Abstract
The aim of the present work is the assessment of a new sorbent, prepared using silica gel coated with a pyrimidine derivative (allyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate), for extraction and preconcentration trace amount of lead from different samples prior to determination by flame atomic absorption spectrometry. Common coexisting ions did not interfere with the separation and determination of lead at pH 6, so that lead ion completely adsorbed on the column. The limit of detection based on three times the standard deviation of the blank was found to be 0.53 ng mL−1 in original solution. Obtained sorption capacity for 1 g sorbent was 5.0 mg Pb. The linearity was maintained in the concentration range of 0.1–30.0 ng mL−1 for the concentrated solution. Eight replicate determinations of 2.0 μg mL−1 of lead in the final solution gave relative standard deviation of ±2.6 %. The proposed method was successfully applied to the determination trace amounts of lead in the environmental samples such as carrot, rice, zardchoobe, and real water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead has been used at least since biblical times in a variety of products, mainly in inorganic form, but its impact as a major environmental pollutant was recognized in the last century. It virtually affects every system in the body. Blood lead levels as low as 100 μg L−1 are associated with adverse health effects in children (Dadfarnia et al. 2008). In recent years there has been an increased concern over the content of lead in drinking and natural water. The World Health Organization recommended a limit of 10 μg L−1 of lead in drinking water (Baird 1999), which requires a very sensitive measurement technique. Currently, the most common analytical methods for the lead trace determination are flame atomic absorption spectrometry (FAAS) (Dos Santos et al. 2004; Silva and Roldan 2009; Soylak et al. 2006; Tokalioglu et al. 2009; Tuzen et al. 2006), electrothermal atomic absorption spectrometry (Martinis et al. 2010; Jiang and Hu 2008; Liang et al. 2008; Minami et al. 2005), inductively coupled plasma atomic emission spectrometry (Liu et al. 2005), and inductively coupled plasma-mass spectrometry (Diedjibegovic et al. 2012; Diang and Hu 2012). FAAS is still being used because it combines a fast analysis time, a relative simplicity and a cheaper cost. Nevertheless, the detection of metal trace elements in aqueous samples is difficult due to various factors, particularly their low concentration and the matrix effects (Chen et al. 2005).
Solid-phase extraction (SPE) technique has become increasingly popular compared with other techniques such as liquid–liquid extraction because of its advantages of high enrichment factor, high recovery, rapid phase separation, low cost, low consumption of organic solvents, and the combining ability with different detection techniques in the form of online or offline mode.
The choice of sorbent is a key point in SPE because it can control the analytical parameters such as selectivity, affinity, and capacity. Preparation of new material for selective solid-phase extraction of analytes is an important trend of solid-phase extraction (Pool 2003). Reagents can be modified on organic or inorganic support as solid-phase extractant. Silica gel is one of the most used solid materials due to its well-established particle size and well-defined porosity and high surface area. It presents other advantages such as no swelling, fast kinetics, mechanical, thermal, and chemical stability under various conditions (Jal et al. 2004). Therefore; it is a widely used support for various solid-phase extractants.
This paper describes an analytical procedure for preconcentration of lead in water and plant samples by using silica gel coated with allyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (DHPM) (Fig. 1) in a column system and determination by FAAS.
Experimental
Apparatus and reagents
An atomic absorption spectrometer model Sens AA (Dandenong, Australia, (http://www.gbcaustralia.com) equipped with deuterium lamp background corrector was used for determination of lead in air–acetylene flame. The instrumental settings of the spectrometer were as follows: wavelength, 217.0 nm; slit width, 1.0 nm; lamp current, 5 mA; acetylene flow, 1.5 L min−1; and air flow, 3.5 L min−1. A mechanical shaker KS 130 basic (Deutschland, Germany, www.ika.net) having speed control and timer was used for preparation of the sorbent. Funnel-tipped glass tube (5 × 100 mm) equipped with stopcock was used as a column for the preconcentration purposes. A 691 Metrohm pH meter (Herisau, Switzerland, http://www.metrom.com) was employed for pH measurements.
All reagents were of analytical grade. Deionized and distilled water was used in all experiments. The stock solution of 1,000.0 μg mL−1 Pb (II) was prepared by dissolving an appropriate amount of Pb(NO3)2 99.99 % (Merck, Darmstadt, Germany) in distilled water and by diluting to 100.0 mL. The standard working solutions were diluted daily prior to use. A 0.2 % (w/v) solution of the ligand was prepared by dissolving 0.20 g of DHPM in chloroform and diluting to 100.0 mL. Silica gel (0.063–0.2 mm or 70–230 mesh ASTM) (Merck) was used as sorbent.
Preparation of the ligand DHMP
A mixture of the appropriate benzaldehyde (2.00 mmol), ally acetoacetate (2.00 mmol), thiourea (3.00 mmol), and concentrated HCl (0.5 % mol.) as catalyst was stirred vigorously at 100 °C without solvent for 30 min. After cooling down to room temperature, the reaction mixture was poured onto crushed ice. Stirring was continued for several minutes. The desired insoluble 3,4-DHPM was collected by filtration and was purified by recrystallization by EtOH/water and subsequently dried (Mp 154–155 °C) (Desai et al. 2006).
Preparation of the sorbent
Firstly, silica gel was activated with concentrated hydrochloric acid for 4 h under reflux conditions, and then filtered off and washed thoroughly with doubly distilled water till it was acid-free and finally at 150 °C for 6 h (Madrakian et al. 2006).
For the synthesis of the sorbent, 4–5 g of activated silica gel was added to 50.0 mL of the solution containing 0.2 % DHPM, and the mixture was shaken at room temperature for 24 h. The reagent-coated silica gel was filtered, washed with distilled water, and dried at room temperature.
The modified silica gel was confirmed by IR analysis (Fig. 2). In the comparison of the IR spectrum of bare silica gel with modified silica gel, many new peaks appeared in the spectrum. The infrared spectrum of the ligand showed strong absorption bands at 3,327 and 3,258 (N–H); 3171, 3,099 and 2,935 (C–H); 1,702 (C = O), 1,649 (C = C), 1,594 (C = S), 1,410 (C–N), 1,185 (C–O–C), and 694 (phenyl bending). The silica adsorbent (Fig. 2a) showing the characteristic peaks at 470 and 810 cm−1 are ascribed to the Si–O–Si bending vibration, that at 1,100 cm−1 to the Si–O stretching vibration, and that at 960 cm−1 to the Si–OH stretching vibration and strong peaks at about 3,400–3,450 cm−1 hydroxyl groups on the surface of the silica.
However, the IR-spectrum of modified silica adsorbent with ligand is dominated by the peaks corresponding to the silica matrix and some of the band corresponding to ligand, for example, at 3,174, 3,099, and 2,935 (C–H); 1,702 (C = O); 1,649 (C = C); 1,594 (C = S); 1,185 (C–O–C); and 694 (phenyl bending) (Fig. 2b). Consequently, the above experimental results suggest that silica is successfully modified by the ligand DHPM.
Analysis of real samples
Various plant samples were dried in 80 °C for 72 h. Two grams of the powder of dry samples (tea, carrot, rice, and zardchoobe) was weighted exactly into a 250-mL beaker, separately, and dissolved in concentrated nitric acid and perchloric acid (3:1) by heating on a heater. The solutions were cooled, diluted, and filtered. The filtered solution was diluted to 100.0 mL with distilled water in a calibration flask (Malekpour et al. 2009). Twenty-five milliliters of the pretreated sample solution was taken individually, and lead was determined by the proposed procedure.
The proposed preconcentration procedure has been also applied to determine lead in water different samples including tap water from Payame Noor University and river and spring water from Lalezar, Bardsirand Shahdad in Kerman. The water samples were filtered through a cellulose membrane filter (Millipore) of 0.45-μm pore size. Fifty milliliters of water samples was transferred to a beaker, and pH was adjusted to 6 by addition of the buffer solution. Then the proposed procedure was applied to these samples.
General procedure
A small amount of glass wool was placed in the end of the columns to prevent loss of the sorbent during sample loading. Then, the columns were packed with 100.0 mg of the silica gel coated with DHPM and conditioned with distilled water. The bed height of the adsorbent in the column was approximately 10 mm. An aliquot of the sample solution containing Pb(II) (0.5–150.0 μg) was taken in a 50-mL beaker. The pH of the solution was adjusted to 6 with diluted nitric acid or sodium hydroxide. The total volume of the solution was made up of about 30 mL with distilled water. It was then passed through the column with flow rate of 3.0 mL min−1. The retained lead ions were eluted from the solid phase with 5.0 mL of 1.0 mol L−1 HCl solution. This solution was aspirated into an air–acetylene flame for the determination of Pb by FAAS.
Results and discussion
The optimum sorption and desorption properties of the new sorbent for Pb was found by using the column technique. The preliminary experiments showed that the bared silica gel can adsorb a lot of metal ions, but adsorption was not selective and the recovery was incomplete. By immobilization of the ligand DHPM on the silica gel, only Pb can be adsorbed in the specific pH. In order to achieve the best performance, the separation/preconcentration procedure was optimized for various analytical parameters, such as pH of the sample, the flow rate of eluent and sample solution, amount of adsorbent, volume and type of the eluent solution, and volume of the sample solution. Various ion interference effects were also investigated.
Effect of pH
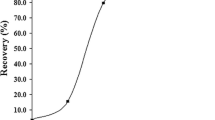
The pH of the sample solution plays an important role in retention of metal ions. Thus, the effect of pH on the recovery of Pb(II) was examined. The pH value of the sample solution was adjusted in a range of 2–10 with diluted HNO3 or NaOH. The obtained solutions were passed through the column at a flow rate of 3.0 mL min−1. The lead ions were then eluted by an appropriate eluent (5.0 mL of 1.0 mol L−1 HCl solution) and determined by FAAS. As can be seen in Fig. 3, quantitative recovery (>95 %) was found in the pH > 5 for lead. A pH of 6 was selected for further studies.
Effect of type and concentration of eluent
There are many different methods for desorption of analyte on solid phase. One of them is simply to wash the modified adsorbent with a small amount of an organic solvent, such as acetone, acetonitrile, or methanol. This will usually dissolve the metal complexes and cause them to elute quickly and completely. Another possibility is to wash the column with an aqueous solution that is sufficiently acidic to break up the metal organic complex. A combination of these two, as the third desorption approach, is possible; a solution of strong mineral acid in an organic solvent will simultaneously break up the complex and eluent analyte. In order to choose the most effective eluent for desorbing of the retained Pb(II) from the column, a series of selected eluent solutions such as different acids were tested. The results showed that the recovery is best when hydrochloric acid was used as the eluent. Five milliliters of 1.0 mol L−1 HCl solution was applied for further works.
Effect of flow rate of sample and eluent solution
The retention of an element on a sorbent also depends on the flow rate of the sample solution. Thus, the effect of flow rate of the sample and elution solution on the retention and recovery of lead ions was investigated under optimum conditions. The solution containing Pb(II) was passed through the column with the flow rates adjusted in a range of 0.15–4.0 mL min−1. It was observed that, at flow rates greater than 3.0 mL min−1, there was a decrease in the recovery Pb. The reason for this decrease is probably insufficient contact of the metal ions and the sorbent to reach equilibrium. Therefore, a flow rate of 3.0 mL min−1 was selected for subsequent experiments.
For desorption of lead ions, flow rate was varied between 0.5–3.0 mL min−1. The flow rate of 2.0 mL min−1 was adequate for desorption of the analyte. Therefore, a flow rate of 3.0 mL min−1 was used in the sorption step and flow rate of 2.0 mL min−1 for desorption and adjusted with a stopcock in end of the column.
Effect of amount of the sorbent
The amount of sorbent is another important parameter that affects the recovery. A quantitative retention is not obtained when the amount of sorbent is less. On the other hand, an excess amount of sorbent prevents the elution of the retained sample by a small volume of elution solution. For this purpose, different amounts of the sorbent (3.0–100.0 mg) were examined by using 5.0 mL of elution solution. A 100.0-mg sorbent gave the highest recovery with using 5.0 mL of elution solution. So, in order to achieve higher recovery, subsequent extraction experiments were carried out with 100.0 mg of the sorbent.
Effect of the sample volume (breakthrough volume)
The measurement of breakthrough volume is important in solid-phase extraction because breakthrough volume represents the sample volume that can be preconcentrated without loss of analyte during elution of the sample. The volume of the first aqueous phase, containing a fixed amount of analyte (5.0 μg Pb) was varied in the range of 50.0–1,000.0 mL under the optimum conditions, keeping other variables constant, and was passed through a column for preconcentration. It was observed that adsorption was constant up to 750.0 mL. At higher sample volumes, the recoveries decreased gradually with increasing volume of sample solution. Since the final elution volume was 5.0 mL, preconcentration factor of 150 was obtained for lead.
Sorption capacity and stability
The ratio of sample to sorbent or sorption capacity, as milligrams per gram, is an important factor, because it determines how much sorbent is required to quantitatively remove a specific amount of metal ions from the solution. To determine the amount of analyte retained on the column, for a specific mass of sorbent, several solutions were made by different concentrations and introduced into the column. The outlet solutions were collected, and the presence of the analyte was tested in each of them by FAAS. When Pb was detected in the outlet solution, the test was stopped and the sorption capacity was calculated. The sorption capacity was found to be 5.0 mg lead for 1.0 g sorbent.
The stability of the sorbent was examined, and it is found that after ten runs, the recovery of the Pb(II) slightly decreased to below 95 %. The column filled with 100 mg adsorbent can be regenerated over ten cycles of adsorption–desorption without any significant change in the retention of Pb. It can be recovered with 10–15 mL of distilled water.
Analytical features
Calibration curve for the determination of lead was prepared according to the proposed procedure under the optimum conditions. The linearity was maintained in the concentration range of 0.1–30.0 μg mL−1 in the final solution or 0.66–2.0 × 102 ng mL−1 in the original solution. The equation of the line is A = 0.0227C + 0.0176 with the regression coefficient of 0.9992, where A is the absorbance and C is the concentration of the metal ion (in micrograms per milliliter) in the final solution.
The limit of detection (LOD) of a method is the lowest analyte concentration that produces a response detectable above the noise level of the system, typically, three times the standard deviation of the blank (n = 8); on a sample volume of 750.0 mL, it was found to be 0.53 ng mL−1. In eight replicate determinations, a mixture of 2.0 μg mL−1 of lead in the final solution gives a mean absorbance of 0.04 with relative standard deviation (RSD %) of ±2.6 %.
Effect of interference ions
In order to assess the possible analytical procedure, the effect of some foreign ions which interfere with the determination of trace of target ion or/and often accompany analyte ion in various real environmental samples, was examined with the optimized conditions. The results are given in Table 1. The tolerance limit was set as the concentration of the ion required to cause ±3 % error. The experiments (Table 1) indicated that within ±3 % error range, interfering effects from most of inorganic cation and some anions were not observed with the determination, and no further sample treatment or masking reagents are needed. This suggests that the new solid-phase extractant has good selectivity and can be used for determination of Pb in the plant and water samples.
Analysis of standard samples
To evaluate accuracy of the proposed method, a standard reference material containing lead should be analyzed. Standard alloys Nippon Keikinzoku Kogyo (NKK) no. 1021, Al, Si, Cu, Zn Alloy and NKK no. 920, Aluminum Alloy, Japanese Standards of Iron and Steel were applied to this purpose. A 0.1-g sample of the standard alloy was completely dissolved in hydrochloric acid (1:1) by heating and then 1 mL of 30 % (v/v) hydrogen peroxide was added to it. The excess of peroxide was decomposed by heating the sample on a water bath. The solution was cooled and diluted to 100.0 mL with distilled water in a standard flask. An aliquot of this sample was taken and then the proposed procedure was applied and lead determined. The results are given in Table 2.
Conclusions
The present study proves the capability and effectiveness of newly modified silica gel with DHPM for separation and preconcentration of lead from various water and plant samples. The preparation of the sorbent is simple, rapid, and of low cost. The use of 100.0 mg of the modified silica gel allows the enrichment of Pb(II) by a factor 150, and the modified material can be reused ten times. Moreover, as lead can be preconcentrated quantitatively over a wide pH range (5–10), no buffer is required to control the pH value precisely, which minimize contamination. The applied method provides good precision with relative standard deviation and high accuracy obtained with the quantitative recoveries of standard materials and spiked materials (Tables 3 and 4). Thus, it may be concluded that the method is an effective approach in preconcentration and selective separation of lead from the complex matrices.
Comparative data from some recent papers on solid-phase extraction of lead on the various adsorbents for the figure of merits are summarized in Table 5. The analytical performance of the present method is comparable with other preconcentration methods.
References
Aydin, F. A., & Soylak, M. (2007). A novel multi-element coprecipitation technique for separation and enrichment of metal ions in environmental samples. Talanta, 73, 134–141.
Aydin, F. A., & Soylak, M. (2010). Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium(IV), titanium(IV), iron(III), lead(II) and chromium(III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin. Journal of Hazardous Materials, 173, 669–674.
Baird, C. (1999). Environmental chemistry (2nd ed.). NY: W.H. Freeman Company.
Bulut, V. N., Gundogdu, A., Duran, C., Senturk, H. B., Soylak, M., Elci, L., et al. (2007). A multi-element solid-phase extraction method for trace metals determination in environmental samples on Amberlite XAD-2000. Journal of Hazardous Materials, 146, 155–163.
Chen, J., Xiao, S., Wu, X., Fang, K., & Liu, W. (2005). Determination of lead in water samples by graphite furnace atomic absorption spectrometry after cloud point extraction. Talanta, 67, 992–996.
Dadfarnia, S., Salmanzadeh, A. M., & HajiSabani, A. M. (2008). A novel separation/preconcentration system based on solidification of floating organic drop microextraction for determination of lead by graphite furnace atomic absorption spectrometry. Analytica Chimica Acta, 623, 163–167.
Desai, B., Dallinger, D., & Oliver Kappe, C. (2006). Microwave-assisted solution phase synthesis of dihydropyrimidine C5 amides and esters. Tetrahedron, 62, 4651–4664.
Diang, Z., & Hu, X. (2012). Transfer of Heavy metals (Cd, Pb, Cu and Zn) from roadside soil to ornamental plants in Nanjing, China. Advanced Materials Research, 356–360, 3051–3054.
Diedjibegovic, J., Larssen, T., Skrbo, A., Marganovic, A., & Sober, M. (2012). Contents of cadmium, copper, mercury and lead in fish formateNeretva river (Bosnia and Herzegovina) determined by inductively coupled plasma mass spectrometry. Food Chemistry, 131, 469–476.
Dos Santos, W. L., Dos Santos, C. M. M., Costa, J. L. O., Andrade, H. M. C., & Ferreira, S. L. C. (2004). Multivariate optimization and validation studies in on-line pre-concentration system for lead determination in drinking water and saline waste from oil refinery. Microchemical Journal, 77, 123–129.
Jal, P. K., Patel, S., & Mishra, B. K. (2004). Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta, 62, 1005–1028.
Javanbakht, M., Rudbaraki, H., Sohrabi, M. R., Attaran, A. M., & Badiei, A. (2010). Separation, pre-concentration and determination of trace amounts of lead(II) ions in environmental samples using two functionalized nanoporous silica gels containing a dipyridyl sub-unit. International Journal of Environmental Analytical Chemistry, 90(13), 1014–1024.
Jiang, H., & Hu, B. (2008). Determination of trace Cd and Pb in natural waters by direct single drop microextraction combined with electrothermal atomic absorption spectrometry. Microchimica Acta, 161(1–2), 101–107.
Liang, P., Liu, R., & Cao, J. (2008). Single drop microextraction combined with graphite furnace atomic absorption spectrometry for determination of lead in biological samples. Microchimica Acta, 160(1–2), 135–139.
Liu, Y., Liang, P., & Guo, L. (2005). Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta, 68, 25–30.
Madrakian, T., Afkhami, A., Zolfigol, M. A., & Solgi, M. (2006). Separation, preconcentration and determination of silver ion from water samples using silica gel modified with 2,4,6-trimorpholino-1,3,5-triazin. Journal of Hazardous Materials, 128, 67–72.
Malekpour, A., Hajialigol, S., & Taher, M. A. (2009). Study on solid-phase extraction and flame atomic absorption spectrometry for the selective determination of cadmium in water and plant samples with modified clinoptilolite. Journal of Hazardous Materials, 172, 229–233.
Martinis, E. M., Berton, P., Altamirano, J. C., Hakala, U., & Wuilloud, R. G. (2010). Tetradecyl(trihexyl)phosphonium chloride ionic liquid single-drop microextraction for electrothermal atomic absorption spectrometric determination of lead in water samples. Talanta, 80, 2034–2040.
Matoso, E., Kubota, L. T., & Cardore, S. (2003). Use of silica gel chemically modified with zirconium phosphate for preconcentration and determination of lead and copper by flame atomic absorption spectrometry. Talanta, 60, 1105–1111.
Minami, T., Sohrin, Y., & Ueda, J. (2005). Determination of chromium, copper and lead in river water by graphite-furnace atomic absorption spectrometry after coprecipitation with terbium hydroxide. Analytical Sciences, 21(12), 1519–1522.
Pool, C. F. (2003). Trends in Analytical Chemistry, 22, 362.
Pourreza, N., & Hoveizavi, R. (2005). Simultaneous preconcentration of Cu, Fe and Pb as methylthymol blue complexes on naphthalene adsorbent and flame atomic absorption determination. Analytica Chimica Acta, 549, 124–128.
Silva, E. L., & Roldan, P. S. (2009). Simultaneous flow injection preconcentration of lead and cadmium using cloud point extraction and determination by atomic absorption spectrometry. Journal of Hazardous Materials, 161, 142–147.
Soylak, M., Yuzen, M., & Narin, I. (2006). Solid phase extraction of iron and lead in environmental matrices on amberlite XAD-1180/PV. Qimica Nova, 29, 203–207.
Tokalioglu, S., Yilmaz, V., Kartal, S., Delibas, A., & Soykan, C. (2009). Synthesis of a novel chelating resin and its use for selective separation and preconcentration of some trace metals in water samples. Journal of Hazardous Materials, 169, 593–598.
Tuzen, M., Soylak, M., & Elci, L. (2005). Multi-element pre-concentration of heavy metal ions by solid phase extraction on Chromosorb 108. Analytica Chimica Acta, 548, 101–108.
Tuzen, M., Melek, E., & Soylak, M. (2006). Celtek clay as sorbent for separation-preconcentration of metal ions from environmental samples. Journal of Hazardous Materials, 136, 597–603.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabermahani, F., Hassani, Z. & Faramarzpoor, M. The use of silica gel modified with allyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate for selective separation and preconcentration of lead in environmental samples. Environ Monit Assess 185, 4969–4976 (2013). https://doi.org/10.1007/s10661-012-2918-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2918-0