Abstract
In this study, a novel method for determination of Pd(II) ions at trace level was developed using on-line preconcentration onto polyamine group bonded silica (PA-SG) with flame atomic absorption spectrometric determination. Pd(II) uptake dynamics of PA-SG was studied batchwise by investigating acidity and chloride concentration of aqueous phase, contact time and initial concentration of Pd(II) ions. The Pd(II) adsorption capacity of PA-SG was found to be 158.7 mg g−1 from aqueous phase containing 0.1 M HCl. The on-line preconcentration procedure of Pd(II) was optimized with main analytical parameters including sample and eluent flow rate, eluent type and volume and matrix ions. The optimum eluent type and flow rate of sample and eluent were found to be 1.0% thiourea in 1.0 mol L−1 HCl and 7.5 mL min−1, respectively. The preconcentration factor and sampling frequency were calculated to be 23.9 and 20 h−1, respectively. The calibration graph was linear over the range 10–200 µg L−1. The limits of detection (3σ) and quantification (10σ) values were computed to be 3 µg L−1 and 10 µg L−1, respectively. The RSD, % was found to be 4.6% for five measurement of 25 µg L−1 of Pd(II) ions. The accuracy of the developed method was successfully checked by determination of Pd(II) level of certified reference material platinum ore (SARM 7B). The proposed method was successfully applied for Pd(II) determination in various environmental water samples such as river, lake, sea and tap water, and spent auto catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, platinum group elements (PGE) have been widely used in the pharmaceutical industry, especially in many cancer drugs and in the automotive industry due to new applications in catalyst production (Muzikar et al. 2006). Among the precious metals, especially palladium, finds wide utilize in the electrical industry for contacts in the telephone relay and in the electrodes circuits for electronic tubing and high quality buzzers. Palladium is released as a major pollutant in the environment, in particular by the use of catalysts containing active palladium metal (Jamali et al. 2007). The release of the palladium ions has been found to increase contamination levels of soil, plant and road sediments near highways (Muzikar et al. 2006).
The development of analytical methods for accurate and precise determination of Pd(II) ions are important both in monitoring the environmental impacts, quality control at industrial products and in the development of procedure for the recovery of palladium ions from waste materials (Nakajima et al. 2009; Zhou et al. 2014a, b). Several sensitive and selective techniques for determination of palladium ions involving as flame atomic absorption spectrometry (FAAS) (Hassanien 2009), inductively coupled plasma optic emission spectrometry (ICP-OES) (Niemelä et al. 2012), inductively coupled plasma mass spectrometry (ICP-MS) (Krishna et al. 2009), inductively coupled plasma atomic emission spectrometry (ICP-AES) (Zheng et al. 2007), instrumental neutron activation analysis (INAA) (Avino et al. 2011), cyclic voltammetry (SV) (Velmurugan et al. 2017), X-ray fluorescence spectrometry (Messerschmidt et al. 2000) have been used.
Determination of Pd(II) ions at low concentration in the samples containing high matrix ions makes its direct determination difficult due to interferences from matrix ions and/or insufficient detection limit of the instrument. Therefore, preconcentration and separation procedure before the measurement step is required for determination of Pd(II) ions in environmental samples (Bruzzoniti et al. 2003). Up to now, many different techniques such as co-precipitation (Soylak and Tuzen 2008), solvent extraction (Kondo et al. 2015), liquid–liquid extraction (LLE) (Anthemidis et al. 2001), ion exchange (Schoeman et al. 2017), solid phase extraction (SPE) (Sharma et al. 2012), electrodeposition (Booth et al. 2017), and cloud point extraction (CPE) (Tavakoli et al. 2008) have been used for separation and preconcentration of the Pd(II) ions.
Among the above-mentioned methods, the solid phase extraction method is most often used due to its high preconcentration factor (PF), low cost, low solvent consumption, high recovery, fast phase separation and suitability with different on-line or off-line detection/determination techniques. Many sorbents such as activated carbon (Tavakkoli et al. 2014), modified alumina (Golshaei et al. 2015), multiwalled carbon nanotube (Ghaedi et al. 2013), Amberlite XAD resins (Kovalev et al. 2000), polystyrene-based sorbent (Saitoh et al. 2005), modified silica gel (Mladenova et al. 2012) and polymeric resins (Godlewska-Żyłkiewicz et al. 2012) have been used as a sorbent for off-line solid phase extraction of Pd(II) ions. The main disadvantage of off-line SPE is that it requires a large amount of sample and long time. The on-line SPE methods eliminate these problems and provide advantages such as decrease of sample and eluent consumption, accuracy and precision of analytical results reduction of contamination from laboratory environment due to closed system and enable a fast operation (Nakajima et al. 2009). However, the on-line SPE method is not free from problems. The sorbents used in this method should have a fast metal uptake ability from solution containing high matrix ions and the adsorbed metal ions onto sorbent should be eluted quickly and easily. For this purpose, synthesis of novel sorbents having these properties and its applications to on-line solid phase extraction has been focused in recent years (Antonio et al. 2016; Bosch Ojeda et al. 2007; Zolfonoun and Yousefi 2016; Jia et al. 2016; Rojas et al. 2006; Rossi et al. 2017; Tarley et al. 2017a, b; Sivrikaya et al. 2016; Karadaş and Kara 2013; Karadaş et al. 2013).
The present work describes a novel on-line preconcentration method using polyamine silica gel coupled with FAAS for determination of Pd(II) ions in environmental samples, spent auto catalyst and certified reference material platinum ore. The Pd(II) adsorption performance of the sorbent was investigated and then on-line preconcentration method was optimized by studying effective factors such as sample and eluent flow rate, eluent type and volume and matrix ions and then, the analytical figures of merit of the developed method was computed.
Experimental
Instrumentation
The measurement of the Pd(II) level in the samples was carried out by a Shimadzu AA6701F flame atomic absorption spectrometer (FAAS) (Shimadzu Corporation, Kyoto, Japan). The FAAS operating conditions were adjusted to optimal condition according to user manual book to obtain the best sensitivity for Pd(II). Air/acetylene flame with acetylene flow rate of 1.8 mL min−1, slit width of 0.5 nm, lamp current of 10 mA, wavelength of 244.8 nm and deuterium background correction were used. The signals were measured as peak height. The pH values were monitored with a Schott Model CG 840 pH meter (Schott AG, Mainz, Germany) equipped with a combined pH electrode. The Heidolph RZR 2020 model mechanical stirrer (Heidolph Instruments GmbH & Co. KG, Schwabach, Germany) and IKA MSH Basic model heated magnetic stirrer (IKA Werke GmbH, Staufen, Germany) were used at synthesis of PA-SG. IKA 4000i model orbital shaker (IKA Werke GmbH, Staufen, Germany) was used for the batch adsorption experiments.
In on-line preconcentration system, to forward the sample solution through mini column to FAAS, a peristaltic pump (Ismatec, Cole-Parmer GmbH, Wertheim, Germany) having four channels was used. 3-stop tubings of Tygon® were fixed to the peristaltic pump. The peristaltic pump was connected with polyethylene tubings to 2 two-way valves and then to glass mini column (0.4 mm i.d., 6.0 cm long) (Omnifit, Cambridge, UK). Outlet of the column was connected to FAAS. The valve have a loop can be changeable at a desired volume of eluent. The schematic diagram of the on-line preconcentration system is shown in Fig. 1.
Chemicals and Solutions
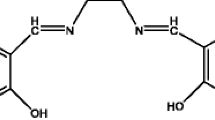
The chemicals used in this study were of analytical reagent grade. Distilled-deionized water with a chemical resistivity of 18 MΩ.cm was used in all analyses. Distilled water was prepared from tap water using Nüve ND 12 water distiller apparatus (Nüve A.Ş., Ankara, Turkey). Deionized water was prepared from the distilled water using Milli-Q® integral water purification system (Millipore, Bedford, MA, USA). The working and standard solutions were prepared by daily stepwise dilution of spectroscopic grade Pd(II) standard solution (1000 mg L−1 Pd(II), Merck KGaA, Darmstadt, Germany). Silica gel, 3-chloropropyltrimethoxysilane, triethylamine (Merck KGaA, Darmstadt, Germany), pentaethylenehexamine (Fluka, Steinheim, Germany), were used without any purification. The acidity of Pd(II) solutions were determined by adding dilute HCl, HNO3 or NaOH solution (Merck KGaA, Darmstadt, Germany) and controlled with pH meter. The platinum ore SARM 7B from The South African Bureau of Standards, which certified reference material was used for testing accuracy of the proposed method. Synthesis and characterization of silica gel modified by covalent attachment of polyamine group was given in the previous study (Sivrikaya et al. 2014). Proposed structure of polyamine silica gel is illustrated in Fig. 2. The content of pentaethylenehexamine on the modified silica gel was found to be 0.53 mmol g−1 (Sivrikaya et al. 2014).
Batch Adsorption Studies
The adsorption dynamics of Pd(II) ions on PA-SG were investigated with a batch adsorption method. The effects of solution acidity and chloride concentration, Pd(II) initial concentration and contact time on the adsorption of Pd(II) ions were investigated. The solutions containing 50 mL Pd(II) ions at different concentrations (50 or 100 mg L−1) were shaken with 50 mg of PA-SG at ambient temperature for various times using the orbital shaker. Then, mixture was filtered off and the concentrations of Pd(II) ions remaining in the solution was determined by FAAS, and the amounts of Pd(II) adsorbed onto PA-SG were computed by Eq. (1).
where q e is amount of Pd(II) adsorbed on PA-SG (mg g−1); C o is initial Pd(II) concentration (mg L−1); C e is concentration of Pd(II) remained in the solution (mg L−1); V is volume of Pd(II) solution (L); m is amount of PA-SG (g) (Karaçetin et al. 2014).
On-line Preconcentration Procedure
The mini column was filled about 120 mg of PA-SG and then preconditioned at desired acidic condition using appropriate acid solution (by 0.1 M HCl in optimal condition) for 30 s at 5 mL min−1 flow rate. The sample was passed at 5 or 7.5 mL min−1 for 60 s. The eluent was filled to the loop. The column was washed with 0.1 M HCl solution for 20 s to eliminate the matrix ions. After sample loading and cleaning, the valve was shifted to the inject position and the eluent was passed through the column at 7.5 mL min−1 flow rate. The eluate containing Pd(II) was reached directly into the FAAS and the observed peak height was recorded. After this cycle, the column was cleaned with 0.1 M HCl solution for 45 s and the system was ready for the next operation. All experiments were carried out three times and the average of the results was submitted in this study.
Sample Preparation
Spent auto catalyst from local auto service from Sakarya, Turkey was carefully grinded using agate mortar. The certified platinum ore SARM 7B and spent auto catalyst was digested using concentrated HCl–HNO3 solutions according to literature. Briefly, 2.00 g of the CRM or catalyst was mixed with 40 mL of reverse aqua regia and the suspension was evaporated to dryness. Then, 20 mL of 1/1 (v/v) concentrated HCl–HNO3 solution was added over the residue. After evaporation to dryness; 30 mL of 10% HCl solution was added to the beaker and the suspension was filtered off and the filtrate was completed to 100 mL. After adjustment of the pH of the sample to 1.0, the developed on-line SPE procedure was performed for the determination of Pd(II) level in the samples (Sivrikaya et al. 2011; Imamoglu et al. 2005).
The proposed method was also employed for determination of the Pd(II) levels in some environmental water samples such as Sakarya River, laboratory tap water, Sapanca lake and Marmara seawater. The water samples were immediately acidified with HNO3 to a pH of 2.0 and then filtered using 0.45 µm cellulose acetate membranes. After spiking of 0.1 M chloride in the final samples, the developed method was used for the determination of Pd(II) level in the samples. Spiking and recovery test was also carried out to test accuracy of the developed method.
Results and Discussion
Optimization of Sample Solution Acidity and Chloride Concentration for Pd(II) Uptake
In order to study the effect of solution acidity on the Pd(II) adsorption by PA-SG, the HNO3 level was changed between 0.025 and 5.0 M in 50 mL of Pd(II) solutions containing 50 or 100 mg L−1 Pd(II) and 0.1 M chloride. Then, 50 mg of PA-SG was added to these Pd(II) solutions. After their shaking for 240 min, the concentration of Pd(II) ions remaining in the filtrate was measured by FAAS. The changes in adsorption of Pd(II) ions with the acidity of the solutions are shown in Fig. 3. Pd(II) adsorption is found to be increased by increasing of HNO3 concentration in the solution from 0.025 to 0.1 M. Further increasing of the solution acidity decreases the adsorption of Pd(II) and does not even lead to Pd(II) adsorption in the solution containing 5.0 M HNO3. On the other hand, it is known that adsorption of polyamine with other metal ions [Fe(III), Cu(II), Ni(II), etc.] also occurs at lower nitric acid concentrations than < 0.01 M (or pH 2 and above) (Fan et al. 2012; Radi et al. 2013; Wang et al. 2014). The adsorption of Pd(II) at lower HNO3 level than < 0.025 M was not investigated to eliminate adsorption of these metals. For this reason, it was decided that the acidity of the sample solution for the efficient adsorption of Pd(II) ions was 0.1 M HNO3 containing solutions.
The effect of chloride concentration on the Pd(II) uptake of PA-SG was examined batchwise. For this purpose, chloride concentrations ranging from 0.0 to 3.0 M were added to Pd(II) solution (50 mL) in 0.1 M HNO3. Then, 50 mg of PA-SG was suspended with the solutions and shaken for 360 min. The change of Pd (II) adsorption by the chloride concentration is shown in the Fig. 4. Increasing of chloride concentration from 0.0 to 0.1 M caused an about 5% increase in adsorption of Pd(II) and further increase in chloride level not caused positive or negative effect on the adsorption. Therefore, to obtain the most efficient Pd(II) adsorption with PA-SG, optimal chloride level of Pd(II) solutions were found to be 0.1 M. On the other hand, Pd(II) adsorption ability of PA-SG from solution containing 3.0 M chloride also suggests that the interaction between Pd(II) and polyamine silica gel is mainly due to formation of the chelating complex. However, since the presence of chloride ions enhances the adsorption, the ion exchange mechanism also contributes to Pd(II) adsorption.
Effect of Contact Time and Kinetics of Pd(II) Ions on PA-SG
The effect of contact time on the Pd(II) uptake of PA-SG was examined batchwise. To investigate the adsorption kinetic of Pd(II) ions and to find the equilibrium time, 20 mg of PA-SG was added to 200 mg L−1 Pd(II) solutions (50 mL) containing 0.1 M chloride ions and stirred at ambient temperature for various times ranged from 5 to 600 min. The adsorption of Pd(II) ions on PA-SG by contact time is shown in Fig. 5. The adsorption of Pd(II) was rapidly increased in first 30 min and then, the adsorption was increased slowly until reach the equilibrium conditions at 360 min. Even if the Pd(II) adsorption on PA-SG seems to be long, the PA-SG can adsorb 34.60 mg Pd(II) ions in the first 5 min. This capacity is quite sufficient for uptake trace level of Pd(II) from solution and so, PA-SG can be reliably used for on-line preconcentration Pd(II) ions.
The adsorption of Pd (II) ions on PA-SG was applied to two different kinetic models that pseudo first order and pseudo second model.
Pseudo first order kinetic model equation can be given by Eq. (2) by:
where q e (mg g−1) and q t (mg g−1) show amount of Pd(II) adsorbed on PA-SG at equilibrium and at time t and k 1 (min−1) is pseudo first order rate constant. A plot of ln(q e–q t) versus t is a straight line, where q e and k 1 are calculated from intercept and gradient of the line, respectively (Lagergren 1898).
Pseudo second order kinetic model is given by the following Eq. (3):
where k 2 (g mg−1 min−1) is second order rate constant. A plot of t/q t versus t gives a straight line, and q e and k 2 are calculated from gradient and intercept of the line, respectively (Ho and McKay 1998).
Pseudo first order and pseudo second order kinetic models constants for Pd(II) adsorption by PA-SG are given in Table 1. The calculated q e values (q e cal) for the pseudo first and second order kinetic models are close to the experimental q e values (q e exp). However, when the correlation coefficients of the two kinetic models are compared, it is seen that the correlation coefficient of the pseudo second order kinetic model is closer to unity. Hence, Pd(II) adsorption onto PA-SG is more compatible with pseudo second order kinetic model.
Pd(II) Adsorption Isotherms
The Langmuir and Freundlich isotherms have been successfully applied to identification of Pd(II) adsorption data (Langmuir 1918; Freundlich 1906). The Langmuir and Freundlich isotherms are expressed by the following Eqs. (4 and 5), respectively:
where K L and q max are Langmuir constants related to free energy or adsorption enthalpy (L mg−1) and maximum monolayer adsorption capacity (mg g−1), respectively, and Freundlich coefficients n and K F are related to adsorption intensity and adsorption capacity, respectively (Ghasemi and Asadpour 2007; Moazezi and Moosavian 2016). The curves of Langmuir and Freundlich isotherms are shown in Fig. 6 and isotherm coefficients of both models are given in Table 2.
Considering the correlation coefficients of Langmuir and Freundlich isotherms for the adsorption of Pd(II) ions with PA-SG, it is seen that the correlation coefficient of Langmuir isotherm is higher than Freundlich isotherm and closer to unity. Furthermore, the maximum adsorption capacity experimentally found was more accurately estimated by the Langmuir isotherm. Therefore, it was concluded that adsorption of Pd(II) ions with PA-SG is more compatible with Langmuir isotherm equation.
The maximum Pd(II) adsorption capacities of different adsorbents are listed in Table 3 for comparison. Although higher capacity sorbents than the Pd(II) adsorption capacity of PA-SG were reported in the literature, PA-SG can also be listed among the high Pd(II) adsorption capacity sorbents and this capacity is enough for the on-line preconcentration of trace level of Pd(II) ions.
Optimization of the On-line Preconcentration Procedure
Optimization of the Eluent
To determine the most efficient eluent for Pd(II) ions from the PA-SG, solutions of thiourea in HCl were tested as eluent. For this purpose, Pd(II) solution was passed through the column at a flow rate of 5 mL min−1 and adsorbed Pd(II) ions eluted with different thiourea in HCl solution at a flow rate of 5 mL min−1. The results in Table 4 demonstrated that the absorbance was the best when 1.0 and 3.0% thiourea in 1.0 M HCl was used as eluent. 1.0% thiourea in 1.0 M HCl solution was selected as the optimal eluent due to contain fewer dissolved matter.
Different eluent volumes (68–500 μL) were tested to optimize the on-line preconcentration system. For this purpose, the sample solution was passed through the column at a flow rate of 5 mL min−1 for 1 min and then, retained Pd(II) ions on PA-SG were eluted at a flow rate of 7.5 mL min−1. The results in Fig. 7 show that full elution of adsorbed Pd(II) on the PA-SG is not achieved when 68 μL of the eluent volume is used. Full elution was done by 250 μL volume of the eluent. Therefore, 250 μL of the eluent volume was optimized in this study.
Effect of eluent was studied at flow rates in the range from 2.5 to 7.5 mL min−1 using 250 μL of 1% thiourea in 1 M HCl and the highest absorbance was obtained at 7.5 mL min−1. So, the 7.5 mL min−1 was selected as the optimum eluent flow rate.
Optimization of the Sample Flow Rate and Volume
The flow rate of the sample solution determines the contact time between the analytes and the sorbent in the on-line preconcentration procedures. Hence, sorbents should have fast kinetics of sorption of analytes for success of on-line preconcentration method. High flow rate of both sample and elution solutions are preferred in on-line preconcentration systems. The sampling frequency increases by high flow rate of sample solutions. The low flow rate of eluent solution causes to peak expansion.
The sample solution (5 mL) containing 0.1 mg L−1 Pd(II) was passed through the mini column at various flow rates ranged from 2.5 to 7.5 mL min−1. It was found that there was no change in the absorbance by the increasing of sample flow rate. Therefore, the highest flow rate of 7.5 mL min−1 was chosen as the optimum sample flow rate.
Different volumes of sample solutions in 0.1 M HCl containing same amount of Pd(II) (0.5 μg) were passed through the mini column and then, adsorbed Pd(II) ions were eluted using 250 μL of % 1 thiourea in 0.1 M HCl at flow rate of 7.5 mL min−1. The Pd(II) absorbances were not decreased up to 30 mL of sample volume. So, it can be concluded that the method can reliably be used up to 30 mL sample volume when the concentration of Pd(II) is low to achieve high PF or to obtain smaller LOD values.
Effect of Matrix Ions
To examine the effects of the matrix ions for the determination of Pd(II) ions at the on-line preconcentration system, various heavy metal ions and some major cations and anions were spiked to 0.1 mg L−1 Pd(II) solutions. Then, the solutions were passed through the mini column for 1 min at a flow rate of 7.5 mL min−1, and the elution was done using 250 μL of %1 thiourea in 0.1 M HCl at a flow rate of 7.5 mL min−1. The concentrations of matrix ions given in Table 5 caused a relative error of less than 5% in the on-line determinations of Pd(II). So, the developed on-line preconcentration technique for Pd(II) can reliably be used for the determination of Pd(II) levels in real samples containing similar matrix ions.
Analytical Figures of Merit
Experimental parameters of the developed on-line preconcentration method for Pd(II) ions are listed in Table 6 and the analytical figures of merit of the method containing calibration curve equation, linear range, limit of detection (LOD), limit of quantification (LOQ), and preconcentration factor is listed in Table 7.
The LOD of 3 µg L−1 and LOQ of 10 µg L−1 were described as 3σ/m and 10σ/m, respectively, where σ is the standard deviation of ten replicates of blank signal and m is the slope of the calibration curve after preconcentration system. The relative standard deviation (RSD) for the 25 µg L−1 of Pd(II) was 4.6% at five replicate measurements. Additionally, RSD was calculated from the real samples analysis results of CRM platinum ore SARM 7B and was found to be 5.7% (n = 3). The calibration curve was linear in the range of 10–200 µg L−1. The preconcentration factor of 23.9 was obtained from the ratio of calibration curves slopes obtained with the on-line preconcentration and direct aspiration. The sampling frequency of the on-line preconcentration method was calculated to be 20 h−1 which showing the rapidity of the developed method.
Accuracy of the On-line Preconcentration Method
To test the accuracy of the proposed method, the proposed method was applied to the certified reference material platinum ore SARM 7B for the determination of Pd level. The obtained results in Table 8 show that the found Pd level by the developed on-line preconcentration method was determined with a relative error of − 8.4%. Student’s test (t test) at 95% confidence level was performed to examine the compliance of obtained value and the certified reference value. It was found that the t exp (experimental value) value of 2.81 was lower than t crit (critical value) of 4.30. Therefore, there is no significant difference between the obtained and the certified values at 95% confidence level.
Determination of Pd(II) Level in Various Samples
This developed method was successfully applied to the determination of Pd(II) levels in spent auto catalyst and water samples. To check the accuracy of the method, different amounts Pd(II) ions were spiked to these water samples and recoveries were computed after measuring of Pd(II) levels by on-line preconcentration system coupled with FAAS. The found Pd(II) concentrations in environmental water samples, spiked and recovery values of Pd(II) are presented in the Table 9. The level of Pd(II) was found to be below LOD level of the method. The recoveries of Pd(II) ions from spiked samples are in the range of 98.0–108% showing the method can be used successfully for Pd(II) determination in environmental samples in the presence of different matrices. Application of the method to solid material for the determination of Pd(II) level was also carried out using spent auto catalyst sample. The Pd(II) concentration in the spent auto catalyst was found to be 3530 ± 70 µg/g. The found level of Pd(II) in spent auto catalyst is similar to the results in the literature (Saçmacı et al. 2013).
Comparison of the On-line Preconcentration Method
Analytical figures of merit of different methods reported in the literature for the on-line preconcentration of Pd(II) ions are summarized in Table 10. The PF of two methods was reported to be 125 (Krishna et al. 2009) and 335 (Sharma et al. 2012) and the others were in the range of between 8.7 and 29. The PF of the developed method in this study is 23.9 and it could be said that similar PF is obtained with many other studies. The LOD of the on-line preconcentration methods coupled with FAAS is in the range of 3.0 and 16 μg L−1 and two instruments (GFAAS and ICP-MS) have a lower LOD values than FAAS. The LOD value of the developed method has the lowest LOD values among the FAAS based methods.
Conclusion
The on-line preconcentration and adsorption of Pd(II) by polyamine attached silica gel was fully investigated for the development novel method for determination of Pd(II) levels. The PA-SG can efficiently uptake Pd(II) ions from solution containing 0.1 M H3O+ and Cl−. The batch adsorption of Pd(II) was reached to equilibrium at 360 min and PA-SG has Pd(II) uptake capacity of 158.7 mg g−1. The on-line preconcentration of Pd(II) using PA-SG can adsorb and release Pd(II) ions at high flow rate of 7.5 mL min−1. The limit of detection of the method for Pd(II) was 3 µg L−1. The required time for the determination of Pd(II) ions by on-line preconcentration system was 3 min and sampling frequency was found to be 20 h−1. The precision and accuracy of this developed method was satisfactory. The method offers many advantages such as simplicity, usability, rapid sample loading, fast elution, and being environmentally friendly and has also very good precision and accuracy for on-line preconcentration of Pd(II) ions.
References
Anthemidis AN, Themelis DG, Stratis JA (2001) Stopped-flow injection liquid-liquid extraction spectrophotometric determination of palladium in airborne particulate matter and automobile catalysts. Talanta 54:37–43. doi:10.1016/S0039-9140(00)00620-2
Antonio F, Cajamarca S, Zanetti M, Caroline M, Douglas P, Dragunski C, Rocker C (2016) Investigation on the performance of chemically modified aquatic macrophytes-salvinia molesta for the micro-solid phase preconcentration of Cd (II) on-line coupled to FAAS. Bull Environ Contam Toxicol 97:863–869. doi:10.1007/s00128-016-1923-3
Avino P, Santoro E, Sarto F, Violante V, Rosada A (2011) Neutron activation analysis for investigating purity grade of copper, nickel and palladium thin films used in cold fusion experiments. J Radıoanal Nucl Chem 290:427–436. doi:10.1007/s10967-011-1296-3
Booth SG, Chang SY, Uehara A, La Fontaine C, Cibin G, Schroeder SLM, Dryfe RAW (2017) In situ XAFS Study of palladium electrodeposition at the liquid/liquid interface. Electrochim Acta 235:251–261. doi:10.1016/j.electacta.2017.03.059
Bosch Ojeda C, Sánchez Rojas F, Manuel Cano Pavón J (2007) On-line preconcentration of palladium(II) using a microcolumn packed with a chelating resin, and its subsequent determination by graphite furnace atomic absorption spectrometry. Microchim Acta 158:103–110. doi:10.1007/s00604-006-0700-0
Bruzzoniti MC, Mucchino C, Tarasco E, Sarzanini C (2003) On-line preconcentration, ion chromatographic separation and spectrophotometric determination of palladium at trace level. J Chromatogr A 1007:93–100. doi:10.1016/S0021-9673(03)00931-2
Fan Z, Shen J, Li R, Li S (2012) Synthesis and adsorption behavior of surface Cu(II) Ion-imprinted Poly(allylamine)-Silica gel material. Polym-Plast Technol 51:1289–1295. doi:10.1080/03602559.2012.700543
Freundlich HMF (1906) Uber die adsorption in losungen. Z Phys Chem 57:385–470
Fujiwara K, Ramesh A, Maki T, Hasegawa H, Ueda K (2007) Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto L-lysine modified crosslinked chitosan resin. J Hazard Mater 146:39–50. doi:10.1016/j.jhazmat.2006.11.049
Ghaedi M, Montazerozohori M, Nazari E, Nejabat R (2013) Functionalization of multiwalled carbon nanotubes for the solid-phase extraction of silver, cadmium, palladium, zinc, manganese and copper by flame atomic absorption spectrometry. Hum Exp Toxıcol 32:687–697. doi:10.1177/0960327112467039
Ghasemi J, Asadpour S (2007) Thermodynamics’ study of the adsorption process of methylene blue on activated carbon at different ionic strengths. J Chem Thermodyn 39:967–971. doi:10.1016/j.jct.2006.10.018
Godlewska-Żyłkiewicz B, Leśniewska B, Wilczewska AZ (2012) Evaluation of ion imprinted polymers for the solid phase extraction and electrothermal atomic absorption spectrometric determination of palladium in environmental samples. Int J Envıron Anal Chem 7319:1–16. doi:10.1080/03067319.2012.656096
Golshaei R, Shemirani F, Davudabadi Farahani M (2015) Combination of solid phase extraction based on nano alumina and liquid-liquid extraction for selective determination of palladium in food samples. J Anal Chem 70:310–315. doi:10.1134/S1061934815030089
Gurung M, Babu B, Morisada S, Kawakita H, Ohto K (2013) N-aminoguanidine modified persimmon tannin: a new sustainable material for selective adsorption, preconcentration and recovery of precious metals from acidic chloride solution. Bioresource Technol 129:108–117. doi:10.1016/j.biortech.2012.11.012
Hassanien MM (2009) FAAS determination of palladium after its selective recovery by silica modified with hydrazone derivative. Microchim Acta 167:81–89. doi:10.1007/s00604-009-0219-2
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. J Environ Sci Health Part B 76:183–191. doi:10.1205/095758298529326
Iglesias M, Anticó E, Salvadó V (2003) On-line determination of trace levels of palladium by flame atomic absorption spectrometry. Talanta 59:651–657. doi:10.1016/S0039-9140(02)00616-1
Imamoglu M, Aydin AO, Dundar MS (2005) Determination of gold, palladium and copper by flame atomic absorption spectrometry after preconcentration on silica gel modified with 3-(2-aminoethylamino) propyl group. Cent Eur J Chem 3:252–262. doi:10.2478/BF02475994
Jamali MR, Assadi Y, Shemirani F, Salavati-Niasari M (2007) Application of thiophene-2-carbaldehyde-modified mesoporous silica as a new sorbent for separation and preconcentration of palladium prior to inductively coupled plasma atomic emission spectrometric determination. Talanta 71:1524–1529. doi:10.1016/j.talanta.2006.07.034
Jia X, Gong D, Wang J, Huang F, Duan T, Zhang X (2016) Arsenic speciation in environmental waters by a new specific phosphine modified polymer microsphere preconcentration and HPLC–ICP-MS determination. Talanta 160:437–443. doi:10.1016/j.talanta.2016.07.050
Karaçetin G, Sivrikaya S, Imamoglu M (2014) Adsorption of methylene blue from aqueous solutions by activated carbon prepared from hazelnut husk using zinc chloride. J Anal Appl Pyrol 110:270–276. doi:10.1016/j.jaap.2014.09.006
Karadaş C, Kara D (2013) On-line preconcentration and determination of trace elements in waters and reference cereal materials by flow injection–FAAS using newly synthesized 8-hydroxy-2-quinoline carboxaldehyde functionalized Amberlite XAD-4. J Food Compos Anal 32:90–98. doi:10.1016/j.jfca.2013.07.003
Karadaş C, Turhan O, Kara D (2013) Synthesis and application of a new functionalized resin for use in an on-line, solid phase extraction system for the determination of trace elements in waters and reference cereal materials by flame atomic absorption spectrometry. Food Chem 141:655–661. doi:10.1016/j.foodchem.2013.03.042
Kondo K, Kanazawa Y, Matsumoto M (2015) Adsorption of noble metals using silica gel modified with surfactant molecular assembly containing an extractant. Separat Sci Technol 50:1453–1460. doi:10.1080/01496395.2014.976877
Kovalev IA, Bogacheva LV, Tsysin GI, Formanovsky AA, Zolotov YA (2000) FIA-FAAS system including on-line solid phase extraction for the determination of palladium, platinum and rhodium in alloys and ores. Talanta 52:39–50. doi:10.1016/S0039-9140(00)00314-3
Krishna MVB, Ranjit M, Chandrasekaran K, Venkateswarlu G, Karunasagar D (2009) On-line preconcentration and recovery of palladium from waters using polyaniline (PANI) loaded in mini-column and determination by ICP-MS; elimination of spectral interferences. Talanta 79:1454–1463. doi:10.1016/j.talanta.2009.06.008
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. K Sven Vetensk Handl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Leśniewska BA, Godlewska I, Godlewska—Żyłkiewicz B (2005) The study of applicability of dithiocarbamate-coated fullerene C60 for preconcentration of palladium for graphite furnace atomic absorption spectrometric determination in environmental samples. Spectrochim Acta B 60:377–384. doi:10.1016/j.sab.2004.11.013
Limbeck A, Rudolph E, Hann S, Koellensperger G, Stingeder G, Rendl J (2004) Flow injection on-line pre-concentration of platinum coupled with electrothermal atomic absorption spectrometry. J Anal Atom Spectrom 19:1474. doi:10.1039/B407546N
Liu P, Pu Q, Su Z (2000) Synthesis of silica gel immobilized thiourea and its application to the on-line preconcentration and separation of silver, gold and palladium. Analyst 125:147–150. doi:10.1039/A906074J
Liu L, Liu S, Zhang Q, Li C, Bao C, Liu X, Xiao P (2013) Adsorption of Au(III), Pd(II), and Pt(IV) from aqueous solution onto graphene oxide. J Chem Eng Data 58:209–216. doi:10.1021/je300551c
Messerschmidt J, von Bohlen A, Alt F, Klockenkamper R (2000) Separation and enrichment of palladium and gold in biological and environmental samples, adapted to the determination by total reflection X-ray fluorescence. Analyst 125:397–399. doi:10.1039/b000471p
Mladenova E, Dakova I, Karadjova I, Karadjov M (2012) Column solid phase extraction and determination of ultra-trace Au, Pd and Pt in environmental and geological samples. Microchem J 101:59–64. doi:10.1016/j.microc.2011.10.007
Moazezi N, Moosavian MA (2016) Removal of rubidium ions by polyaniline nanocomposites modified with cobalt-Prussian blue analogues. J Environ Chem Eng 4:2440–2449. doi:10.1016/j.jece.2016.04.018
Muzikar M, Fontàs C, Hidalgo M, Havel J, Salvadó V (2006) A preconcentration system using polyamine metalfix-chelamine resin for the on-line determination of palladium(II) and platinum(IV) by inductively coupled plasma optical emission spectrometry. Talanta 70:1081–1086. doi:10.1016/j.talanta.2006.02.021
Nakajima J, Ohno M, Chikama K, Seki T, Oguma K (2009) Determination of traces of palladium in stream sediment and auto catalyst by FI-ICP-OES using on-line separation and preconcentration with QuadraSil TA. Talanta 79:1050–1054. doi:10.1016/j.talanta.2009.02.035
Niemelä M, Pitkäaho S, Ojala S, Keiski RL, Perämäki P (2012) Microwave-assisted aqua regia digestion for determining platinum, palladium, rhodium and lead in catalyst materials. Microchem J 101:75–79. doi:10.1016/j.microc.2011.11.001
Radi S, Toubi Y, Bacquet M, Degoutin S, Cazier F (2013) 1-(Pyridin-2-yl) Imine functionalized silica gel: synthesis, characterization, and preliminary use in metal ion extraction. Separat Sci Technol 48:1349–1355. doi:10.1080/01496395.2012.726309
Ramakul P, Yanachawakul Y, Leepipatpiboon N, Sunsandee N (2012) Biosorption of palladium (II) and platinum (IV) from aqueous solution using tannin from Indian almond (Terminalia catappa L.) leaf biomass: kinetic and equilibrium studies. Chem Eng J 193:102–111. doi:10.1016/j.cej.2012.04.035
Rojas FS, Ojeda CB, Pavón JMC (2006) Automated on-line separation preconcentration system for palladium determination by graphite furnace atomic absorption spectrometry and its application to palladium determination in environmental and food samples. Talanta 70(5):979–983. doi:10.1016/j.talanta.2006.05.048
Rossi E, Errea MI, de Cortalezzi MMF, Stripeikis J (2017) Selective determination of Cr(VI) by on-line solid phase extraction FI-SPE-FAAS using an ion exchanger resin as sorbent: an improvement treatment of the analytical signal. Microchem J 130:88–92. doi:10.1016/j.microc.2016.08.004
Saçmacı Ş, Şahan S, Saçmacı M, Şahin U, Ülgen A, Kartal Ş (2013) On-line determination of palladium by flame atomic absorption spectrometry coupled with a separation/preconcentration minicolumn containing a new sorbent. Intern J Environ Anal Chem 93:1223–1235. doi:10.1080/03067319.2012.727809
Saitoh T, Suzuki S, Hiraide M (2005) Solid phase extraction of some precious metals from hydrochloric acid to polystyrene-divinylbenzene porous resin impregnated with polyoxyethylene-type nonionic surfactant. J Chromatogr A 1097:179–182. doi:10.1016/j.chroma.2005.10.002
Sayın M, Can M, Imamoglu M, Arslan M (2015) 1,3,5-Triazine-pentaethylenehexamine polymer for the adsorption of palladium (II) from chloride-containing solutions. React Funct Polym 88:31–38. doi:10.1016/j.reactfunctpolym.2015.02.003
Schoeman E, Bradshaw SM, Akdogan GA, Snyders CAJJ (2017) Extraction of platinum and palladium from a heap leach cyanide solution using strong base anion exchange resins. Int J Miner Process 162:27–35. doi:10.1016/j.minpro.2017.02.017
Sharma RK, Pandey A, Gulati S, Adholeya A (2012) An optimized procedure for preconcentration, determination and on-line recovery of palladium using highly selective diphenyldiketone-monothiosemicarbazone modified silica gel. J Hazard Mater 209–210:285–292. doi:10.1016/j.jhazmat.2012.01.022
Sivrikaya S, Altundag H, Zengin M, Imamoglu M (2011) Separation, preconcentration, and recovery of Pd(II) Ions using newly modified silica gel with Bis(3-Aminopropyl)Amine. Separat Sci Technol 46:2032–2040. doi:10.1080/01496395.2011.572111
Sivrikaya S, Imamoglu M, Kara D (2014) On-line solid phase extraction of nickel, copper, and cadmium using a newly synthesized polyamine silica gel-loaded mini-column for flame atomic absorption spectrometric determination. Atomic Spectrosc 35:168–176
Sivrikaya S, Imamoglu M, Yıldız SZ, Kara D (2016) Novel functionalized silica gel for on-line preconcentration of Cadmium (II), Copper (II), and Cobalt (II) with determination by flame atomic absorption spectrometry. Anal Lett 49:943–957. doi:10.1080/00032719.2015.1067812
Soylak M, Tuzen M (2008) Coprecipitation of gold(III), palladium(II) and lead(II) for their flame atomic absorption spectrometric determinations. J Hazard Mater 152:656–661. doi:10.1016/j.jhazmat.2007.07.027
Tarley CRT, Corazza MZ, de Oliveira FM, Somera BF, Nascentes CC, Segatelli MG (2017a) On-line micro-solid phase preconcentration of Cd2+ coupled to TS-FF-AAS using a novel ion-selective bifunctional hybrid imprinted adsorbent. Microchem J 131:57–69. doi:10.1016/j.microc.2016.11.013
Tarley T, Diniz KM, Cajamarca FA (2017b) Study on the performance of micro-flow injection preconcentration method on-line coupled to thermospray flame furnace AAS using MWCNTs wrapped with polyvinylpyridine nanocomposites as adsorbent. RSC Adv 7:19296–19304. doi:10.1039/C7RA01220A
Tavakkoli N, Habibollahi S, Keshavarzianpour M (2014) Solid phase extraction of trace amounts of palladium in environmental water samples on multi-walled carbon nanotubes as a new sorbent: comparison with activated carbon. Desalin Water Treat 52:350–356. doi:10.1080/19443994.2013.792143
Tavakoli L, Yamini Y, Ebrahimzadeh H, Nezhadali A, Shariati S, Nourmohammadian F (2008) Development of cloud point extraction for simultaneous extraction and determination of gold and palladium using ICP-OES. J Hazard Mater 152:737–743. doi:10.1016/j.jhazmat.2007.07.039
Velmurugan M, Thirumalraj B, Chen SM, Al-Hemaid FMA, Ajmal Ali M, Elshikh MS (2017) Development of electrochemical sensor for the determination of palladium ions (Pd2+) using flexible screen printed un-modified carbon electrode. J Colloid Interf Sci 485:123–128. doi:10.1016/j.jcis.2016.08.073
Wang H, Li C, Bao C, Liu L, Liu X (2011) Adsorption and determination of Pd (II) and Pt(IV) onto 3′-Nitro-4- amino azobenzene modified chitosan. J Chem Eng Data 56:4203–4207. doi:10.1021/je2007154
Wang Q, Gao W, Liu Y, Yuan J, Xu Z, Zeng Q, Schröder M (2014) Simultaneous adsorption of Cu(II) and SO42- ions by a novel silica gel functionalized with a ditopic zwitterionic Schiff base ligand. Chem Eng J 250:55–65. doi:10.1016/j.cej.2014.03.106
Wu XZ, Liu P, Pu QS, Sun QY, Su ZX (2004) Preparation of dendrimer-like polyamidoamine immobilized silica gel and its application to online preconcentration and separation palladium prior to FAAS determination. Talanta 62:918–923. doi:10.1016/j.talanta.2003.10.011
Ye J, Liu SX, Tian M, Li W, Hu B, Zhou W, Jia Q (2014) Preparation and characterization of magnetic nanoparticles for the on-line determination of gold, palladium, and platinum in mine samples based on flow injection micro-column preconcentration coupled with graphite furnace atomic absorption spectrometry. Talanta 118:231–237. doi:10.1016/j.talanta.2013.10.018
Zalupski PR, Mcdowell R, Dutech G, Zalupski PR, Mcdowell R, Dutech G (2014) The adsorption of gold, palladium, and platinum from acidic chloride solutions on mesoporous carbons. Solvent Extr Ion Exch 32:737–748. doi:10.1080/07366299.2014.951278
Zhang S, Pu Q, Liu P, Sun Q, Su Z (2002) Synthesis of amidinothioureido-silica gel and its application to flame atomic absorption spectrometric determination of silver, gold and palladium with on-line preconcentration and separation. Anal Chim Acta 452:223–230. doi:10.1016/S0003-2670(01)01476-3
Zheng H, Zhang D, Wang WY, Fan YQ, Li J, Han HP (2007) Highly selective determination of palladium(II) after preconcentration using Pd(II)-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Microchim Acta 157:7–11. doi:10.1007/s00604-006-0649-z
Zhou L, Xu J, Liang X, Liu Z (2010) Adsorption of platinum (IV) and palladium (II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J Hazard Mater 182:518–524. doi:10.1016/j.jhazmat.2010.06.062
Zhou F, Qian L, Li G, Zhu K, Peng J, Li C (2014a) On-line flow injection preconcentration of palladium in environmental waters using a microcolumn packed with silica nanoparticles modified by γ-aminopropyltriethoxisilane and determination by flame atomic absorption spectrometry. Anal Lett 47:543–555. doi:10.1080/00032719.2013.845834
Zhou SY, Song N, Liu SX, Chen DX, Jia Q, Yang YW (2014b) Separation and preconcentration of gold and palladium ions with a carboxylated pillar[5]arene derived sorbent prior to their determination by flow injection FAAS. Microchim Acta 181:1551–1556. doi:10.1007/s00604-014-1229-2
Zolfonoun E, Yousefi SR (2016) Simultaneous determination of rare earth elements by ICP OES after on-line enrichment using multi-walled carbon nanotubes coated cellulose acetate membrane. J Braz Chem Soc 27:2348–2353. doi:10.5935/0103-5053.20160131
Acknowledgements
This work was supported by the Sakarya University Research Fund with Project Number 2017-50-01-027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivrikaya, S., Karslı, B. & Imamoglu, M. On-line Preconcentration of Pd(II) Using Polyamine Silica Gel Filled Mini Column for Flame Atomic Absorption Spectrometric Determination. Int J Environ Res 11, 579–590 (2017). https://doi.org/10.1007/s41742-017-0051-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-017-0051-1