Abstract
In this paper, a silica gel-based new sorbent was prepared with a Schiff base, called as N,N′-bis(4-methoxysalicylidene) ethylenediamine (MSE). The modified silica gel (Si-MSE) was used for simultaneous separation and preconcentration of Cd(II) and Pb(II) from aqueous solutions. Determination of analyte concentrations in eluates were achieved by flame atomic absorption spectrometry (FAAS). The analytical variables including pH, flow rate, and sample volume for sorption and flow rate, eluent concentration, and eluent volume for elution, were optimized by central composite design (CCD). HNO3 was used as eluent and maximum preconcentration factor was found to be 200. Limit of detections (LODs) were found to be 49.6 ng L−1 and 1.3 μg L−1 for Cd(II) and Pb(II), respectively. Validation of the developed method was performed by using certified reference material (TMDA-53.3) and mixed metal standard. The methodology was applied for determination of Cd(II) and Pb(II) in natural water samples and satisfactory results were obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Industrial effluents and wastewater disposal raises natural water pollution. Determination of trace metal levels in natural waters is very important due to contamination monitoring. Beside environmental pollution, Pb(II) and Cd(II) heavy metals affect living organisms owing to their cumulative toxicity. Long-term exposure to these metals can cause serious health problems such as damage in organs, especially in liver and kidneys; anemia; and cancer (Eklund et al. 2003; Lara et al. 2001; Yebra et al. 2002; Li et al. 2005; Tian et al. 2011). Therefore, accurate and sensitive methods for determination of Cd(II) and Pb(II) are required. Flame atomic absorption spectrometry (FAAS) is preferred for metal analysis depending on easy operation, selectivity, and cheapness. On the other hand, low sensitivity is the main problem for direct determination of metal concentrations at ppb level. In order to overcome this limitation, preconcentration techniques are frequently used (Ozcelik et al. 2012; Sabermahani et al. 2013; Shemirani et al. 2004). According to the reports, inorganics were preconcentrated/separated by various techniques such as liquid-liquid extraction (Baran and Yaşar 2010; Soylak and Yilmaz 2011), precipitation (Yuan-Zhen et al. 2012; Komjarova and Blust 2006), cloud point extraction (Silva et al. 2009), and solid-phase extraction (SPE) (Sabermahani et al. 2013; Shemirani et al. 2004) prior to FAAS determination.
Solid-phase extraction of trace metals is preferred to other preconcentration techniques according to simplicity and minimal waste of organic solvent generation. Moreover, high preconcentration factor can be obtained at optimal conditions and the technique is able to combine with analytical instruments. Organic- and inorganic-based solid-phase materials such as XAD-2 and XAD-4 resins (Guo et al. 2004), polyurethane foam (Ferreira et al. 2003), alumina (Ahmed 2008), magnesia (Yu et al. 2006), zirconia (Li et al. 2012), and silica gel (Ozcelik et al. 2012; Sabermahani et al. 2013; Shemirani et al. 2004) have been widely used for separation and preconcentration. Besides thermal, chemical, and mechanical stability, and cheapness of silica gel, increases the usage as solid support, as compared to other organic and inorganic solid materials. The presence of silanol groups allows modification of silica surface with selective chelating agents such as N-(2-aminoethyl)-salicylaldimine (Ozcelik et al. 2012), N,N′-bis-(α-methylsalicylidene)-2,2-dimethyl-1,3-propanediamine (Bartyzel and Cukrowska 2011), and N,N′-bis(salicylidene) phenylene-1,3-diamine (Shemirani et al. 2004). Experimental conditions of SPE procedures are optimized by various chemometric techniques that are widely used. Beside rapidity, practicality, and economical benefits, optimization procedures make it possible to understand interactions between the factors that influence the analytical response, consequently recoveries (Zarei and Shemirani 2012; Shah et al. 2013; Ferreira et al. 2002; Lemos et al. 2009).
In the present work, silica gel was employed for production of a new solid phase. Schiff base, shown in Fig. 1 and named N,N′-bis(4-methoxysalicylidene) ethylenediamine (MSE) was used for modification of silica gel. The synthesized and characterized new sorbent (Si-MSE) was utilized for preconcentration of Cd(II) and Pb(II) from natural water samples. Determination of metal concentrations was achieved by FAAS after preconcentration procedure. The experimental conditions were optimized by central composite design (CCD).
2 Experimental
2.1 Reagents and Chemicals
The analytical-grade deionized water was obtained by reverse osmosis system. All containers and glassware were kept overnight in 10 % nitric acid and rinsed three times with water prior to use. One thousand milligrams per liter stock metal standard solutions were prepared from Cd(NO3)2.4H2O and Pb(NO3)2 (Merck) and diluted as required to the micrograms per liter levels. In interference study, cations were added as nitrates and the anions were added as sodium salts. Silica gel (70–230 mesh) was purchased from Merck. N,N′-bis(4-methoxysalicylidene) ethylenediamine (MSE) was synthesized by condensation reaction of 4-methoxysalicylidene and diamino ethane (Merck). Feasibility of the suggested method was tested with Lake Ontario water certified reference material (TMDA-53.3, lot 0310) and multi element standard solution (1.11355, Merck).
2.2 Apparatus
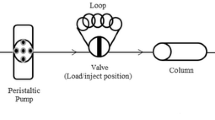
A Philips X Pert-Pro diffractometer (Cu Kα λ = 1.54060 A°, 30 mA, 40 kV), and PerkinElmer Spectrum 65 FTIR-ATR spectrometer were used to confirm the synthesized Si-MSE. Modification period of silica gel was monitored by PG Instrument T80+ UV–Vis spectrometer. Determination of metals in solutions was carried out by PerkinElmer AAnalyst 200 FAAS equipped with deuterium background correction. All measurements were performed in an air/acetylene flame. GFL 3005 orbital shaker having speed and time control was used for preparation of the sorbent. During the solid-phase extraction experiments, VELP Scientifica SP311 peristaltic pump with Tygon tubes was used. A Thermo Orion 5 Star model pH meter, Heidolph MR 3001K model magnetic stirrer, Sartorius TE214S electronic balance, and Eppendorf Research micro pipettes were used for the present work. Funnel-tipped glass tube (10 × 100 mm) equipped with stopcock was used as a column for the preconcentration experiments.
2.3 Preparation of Solid Phase
The commercially available silica gel was activated by refluxing with 0.5 mol L−1 HNO3 for 1 h to remove any adsorbed metal ions. Then, it was filtered and washed with deionized water until the filtrate was neutral.
In order to synthesize physically bonded Si-MSE, 10.0 g of silica gel was added to 50.0 mL acetone containing 50 mg MSE and refluxed for 1 h. The product was filtered off and washed with deionized water and dried at room temperature.
2.4 General Enrichment Procedure
First, 1.0 g of Si-MSE was filled in the column and the effective factors on the preconcentration including pH, flow rate, and selection of eluent type, were investigated using 50-mL model solutions containing 5 μg Cd(II) and 10 μg Pb(II). Determination of metal ions in 5-mL eluates was achieved by FAAS. According to the precipitation of the metal hydroxides at alkaline environment, pH effect was evaluated within the range of 3.00–7.00. Experiments of flow rate for sorption and elution were performed at 3–20 mL min−1. In order to choose a proper eluent for desorbing Cd(II) and Pb(II) from the Si-MSE surface, different mineral and organic acids were tested.
The proposed analytical procedure for Cd(II) and Pb(II) preconcentration was optimized using three-level full factorial CCD. The optimization procedure was performed separately for sorption and elution steps. Three variables; pH, sample volume, flow rate and eluent volume, eluent concentration, flow rate were regarded as factors for sorption and for elution, respectively. Based on the experimental data, simultaneous preconcentration of certain ions were carried at given conditions for sorption: pH = 5.2, flow rate = 4.0 mL min−1, and sample volume = 50 mL, and for elution: flow rate = 4.0 mL min−1, concentration of HNO3 = 0.5 mol L−1, and volume of eluent = 4.8 mL.
3 Results and Discussion
3.1 Modification and Characterization of Sorbent
Modification period of the solid-phase surface is a critical step in SPE procedures. Accordingly, during mechanical shaking of MSE solution and activated silica gel, 1–2 mL proportion was taken from the liquid phase and the absorbance was measured at 328 nm. This wavelength is specific for MSE in order to obtain maximum absorbance and the absorbance of the solution was monitored with 1-h intervals for 6 h. The time-dependent changes in absorbance, shown in Fig. 2, indicate that 1 h is sufficient for modification of silica gel with MSE.
The modification of silica gel was confirmed by Fourier transform infra red (FTIR) and x-ray diffraction (XRD) analyses. In the comparison of the IR spectrum of bare silica gel with modified silica gel, new peaks were monitored and shown in Fig. 3. The silica gel showing the characteristic peak at 805 cm−1 is ascribed to the Si–O–Si bending vibration and at 1031 cm−1 Si–O stretching vibration. The IR spectrum of the modified silica gel has some different peaks that corresponding to ligand at 2932 and 2988 cm−1 (C–H) and 1667 cm−1 (C=N).
According to the literature data about XRD analysis, silica gel and its functionalized derivatives appeared at 24 °, which is the amorphous diffraction peak of silica gel. Also, it is reported that organic moieties decrease the intensity of the diffraction peak (Qu et al. 2008). The spectra of Si-MSE and silica gel obtained from XRD analyses depicted in Fig. 4 are in agreement with literature. According to experimental results, silica gel is successfully modified with MSE.
3.2 Effect of pH
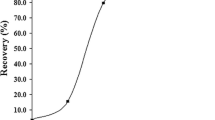
The pH of the sample solution plays an important role in retention of metals on sorbent. The pH of the model solutions containing 5 μg Cd(II) and 10 μg Pb(II) were adjusted to certain value using diluted HNO3 and NaOH. As shown in Fig. 5, the recovery results were not dramatically affected by the change in pH between 4.00 and 7.00 for Cd(II) and Pb(II). Accordingly, center value of pH was chosen as 5.00 for both of analytes for the optimization procedure.
3.3 Selection of Desorption Reagent
For desorption of Cd(II) and Pb(II) from Si-MSE, 0.5 mol L−1 of HNO3, HCl, CH3COOH, H2SO4, and H2O2 were tested. Except for HNO3 elution experiments, the recovery percentages were varied between 5.7 and 87.9 % and 1.3 and 104.1 % for Cd(II) and Pb(II), respectively. The recovery percentages of HNO3 elution experiments were 94.2 ± 1.5 % for Cd(II) and 104.2 ± 1.4 for Pb(II). It was observed that HNO3 is the best eluent for Cd(II) and Pb(II) and suitable for simultaneous desorption of certain metal ions.
3.4 Effect of Flow Rate on Sorption and Elution
The retention of a metal ion on the sorbent also depends on the flow rate of the sample solution. Thus, the effect of flow rate on sorption and desorption of Cd(II) and Pb(II) ions were investigated between 3 and 20 mL min−1. Results showed that sorption of Cd(II) and Pb(II) ions are not quantitatively (>95 %) affected by flow rate up to 10.0 and 6.0 mL min−1, respectively. Increased flow rate, decreases the retention times and sorption percentages of ions. Similarly, quantitative recoveries could be obtained with elution flow rate up to 8.0 mL min−1 for Cd(II) and 5.0 mL min−1 for Pb(II). In order to avoid an abrupt change in adsorption and increase the contact time of the sample solution with the sorbent, center value of flow rate was selected as 4 mL min−1 for optimization of sorption and elution steps.
3.5 Effect of Sample Volume
The preconcentration studies were applied to solutions within the range of 25.0–1000.0 mL and the possibility of enriching at low concentration with high enrichment factor was explored. Model solutions, containing 5 μg amount of Cd(II) and 10 μg Pb(II) were separately passed through the Si-MSE filled column. After eluting with suitable eluent, metal concentrations were determined with FAAS. The recovery value was obtained as 98.7 % at 1000 mL sample volume by analyzing 5-mL eluate and the highest preconcentration factor was found to be 200 for Cd(II). Similarly, preconcentration of Pb(II) was quantitatively achieved up to 1000.0 mL with 98.7 % recovery and the preconcentration factor was calculated as 200. It was seen that, without damaging the structure of Si-MSE, enrichment process could be able to apply in a wide range of sample volumes. In optimization procedure, sample volume was considered as a factor and 50.0 mL was chosen as center value for both of metal ions.
3.6 Optimization of Variables
Optimization of the preliminary studies was achieved by a three-level full factorial CCD with 20 runs. Table 1 lists the maximum, minimum, and center values of the variables for sorption and elution. The metal concentration in solutions was determined with external standard calibration method by FAAS. The results including recovery percentages and responses for sorption and elution are given in Table 2. The obtained experimental data were applied in the CCD and the quadratic equations (Eqs. (1) and (2)) show the relationship of pH (pH), retention flow rate (F), and sample volume (V S) for sorption of Cd(II) and Pb(II), respectively. Also, Eqs. (3) and (4) show the relationship between flow rate (F), concentration (C E), and volume (V E) of eluting solution for desorption of Cd(II) and Pb(II), respectively.
pH, F, and V S, and F, C E, and V E represents the first, second, and third factors for sorption and elution, respectively. Derivatives of the equations were equalized to zero and solved using Microsoft® Excel. The real values presented in Table 3 were obtained by CCD and used as optimal conditions for further experiments.
As can be seen in Table 3, the optimum values in pH and flow rate for both metals are so close. As mentioned before, the sample volume has no dramatic effect on sorption efficiencies between the range of 25.0 and 1000.0 mL. Therefore, Cd(II) and Pb(II) preconcentration experiments can be performed in the same conditions. The sorption of Cd(II) and Pb(II) were done together at following conditions: pH = 5.2, flow rate 4.0 mL min−1, and sample volume 50.0 mL. In a similar approach, the simultaneous elution was done in these conditions: flow rate 4.0 mL min−1, concentration of HNO3 0.5 mol L−1, and volume of eluent 4.8 mL.
3.7 Interference Effects
The preconcentration procedures of trace metal ions can be strongly affected by other ions. The effects of matrix ions such as SO4 2−, Cl−, NO3 −, Na+, Ca2+, Mg2+, K+, Fe3+, Mn2+, Co2+, Cr3+, Zn2+, Ni2+, Pb2+, and Cu2+ on preconcentration and on the selectivity of the developed method were investigated under optimal conditions. Model solutions, which includes 100 μg L−1 Cd(II) and 2 mg L−1 Pb(II) and different amounts of other ions were prepared and the suggested SPE procedure was applied. The tolerance limits were given in Table 4 and are defined as the interfering ion concentration causing a relative error smaller than ±5 % related to the preconcentration of analytes. The experiments indicated that no further sample treatment or masking reagents are needed for preconcentration of Cd(II) and Pb(II).
3.8 Analytical Figures of Merit
The accuracy (recovery, %) and precision (RSD, %) of the proposed solid-phase extraction procedure under optimal conditions were investigated (n = 10) as 102.0 ± 4.0 and 3.9 % for Cd(II) and as 98.0 ± 2.0 and 2.0 % for Pb(II), respectively. Limit of detection (LOD) was obtained by using a criterion signal-to-noise ratio of 3. LODs were calculated as 49.6 ng L−1 for Cd(II) and 1.3 μg L−1 for Pb(II).
3.9 Validation and Application of the Improved Method
The developed procedure was validated by Cd (II) and Pb(II) determination in certified reference material. The results given in Table 5 show good agreement with the certified values. The obtained recovery percentages were acceptable and it shows that the presented method can be applied for the preconcentration of Cd(II) and Pb(II) in natural water samples.
3.10 Real Sample Analysis
Applicability of the optimized preconcentration procedure was tested with various water samples such as tap water, Selimiye lake water, mineral water, snow water, and bottled drinking water. The suitability of the method was checked by spike recovery tests. The mean recovery results for each sample are summarized in Table 6. The recovery percentages of the method were between 90.8 and 103.0 % for Cd(II) and 90.5 and 98.8 % for Pb(II). Taking into consideration these results, the improved method was suggested as practical, accurate, precise, effective, simple, and cheap determination method for Cd(II) and Pb(II) in natural water samples.
4 Conclusion
In the present study, a new silica gel-based sorbent has been reported. The work presented offers a useful and efficient simultaneous separation and preconcentration for Cd(II) and Pb(II) in various water samples. The modified silica gel was prepared easily and sorbed Cd(II) and Pb(II) rapidly. The optimized parameters are pH = 5.2, flow rate 4.0 mL min−1, and sample volume 50 mL for sorption and flow rate 4 mL min−1, eluent concentration 0.5 mol L−1, and eluent volume 4.8 mL in elution for simultaneous procedure. Preconcentration factor was found to be 200 for both metal ions. The mean recovery values for spiked water samples were acceptable and confirmed the validity of the method. Modified sorbent can be used for one cycle, but silica gel can be reused plenty of times and be modified again with MSE in a short period. Finally, the proposed method can be suggested as a simple, sensitive, accurate, and repeatable method for simultaneous preconcentration and determination of Cd(II) and Pb(II).
References
Ahmed, S. A. (2008). Alumina physically loaded by thiosemicarbazide for selective preconcentration of mercury(II) ion from natural water samples. Journal of Hazardous Materials, 156, 521–529.
Baran, E. K., & Yaşar, S. B. (2010). Copper and iron determination with [N, N′-Bis(salicylidene)-2,2′-dimethyl-1,3-propanediaminato] in edible oils without digestion. Journal of the American Oil Chemists’ Society, 87, 1389–1395.
Bartyzel, A., & Cukrowska, E. M. (2011). Solid phase extraction method for the separation and determination of chromium(III) in the presence of chromium(VI) using silica gel modified by N,N′-bis-(α-methylsalicylidene)-2,2-dimethyl-1,3-propanediimine. Analytica Chimica Acta, 707, 204–209.
Eklund, G., Linden, A., Tallkvist, J., & Oskarsson, A. (2003). Bioavailability of cadmium from in vitro digested infant food studied in caco-2 cells. Journal of Agricultural Food Chemistry, 51(14), 4168–4174.
Ferreira, S. L. C., Queiroz, A. S., Fernandes, M. S., & Santos, H. C. (2002). Application of factorial designs and Doehlert matrix in optimization of experimental variables associated with the preconcentration and determination of vanadium and copper in seawater by inductively coupled plasma optical emission spectrometry. Spectrochimica Acta B: Atomic Spectroscopy, 57, 1939–1950.
Ferreira, S. L. C., Santos, W. N. L., Bezerra, M. A., Lemos, V. A., & Bosque-Sendra, J. M. (2003). Use of factorial design and Doehlert matrix for multivariate optimisation of an on-line preconcentration system for lead determination by flame atomic absorption spectrometry. Analytical and Bioanalytical Chemistry, 375(3), 443–449.
Guo, Y., Din, B., Liu, Y., Chang, X., Meng, S., & Tian, M. (2004). Preconcentration of trace metals with 2-(methylthio)aniline-functionalized XAD-2 and their determination by flame atomic absorption spectrometry. Analytica Chimica Acta, 504(2), 319–324.
Komjarova, I., & Blust, R. (2006). Comparison of liquid-liquid extraction, solid-phase extraction and co-precipitation preconcentration methods for the determination of cadmium, copper, nickel, lead and zinc in seawater. Analytica Chimica Acta, 576(2), 221–228.
Lara, R. F., Wuilloud, R. G., Salonia, J. A., Olsina, R. A., & Martinez, L. D. (2001). Determination of low cadmium concentrations in wine by on-line preconcentration in a knotted reactor coupled to an inductively coupled plasma optical emission spectrometer with ultrasonic nebulization. Fresenius’ Journal of Analytical Chemistry, 371(7), 989–993.
Lemos, V. A., Novaes, C. G., & Bezerra, M. A. (2009). An automated preconcentration system for the determination of manganese in food samples. Journal of Food Composition and Analysis, 22(4), 337–342.
Li, Q., Ouyang, R., Xu, G., & Liu, G. (2005). Cadmium preconcentration from aqueous environmental samples using microcrystalline phenolphthalein modified by crystal violet. Analytical Letters, 38(12), 1987–1998.
Li, P., Hu, B., & Li, X. (2012). Zirconia coated stir bar sorptive extraction combined with large volume sample stacking capillary electrophoresis-indirect ultraviolet detection for the determination of chemical warfare agent degradation products in water samples. Journal of Chromatography A, 1247, 49–56.
Ozcelik, G., Imamoglu, M., Yildiz, S. Z., & Kara, D. (2012). Chemically modified silica Gel with N-(2-aminoethyl)-salicylaldimine for simultaneous solid phase extraction and preconcentration of Cu(II), Ni(II), Cd(II) and Zn(II) in waters. Water, Air, & Soil Pollution, 223, 5391–5399.
Qu, R., Wang, M., Sun, C., Zhang, Y., Ji, C., Chen, H., Meng, Y., & Yin, P. (2008). Chemical modification of silica-gel with hydroxyl- or amino-terminated polyamine for adsorption of Au(III). Applied Surface Science, 255(5), 3361–3370.
Sabermahani, F., Hassani, Z., & Faramarzpoor, M. (2013). The use of silica gel modified with allyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate for selective separation and preconcentration of lead in environmental samples. Environmental Monitoring Assessment, 185(6), 4969–4976.
Shah, F., Soylak, M., Kazi, T. G., & Afridi, H. I. (2013). Preconcentration of lead from aqueous solution with activated carbon cloth prior to analysis by flame atomic absorption spectrometry: a multivariate study. Journal of Analytical Atomic Spectrometry, 28, 601–605.
Shemirani, F., Mirroshandel, A. A., Niasari, M. S., & Kozani, R. R. (2004). Silica gel coated with Schiff’s base: synthesis and application as an adsorbent for cadmium, copper, zinc, and nickel determination after preconcentration by flame atomic absorption spectrometry. Journal of Analytical Chemistry, 59(3), 228–233.
Silva, E. L., Roldan, P. S., & Gine, M. F. (2009). Simultaneous preconcentration of copper, zinc, cadmium, and nickel in water samples by cloud point extraction using 4-(2-pyridylazo)-resorcinol and their determination by inductively coupled plasma optic emission spectrometry. Journal of Hazardous Materials, 171, 1133–1138.
Soylak, M., & Yilmaz, E. (2011). Ionic liquid dispersive liquid–liquid microextraction of lead as pyrrolidinedithiocarbamate chelate prior to its flame atomic absorption spectrometric determination. Desalination, 275, 297–301.
Tian, Y., Ming Xie, Z., Li Chen, M., & Wang, J. (2011). Cadmium preconcentration with bean-coat as a green adsorbent with detection by electrothermal atomic absorption spectrometry. Journal of Analytical Atomic Spectrometry, 26, 1408–1413.
Yebra, M. C., Salgado, J., Puig, L., & Moreno-Cid, A. (2002). Field preconcentration of cadmium from seawater by using a minicolumn packed with Amberlite XAD-4/4-(2-pyridylazo) resorcinol and its flow-injection-flame atomic absorption spectrometric determination at the ng L(−1) Level. Analytical and Bioanalytical Chemistry, 374(3), 530–534.
Yu, Q., Yang, J., Lin, B., & Feng, Y. (2006). Preparation of pyrenebutyric acid-modified magnesia–zirconia stationary phases using phosphonate as spacers and their application to the separation of fullerenes. Analytica Chimica Acta, 559(1), 79–88.
Yuan-Zhen, P., Yong-Ming, H., Dong-Xing, Y., Yan, L., & Zhen-Bin, G. (2012). Rapid analysis of heavy metals in coastal seawater using preconcentration with precipitation/co-precipitation on membrane and detection with x-ray fluorescence. Chinese Journal of Analytical Chemistry, 40(6), 877–882.
Zarei, Z., & Shemirani, F. (2012). Determination of nickel in food samples by flame atomic absorption spectroscopy after preconcentration and microextraction-based ionic liquids using full factorial and central composite design. Journal of Food Science, 77, 1242–1248.
Acknowledgments
The authors would like to thank the Scientific and Technological Research Council of Turkey (TUBITAK-TBAG project number 105 T153) and Balıkesir University (BAP project number 2012/40) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tokay, F., Bağdat, S. Simultaneous Preconcentration of Cd(II) and Pb(II) with N,N′-bis(4-methoxysalicylidene) Ethylenediamine Coated Silica Gel Prior to Determination by Flame Atomic Absorption Spectrometry. Water Air Soil Pollut 226, 48 (2015). https://doi.org/10.1007/s11270-015-2352-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2352-3