depth where anodophiles or electrophiles like metal- or sulfate-reducing bacteria can be activated may be a much more practical method (An et al. 2013). However, to date, no typical soil depths at which these electron acceptors do not influence anode performance have been identified, perhaps because the concentrations of electron acceptors vary from sediment to sediment (Dichristina 1992; Nealson and Myers 1992). In contrast, pesticide concentration affects the degradation rate, particularly through microbial routes (Buitrón and Moreno-Andrade 2014). Research on the effects of atrazine concentrations on microbial communities is limited and has mostly focused on contaminated soil; much less is known about simultaneous aerobic and anaerobic degradation of atrazine in soil MFCs.
Abundant microbial community and soil organic matter make it feasible to construct soil MFC system. The soil MFC system has expanded an economical and highly efficient way to soil remediation while it does not need extra electric energy. This study evaluated the performance of soil MFCs with the anode embedded at different depths and considered the effects of different atrazine concentrations in the soil. Atrazine degradation was investigated under different soil redox conditions including anaerobic (the bottom of soil MFCs, near the anode), aerobiotic (the top of soil MFCs, near the cathode), and anoxic (the middle of soil MFCs) conditions, and the microbial communities involved in atrazine biodegradation and bioelectricity production were analyzed using 16S ribosomal RNA (rRNA) gene pyrosequencing. The effects of atrazine concentration on degradation were also investigated.
2 Materials and Methods
2.1 Soil MFC Construction and Operation
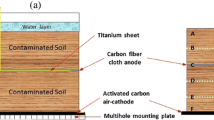
The soil MFCs were inoculated with atrazine; a schematic diagram was provided in a previous study (Wang et al. 2016; Wang et al. 2017). The soil was mixed with atrazine (purity >97%, Energy Chemicals, China) in a laboratory and stirred thoroughly to create a homogeneous mixture (Chan-Cupul et al. 2016). The spiked soil sample was transferred to the soil MFC device and adjusted to 100% of water holding capacity with an excess of 1 mL of distilled water. After application of atrazine, the soil MFCs were incubated at 30 °C in the dark.
To investigate the influence of anode embedding depth on the degradation of atrazine, the experiment was conducted under the following treatment conditions: 4 cm (D1), 6 cm (D2), 8 cm (D3), 10 cm (D4), 12 cm (D5), and 4 cm soil +1 cm water (D1’). The electrode spacing (4 cm) between D1 and D1’ was identical, and all MFCs other than D1’ (D1–D5) included an air cathode. An external resistance of 300 Ω was connected to the anode and cathode. The atrazine theoretical concentration in the soil MFCs was 100 mg/kg.
In another experiment, to evaluate the effect of atrazine concentration on degradation, four sets of duplicate soil MFCs were prepared. The concentrations were 100 mg/kg (D4), 250 mg/kg (C2), 500 mg/kg (C3), and 750 mg/kg (C4) and were operated under a closed circuit condition. The external resistance was held constant at 300 Ω and the electrode spacing was 10 cm.
To investigate atrazine degradation under different soil redox conditions, all the soil MFCs were divided into two or three parts, classified as upper, middle, and lower. Samples were taken and atrazine degradation evaluated every 7 days. The soil samples were freeze dried, sieved, and stored at 4 °C until extraction.
2.2 Microbial Community Analysis
At the end of the experiment, the microbial communities in the anodes were analyzed using 16S rRNA gene pyrosequencing. Microbial DNA was extracted from anode samples using the E.Z.N.A. Stool DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s protocols. The DNA extraction was verified using agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions and quantified using QuantiFluor-ST (Promega, USA). Triplicate PCR reactions were performed using a 20-μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase, and 10 ng of template DNA. Two bacteria primer sets were used: 515F (5′-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′), where the barcode is an eight-base sequence unique to each sample. These primers targeted the V4–V5 region of the bacterial 16S rRNA gene. PCR amplifications were performed using the following program: initial denaturation at 95 °C for 5 min; 27 cycles of denaturation at 94 °C for 30 s; annealing at 55 °C for 30 s and 72 °C for 45 s; and a final extension at 72 °C for 5 min (Song et al. 2017). Purified amplicons were sequenced (2 × 250) using the Illumina platform according to the standard protocols. The identification of the obtained sequences was deposited in the NCBI Sequence Read Archive (SRA) database (Accession Number: SRP041836).
Raw reads were assembled from the final sequences after trimming the adaptors, primers, and low-quality sequence reads. The overlapping criteria were overlapping region lengths larger than 10 bp and a mismatch ratio lower than 0.2. Sequences were clustered into operational taxonomic units (OTUs) with a 97% sequence identity. OTUs with relative abundance values >0.001% (above three tags in at least one sample) were retained (Song et al. 2017).
2.3 Measurements and Calculations
Atrazine was extracted from soil (1 g) by adding 5 mL acetone, 5 mL n-hexane (pesticide analysis grade), and 0.5 g anhydrous sodium sulfate, shaken for 24 h in the dark at room temperature, and then ultrasonificated for 30 min. The tubes were centrifuged for 3 min (6000 rpm) at 4 °C, and the supernatants then further filtered through a 0.45-μm ultrafiltration membrane (Generay Biotech Co., Ltd., Shanghai). The extracts were transferred to a 10-mL graduated test tube with a stopper, nitrogen blown until nearly dried, and diluted with hexane to 1 mL for analysis.
The atrazine concentration was measured using a gas chromatograph–mass spectrometer (GC-MS) (Thermo Fisher Scientific Co., Ltd., USA) with a DB-5 quartz capillary column (30 mm × 0.25 mm × 0.25 μm). The carrier gas was high-purity helium with a flow rate of 1 mL/min. The injector and detector temperatures were 240 and 300 °C, respectively. The initial temperature was set at 40 °C and held for 3 min, then increased to 190 °C in steps of 30 °C/min, where it was maintained for 5 min before being increased again to 250 °C in steps of 30 °C/min, where it was maintained for the final 5 min. The injection volume was 1 μL in the split mode (10:1). The flow rates of carrier gas, hydrogen, and air were 1.0, 3.0, and 60.0 mL/min. The extraction efficiency of this method was 95–105%, and this value was taken into account in the final quantification.
The atrazine removal efficiency and the average degradation rate were calculated using the following equations:
where, A is the initial concentration and B is the residual concentration.
Degradation half-lives were calculated by linear regression of the natural logarithm of the percentage of herbicide remaining against time (Accinelli et al. 2001; Kumar and Singh 2016):
where, C 0 is the initial concentration of the pesticide (mg/kg), C is its concentration (mg/kg) at time t (days), and k obs is the rate constant of the reaction. The half-life (t 1/2) values were calculated from k obs using the following formula:
3 Results and Discussion
3.1 The Effects of Anode Depth on the Performance of Soil MFCs
3.1.1 Electricity Generation
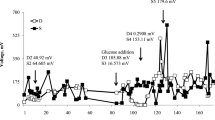
All MFCs were run closed for 63 days with a 300 Ω external resistance. The current was different for different anode depths and these were classified into three groups (Fig. 1). The current reached approximately 0.30 mA in the D3 and D4 MFCs, but the current decreased when the anode depth was shallower or deeper. The D1, D2, and D5 MFCs produced a 150% lower current than the D3 and D4 MFCs. Furthermore, when the cathode was embedded in 1 cm water, the current dropped sharply to 0.10 mA. One possible reason for this is that the cathodic reaction was inhibited when the cathode was embedded below the water layer, while the dissolved oxygen demand increased (Deng et al. 2014). These results indicate that the current was dependent on the anode depth; nevertheless, the current did not increase for deeper anodes. In this study, the optimal depths were 8–10 cm, where currents were higher than those in any other depths. In contrast, it has previously been reported that MFC currents increase with increasing depth (An et al. 2013).
Anode depth or electrode spacing is a key factor affecting the performance of MFCs. The internal resistance of MFCs, especially the ohmic resistance, is greatly dependent on electrode spacing (Fang et al. 2016). However, with increasing distance between the anode and cathode, oxygen from the cathode cannot diffuse to the anode, which could enhance the anodic reaction (Sajana et al. 2014). When the anode is embedded deeper, more electrons and a better negative anode potential are obtained, and the redox conditions are more favorable to anodophiles and electrophiles both thermodynamically and kinetically (An et al. 2013). This might explain the finding that the currents ranged from 0.20–0.30 mA when the anode depths were 8–10 cm. Interestingly, when the electrode spacing was increased to 12 cm, the current decreased and was close to that in D1 and D2. In general, the internal resistance increased with increasing electrode spacing. Actually, in this study, the ohmic resistance was 94, 216, 387, 489, and 568 Ω when the anode depth ranged from 4 to 12 cm, while the ohmic resistance was 217 Ω with the cathode was embedded in 1 cm water (D1’). Therefore, the internal resistance has a great effect on the performance of MFCs (Hong et al. 2009; Sajana et al. 2014).
3.1.2 Atrazine Degradation
Figure 2 presents the effects of anode depth on atrazine degradation. Atrazine was significantly degraded by 17.38 mg/kg during the first 7 days in the D1 MFC. At the end of the experiment, the atrazine concentration was 7.87 mg/kg in D1, lower than that in any other MFC. The concentration differences were closely related to anode depth: D1’, D2, and D5 concentrations were approximately 27 mg/kg, while D3 and D4 concentrations ranged from 14.27–15.66 mg/kg (Fig. 2). These results are in agreement with those of previous studies suggesting that atrazine degradation follows first order reaction kinetics under different redox conditions (Table 1) (Accinelli et al. 2001; Kumar and Singh 2016). When loading a constant external resistance, the atrazine removal efficiency, degradation rate, and half-life depended on the anode depth (Table 1). These indexes were classified into three groups. The highest removal efficiency was 91.6% in D1 MFC, followed by 84% in D3 and D4 MFCs; the lowest was approximately 72% in D1’, D2, and D5 MFCs, with corresponding degradation rates of 1.38, 1.25, and 1.07 mg/(kg day). The half-life was also calculated to further clarify the degradability. The half-life was shortened from 53 days in the D3 and D4 MFCs to 40 days in the D1 MFC, while it was prolonged by 39–51 d in the D1’, D2, and D5 MFCs (Table 1).
To be precise, the atrazine degradation depended on the current generated by the soil MFCs. The electrons could be directly used to degrade organic pollutants, and the anode became the major terminal electron acceptor (TEA), which could enhance their biodegradation (Hamdan et al. 2017; Wang et al. 2017). In other words, in the closed circuit MFCs, the anode was a major sink of electrons for atrazine degradation. Indeed, the higher degradation rate under different anode depths was due to the effects of higher current, which has been confirmed in other studies (Cao et al. 2015; Wang et al. 2017). However, considerably higher removal efficiency was observed in the D1 MFC under a slightly lower current. This was most likely due to evaporation and oxygen. A previous study also reported that the atrazine biotransformation rate is extremely rapid in oxidizing soil (DeLaune et al. 1997). The lowest degradation rate observed in D1’ suggested that the water layer limited the oxygen content and led to a significantly reduced current.
To investigate atrazine degradation under different soil redox conditions, the removal efficiency, degradation rate, and half-life (t 1/2) were calculated, and these values are listed in Table 1. The highest removal rate was observed in the upper layer, followed by that in the lower layer, and the lowest removal rate was observed in the middle layer in all MFCs. Taking the D4 MFC as an example, these indexes were 90.55%, 1.36 mg/(kg day), and 42.57 days in the upper layer, significantly higher than those in the middle layer at 15.93% and 0.24 mg/(kg day) respectively, while the half-life was shorter by 31 days. The substrate (e.g., acetate, glucose) was oxidized to protons and electrons; furthermore, the atrazine obtained electrons and reacted through reductive dechlorination in the anode when the chlorine was replaced by a proton. As mentioned previously, the cathode is another TEA (Logan et al. 2006) and acts as an electron sink for atrazine degradation. Hence, it can be concluded that electrodes play an important TEA role in atrazine degradation in soil MFCs. Atrazine differs significantly from other pollutants (e.g. HCB, PAH), which have higher degradation rates under anodic or anaerobic conditions (Hamdan et al. 2017; Wang et al. 2017). This finding suggests that the characteristics of pollution degradation are dependent on substance properties and are affected by bioelectrochemistry conditions (e.g., soil redox conditions).
3.2 The Effects of Initial Concentration on the Performance of Soil MFCs
3.2.1 Electricity Generation
The current generated from soil MFCs declined significantly from 0.30 to 0.17 mA when the initial concentration increased from 100 to 750 mg/kg (Fig. 3). Soil MFCs were capable of degrading atrazine even for atrazine concentrations up to 750 mg/kg. However, a clear difference in current development was observed among different initial concentrations. Two or three sharp decreases were observed in the C2 to C4 MFCs, while the current was steady in the D4 MFC after a 14-day incubation (Fig. 1). This finding indicates that the acetate substrate was more rapidly depleted when the initial concentration increased. This might be due to the lag period that provides exoelectrogenic bacteria some time to adapt to high pollution concentrations (Fang et al. 2015; Sun et al. 2009). Fang et al. (2015) reported the existence of electron competition between pollution and the electrode under high pollution concentrations.
3.2.2 Atrazine Degradation
The effect of concentration on atrazine degradation was studied at initial concentrations from 100 to 750 mg/kg. Figure 4 shows a higher residual with higher initial concentrations. The atrazine concentrations were 63.04, 187.00, and 353.01 mg/kg for the C2–C4 MFCs, respectively. The removal efficiency in the C4 MFC was only 51.81% lower than in the C2 and C3 MFCs, by 22.35% and 10.07%. The corresponding half-life increased from 74 to 138 days, but the degradation rate was accelerated to 6.03 mg/(kg day), 4.8, 2.1, and 1.3 times greater than in the D4, C2, and C3 MFCs (Table 2).
Higher degradation rates were clearly observed in the upper layer, followed by those in the lower and middle layers, with initial concentrations ranging from 250 to 750 mg/kg. The removal efficiency and degradation rate were 82.29% and 3.19 mg/(kg day) in the upper layer, significantly higher than that in the middle layer (18.27%, 0.71 mg/(kg day)) and the half-life was shortened by 40 days in the upper layer when the initial concentration was 250 mg/kg (Table 2). However, the difference among these layers diminished when the initial concentration was increased up to 750 mg/kg. Indeed, no significant differences were observed between the upper and lower layers at this maximum concentration, with values of 1.63%, 0.19 mg/(kg day), and 5.79 days, respectively (Table 2).
It is clear that the initial concentration of atrazine significantly affected its degradation. In this study, the soil MFCs could not degrade atrazine rapidly, and complete degradation was not observed after 63 days. Atrazine residuals of over 40% were observed when the initial concentration was 500 mg/kg, and this has been confirmed by other studies, where only 20% removal efficiencies were observed for initial concentrations of 500 mg/L (Wang et al. 2014). More electrons are needed for atrazine degradation during reductive dechlorination and other electrophilic processes with increasing initial concentrations. In this study, acetate was oxidized to electrons and protons in the anode, and then the electrons were utilized through two pathways: allocation to the anode that generated the current and utilization by atrazine for degradation. In other words, the anode competes with pollutants for electrons (Fang et al. 2015; Sun et al. 2009), leading to a reduction in the transfer of electrons from the anode to the cathode, affecting atrazine degradation in the cathode. Furthermore, competition with oxygen between the cathode and atrazine was observed in the upper layer. Oxygen is the only electron acceptor in the air-cathode MFCs (Logan et al. 2006), while atrazine degradation also requires some oxygen. Consequently, the relationships between atrazine, electrons, and electrodes determine atrazine degradation. Previous studies have reported that the current or electricity performance of MFCs is the key factor for organic pollution degradation (Cao et al. 2015; Wang et al. 2017), and this result was confirmed in this study.
3.3 Microbial Community Analysis
The composition of bacterial communities on the anode, beneath the anode, and in the MFCs was studied to identify the microorganisms that are likely involved in current generation and atrazine degradation. Bacteriodetes and Proteobacteria were the dominant phylotypes, being present in proportions of 25.59 and 44.43% and 32.70 and 25.61%, in the D4 and D3 soil MFCs, respectively. Furthermore, these two bacteria were also the dominant phylotypes in the lower and upper sections of D3, with values of 30.53 and 27.72% and 33.31 and 31.92%, respectively (Fig. 5a). However, the relative abundance between the lower and the upper layers differed significantly. The relative abundance of Chloroflexi in the anode and lower layer was 10.50% higher than that in the upper layer (0.75%), while in the upper layer, Firmicutes, at 8.06%, was higher than that in the anode and lower layer. Moreover, Bacteriodetes, Proteobacteria, and Chloroflexi were predominant in the anodes and lower layers, and these bacteria are reportedly involved in current generation in sediment MFCs (Cabezas et al. 2015; Hamdan et al. 2017; Logan and Regan 2006).
Deltaproteobacteria was identified under the phylum of Proteobacteria and was present at noticeable levels, decreasing significantly from 36.00 to 15.13% when the atrazine concentration varied from 100 to 750 mg/kg. Additionally, Geobacter, a genus of Proteobacteria, predominated in the soil MFCs while the related abundance was significantly higher (24.30%) in D4 MFCs than in D3 MFCs (3.50%) (Fig. 5b). This observation indicates that atrazine concentration has a profound effect on the development of the microbial community composition in soil MFCs. Deltaproteobacteria emerges and is dominant under restricted anaerobic conditions or anoxic sediments in SMFCs (Logan and Regan 2006), while Deltaproteobacteria and Geobacter are known anode-reducing bacteria related to current generation and produce nanowires that are highly conductive (Cabezas et al. 2015; Jung and Regan 2007; Logan and Regan 2006). The relative abundance of the two main bacteria related to electricity generation varied significantly when the pollutant concentration increased. The difference in microbial communities would also explain the current decrease when the atrazine concentration increased from 100 to 750 mg/kg.
Biodegradation, especially microbial degradation, has been regarded as the major removal mechanism for atrazine degradation in contaminated soils (Chan-Cupul et al. 2016; Ralebitso et al. 2002). Pseudomonas is capable of utilizing atrazine as a source of nitrogen and carbon (Behki and Khan 1986; Ralebitso et al. 2002). However, the relative abundance of Pseudomonas sp. in these samples was low, at 0.51%. Furthermore, no other microbial species were involved in atrazine degradation found in the soil MFCs. These results suggest that microbial degradation is not the main degradation mechanism in the soil MFCs. In contrast, the findings could demonstrate that exoelectrogenic bacteria are the dominant phylotype and that bioelectrochemistry is the dominant mechanism of atrazine degradation in soil MFCs.
4 Conclusion
This study revealed that soil MFCs promoted atrazine degradation and shortened its half-life. The anode depth and initial atrazine concentration significantly affected atrazine degradation. Atrazine degraded most rapidly at an anode depth of 4 cm, with a removal efficiency of 91.69% and a half-life of 40 days. Atrazine degradation was dependent on current generation in the soil MFCs rather than on the electrode spacing. Furthermore, the degradation rate was inhibited when the initial concentration was increased from 100 to 750 mg/kg. The degradation rate differed significantly between different layers. The degradation rate was fastest in the upper layer, followed by that in the lower and middle layers. The exoelectrogenic bacteria Deltaproteobacteria and Geobacter were enriched on the anode and the lower layer of the soil MFCs, while only very low proportions of atrazine-degrading Pseudomonas were observed. In particular, the relative abundances of Deltaproteobacteria and Geobacter were higher under lower initial atrazine concentrations. These results demonstrate that the mechanism of atrazine degradation in soil MFCs is dependent on bioelectrochemistry rather than on microbial degradation.
References
Accinelli, C., Dinelli, G., Vicari, A., & Catizone, P. (2001). Atrazine and metolachlor degradation in subsoils. Biology and Fertility of Soils, 33, 495–500.
An, J., Kim, B., Nam, J., Ng, H. Y., & Chang, I. S. (2013). Comparison in performance of sediment microbial fuel cells according to depth of embedded anode. Bioresource Technology, 127, 138–142.
Assaf, N. A., & Turco, R. F. (2010). Influence of carbon and nitrogen application on the mineralization of atrazine and its metabolites in soil. Pest Management Science, 41, 41–47.
Bastos, A. C., & Magan, N. (2009). Trametes versicolor: potential for atrazine bioremediation in calcareous clay soil, under low water availability conditions. International Biodeterioration and Biodegradation, 63, 389–394.
Behki, R. M., & Khan, S. U. (1986). Degradation of atrazine by pseudomonas—n-dealkylation and dehalogenation of atrazine and its metabolites. Journal of Agricultural and Food Chemistry, 34, 746–749.
Blumhorst, M. R., & Weber, J. B. (2010). Chemical versus microbial degradation of cyanazine and atrazine in soils. Pesticide Science, 42, 79–84.
Buitrón, G., & Moreno-Andrade, I. (2014). Performance of a single-chamber microbial fuel cell degrading phenol: effect of phenol concentration and external resistance. Applied Biochemistry and Biotechnology, 174, 2471.
Cabezas, A., Pommerenke, B., Boon, N., & Friedrich, M. W. (2015). Geobacter, Anaeromyxobacter and Anaerolineae populations are enriched on anodes of root exudate-driven microbial fuel cells in rice field soil. Env Microbiol Rep, 7, 489–497.
Cao, X., Song, H. L., Yu, C. Y., & Li, X. N. (2015). Simultaneous degradation of toxic refractory organic pesticide and bioelectricity generation using a soil microbial fuel cell. Bioresource Technology, 189, 87–93.
Chae, K. J., Choi, M. J., Lee, J. W., Kim, K. Y., & Kim, I. S. (2009). Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresource Technology, 100, 3518–3525.
Chan-Cupul, W., Heredia-Abarca, G., & Rodriguez-Vazquez, R. (2016). Atrazine degradation by fungal co-culture enzyme extracts under different soil conditions. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes, 51, 298–308.
Chung, K. H., Ro, K. S., & Roy, D. (1995a). Atrazine biotransformation in wetland sediment under different nutrient conditions-i: anaerobic. Journal of Environmental Science & Health Part A Environmental Science & Engineering & Toxicology, A30, 121–131.
Chung, K. H., Ro, K. S., & Roy, D. (1995b). Atrazine biotransformation in wetland sediment under different nutrient conditions.1. Anaerobic. J. Environ. Sci. Health Part A-Environ. Sci. Eng. Toxic Hazard. Subt. Control, 30, 109–120.
Coady, K. K., Murphy, M. B., Villeneuve, D. L., Hecker, M., Jones, P. D., Carr, J. A., Solomon, K. R., Smith, E. E., Van, D. K. G., & Kendall, R. J. (2005). Effects of atrazine on metamorphosis, growth, laryngeal and gonadal development, aromatase activity, and sex steroid concentrations in Xenopus Laevis. Ecotoxicology and Environmental Safety, 62, 160.
DeLaune, R. D., Devai, I., Mulbah, C., Crozier, C., & Lindau, C. W. (1997). The influence of soil redox conditions on atrazine degradation in wetlands. Agriculture, Ecosystems & Environment, 66, 41–46.
Delwiche, K. B., Lehmann, J., & Walter, M. T. (2014). Atrazine leaching from biochar-amended soils. Chemosphere, 95, 346–352.
Deng, H., Wu, Y. C., Zhang, F., Huang, Z. C., Chen, Z., Xu, H. J., & Zhao, F. (2014). Factors affecting the performance of single-chamber soil microbial fuel cells for power generation. Pedosphere, 24, 330–338.
Dichristina, T. J. (1992). Effects of nitrate and nitrite on dissimilatory iron reduction by Shewanella putrefaciens 200. Journal of Bacteriology, 174, 1891–1896.
Elsheekh, M. M., Kotkat, H. M., & Hammouda, O. H. (1994). Effect of atrazine herbicide on growth, photosynthesis, protein synthesis, and fatty acid composition in the unicellular green alga Chlorella kessleri. Ecotoxicology and Environmental Safety, 29, 349–358.
Fang, Z., Cheng, S., Cao, X., Wang, H., & Li, X. (2016). Effects of electrode gap and wastewater condition on the performance of microbial fuel cell coupled constructed wetland. Environmental Technology, 1-30.
Fang, Z., Song, H. L., Cang, N., & Li, X. N. (2015). Electricity production from Azo dye wastewater using a microbial fuel cell coupled constructed wetland operating under different operating conditions. Biosensors & Bioelectronics, 68, 135.
Hamdan, H. Z., Salama, D. A., Hari, A. R., Semerjian, L., & Saikaly, P. (2017). Assessment of the performance of SMFCs in the bioremediation of PAHs in contaminated marine sediments under different redox conditions and analysis of the associated microbial communities. Sci. Total Environ., 575, 1453–1461.
Hong, S. W., Chang, I. S., Choi, Y. S., & Chung, T. H. (2009). Experimental evaluation of influential factors for electricity harvesting from sediment using microbial fuel cell. Bioresource Technology, 100, 3029–3035.
Huang, D. Y., Zhou, S. G., Chen, Q., Zhao, B., Yuan, Y., & Zhuang, L. (2011). Enhanced anaerobic degradation of organic pollutants in a soil microbial fuel cell. Chemical Engineering Journal, 172, 647–653.
Jung, S., & Regan, J. M. (2007). Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Applied Microbiology and Biotechnology, 77, 393–402.
Kumar, A., & Singh, N. (2016). Atrazine and its metabolites degradation in mineral salts medium and soil using an enrichment culture. Environmental Monitoring and Assessment, 188, 12.
Logan, B. E., Hamelers, B., Rozendal, R., Schröder, U., Keller, J., Freguia, S., Aelterman, P., Verstraete, W., & Rabaey, K. (2006). Microbial fuel cells: methodology and technology. Environmental Science & Technology, 40, 5181–5192.
Logan, B. E., & Regan, J. M. (2006). Electricity-producing bacterial communities in microbial fuel cells. Trends in Microbiology, 14, 512–518.
Nealson, K. H., & Myers, C. R. (1992). Microbial reduction of manganese and iron: new approaches to carbon cycling. Applied and Environmental Microbiology, 58, 439–443.
Ralebitso, T. K., Senior, E., & Van Verseveld, H. W. (2002). Microbial aspects of atrazine degradation in natural environments. Biodegradation, 13, 11–19.
Rismani-Yazdi, H., Christy, A. D., Carver, S. M., Yu, Z., Dehority, B. A., & Tuovinen, O. H. (2011). Effect of external resistance on bacterial diversity and metabolism in cellulose-fed microbial fuel cells. Bioresource Technology, 102, 278–283.
Ro, K. S., & Chung, K. H. (1995). Atrazine biotransformation in wetland sediment under different nutrient conditions. 2. Aerobic. J. Environ. Sci. Health Part A-Environ. Sci. Eng. Toxic Hazard. Subt. Control, 30, 121–131.
Sajana, T. K., Ghangrekar, M. M., & Mitra, A. (2014). Effect of operating parameters on the performance of sediment microbial fuel cell treating aquaculture water. Aquacultural Engineering, 61, 17–26.
Song, H.-L., Zhang, S., Yang, X.-L., Chen, T.-Q., & Zhang, Y.-Y. (2017). Coupled effects of electrical stimulation and antibiotics on microbial community in three-dimensional biofilm-electrode reactors. Water, Air, & Soil Pollution, 228, 83.
Sparling, G., Dragten, R., Aislabie, J., & Fraser, R. (1998). Atrazine mineralisation in New Zealand topsoils and subsoils: influence of edaphic factors and numbers of atrazine-degrading microbes. Australian Journal of Soil Research, 36, 557–571.
Sun, J., Hu, Y. Y., Bi, Z., & Cao, Y. Q. (2009). Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresource Technology, 100, 3185–3192.
Vanderheyden, V., Debongnie, P., & Pussemier, L. (1997). Accelerated degradation and mineralization of atrazine in surface and subsurface soil materials. Pesticide Science, 49, 237–242.
Wüst, S., & Hock, B. (1992). A sensitive enzyme immunoassay for the detection of atrazine based upon sheep antibodies. Analytical Letters, 25, 1025–1037.
Wang, H., Song, H., Yu, R., Cao, X., Fang, Z., & Li, X. (2016). New process for copper migration by bioelectricity generation in soil microbial fuel cells. Environemental Science and Pollution Research, 23, 13147–13154.
Wang, H., Yi, S., Cao, X., Fang, Z., & Li, X. (2017). Reductive dechlorination of hexachlorobenzene subjected to several conditions in a bioelectrochemical system. Ecotoxicology and Environmental Safety, 139, 172–178.
Wang, J., Zhu, L., Wang, Q., Wang, J., & Xie, H. (2014). Isolation and characterization of atrazine mineralizing Bacillus subtilis strain HB-6. PloS One, 9, e107270.
Wang, X., Cai, Z., Zhou, Q., Zhang, Z., & Chen, C. (2012). Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnology and Bioengineering, 109, 426–433.
Wilhelms, K. W., Cutler, S. A., Proudman, J. A., Carsia, R. V., Anderson, L. L., & Scanes, C. G. (2006). Lack of effects of atrazine on estrogen-responsive organs and circulating hormone concentrations in sexually immature female Japanese quail (Coturnix coturnix japonica). Chemosphere, 65, 674–681.
Acknowledgments
This work was supported by the Provincial Natural Science Foundation of Jiangsu, China (BK20171351), the National Natural Science Foundation of China (21277024), and the Fundamental Research Funds for the Central Universities (2242016K41042) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Li, L., Cao, X. et al. Enhanced Degradation of Atrazine by Soil Microbial Fuel Cells and Analysis of Bacterial Community Structure. Water Air Soil Pollut 228, 308 (2017). https://doi.org/10.1007/s11270-017-3495-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3495-1