Abstract
The use of fertilizers, containing different metals ions such as lead(II), chromium(III), cadmium(II), copper(II) and zinc(II), in the soil, for sugar cane cultivation, may cause impacts on the hydric resources of the adjacent areas. The scope of this study was to evaluate the impacts of sugar cane cultivation based on metal concentrations in sediments and dragonflies (Odonata). The bioavailability of such metals was determined in ten Neotropical streams. Six streams were located on areas with sugar cane cultivation, without riparian vegetation (classified as impacted area) and four streams were located on forested areas (reference sites). The results showed that there are high concentrations of metals in the sediments and dragonflies in streams located on impacted areas. The contamination by metals of aquatic insects of terrestrial adult life cycle, as Odonata organisms, represents a dangerous link for the transference of metals to upper trophic levels, as fishes, reptiles, birds and mammals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In Brazil, several regions of the native land cover vegetation were removed and substituted by agriculture, mainly of sugar cane. This process resulted in deforestation, especially in the Brazilian southeast region (Martins 2001). In Brazil, the sugar cane cultivation passed by different periods, always presenting an increasing of the cultivated area. In the last 15 years, sugar cane cultivation has been expanded, reaching more than 338 million tons/year (Carvalho Filho 2000), which transform the country in the main sugar and alcohol producer in the world, it corresponds to 27% of the worldwide production (Institute of Agro-industrial Development 1998). Sugar cane is the main source of sugar (sucrose) and alcohol production (through a fermentative sugar cane process). Alcohol is exported from Brazil to other countries and it has been also used as an alternative and renewable combustible for vehicles (Carvalho Filho 2000). In the last 5 years, other countries have shown interest for alcohol produced from sugar cane. Therefore, the trend is an increment for sugar cane production in Brazil and also in other countries.
The use of small amounts of pesticides, in addition to the fast growth of the plant, facilitates the control of the erosive process, which is considered positive points for sugar cane cultivation (Nery 2000). However, the use of fertilizers and herbicides in this kind of culture, in addition to the deforestation, are the main negative points (Armas et al. 2005). The use of fertilizers containing different concentrations of lead, nickel, chromium, cadmium and zinc in different periods of cultivation of sugar cane, in addition to the deforestation of riparian vegetation, can cause impacts on the hydric resources of the adjacent areas (Angelotti-Netto et al. 2004). In general, these impacts can be caused by the absence of riparian vegetation, which is responsible for absorption of toxic products which come from the neighboring cultivated areas (Angelotti-Netto et al. 2004; Dudgeon 1989; Martins 2001; Nery 2000; Primavesi et al. 2002).
The metals introduced in the aquatic environment by the sugar cane activity can be absorbed and incorporated into the sediments (Du et al. 2007; Corbi et al. 2006; Haus et al. 2007; Pourang 1996), and in the food chains (Notten et al. 2005; Saiki et al. 2001; Schroder 2005). The accumulation of metals in the sediments results in serious environmental problems to the surrounding areas, affecting water quality, bioassimilation and bioaccumulation of metals by the aquatic organisms. As a consequence, potential long-term implications on human health and on the ecosystems are expected (Mertz 1986; Ip et al. 2007).
Sediments may contribute significantly to the metal concentration in benthic invertebrates, either by absorption/adsorption from interstitial water or ingestion (Clements 1991; Schipper et al. 2008; Vliet et al. 2005). Because of their close association with sediments, abundance in the streams (Corbi and Trivinho-Strixino 2008), and ability of certain species to tolerate and accumulate metals, the Odonata species represents an important link in the transfer of metals to higher trophic levels, like fishes, reptiles, birds, mammals and other organisms (Wayland and Crosley 2006; Warren et al. 1998; Clements 1991). Moreover, larvae of Odonata, as predator organisms, could accumulate more metals than other aquatic insects, as herbivores and detritivores. In this context, knowledgement about the relationship between sugar cane cultivation and its influence on the hydric resources of the adjacent areas is of high importance for Brazilian sustainable development (Ometo et al. 2000).
The metals, found in the fertilizers used in areas with sugar cane cultivation (without riparian vegetation) could enter into the streams through a leaching process and contaminate the sediments and the aquatic invertebrates. In this context, the scope of the present study was to assess the possible impacts of metals in sediments and dragonflies due to sugar cane cultivation in Neotropical streams.
2 Materials and Methods
2.1 Study Sites
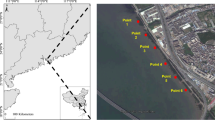
The ten streams were located on Jacaré-Guaçu and Moji-Guaçu River Basin, situated in State of São Paulo, Brazil (Table 1; Fig. 1). All streams are of low order, have low water velocity, low depth, low width and are located at low altitude, from 500 to 700 m. The streams analyzed are located in the areas of Cerrado and for the reason that, have predominantly sand substrates (fine and coarse) (70% of the total). The substrates of the streams located in preserved area are also composed, although in minor fractions, by litter and woods (Corbi and Trivinho-Strixino 2008). The average annual precipitation in the Jacaré-Guaçu and Moji-Guaçu River basin is about 1,400 mm (Ometo et al. 2000). The wet season occurs between October and March, while the dry season occurs from April to September. Sites S1 to S6 are located in extensive areas with sugar cane cultivation, without riparian vegetation, and sites S7 to S10 are located in the forested areas. All streams are free from other anthropic impacts as industrial, domestic or mining activities (Corbi and Trivinho-Strixino 2008).
2.2 Sampling and Storage
Sediments for metals analysis (Fe, Cu, Zn, Cr and Cd) were collected in duplicate from the ten sites using a standard Ekman-birge grab (sampling area of 255 cm2). Larvae of Odonata for metals analysis (Fe, Cu, Zn, Cr, Cd and Al) were collected using a D-frame (Merrit and Cummins 1996) aquatic net (mesh sieve 250 μm). Samples were taken twice at each site, from March to June, 2006. Sediments were stored at 4°C before testing. Larvae of Odonata retained in the net were transferred to acid-washed polypropylene bags and stored in ice until delivered to the laboratory and then frozen at −20°C in order to perform the metal analysis (Pourang 1996). Due to the importance of the amount of organic matter of the sediment in the metal absorption in the aquatic system (Rocha and Rosa 2003), three sediments sub-samples were collected from the streams for the organic matter determination.
2.3 Analytical Procedures
For analytical analysis, deionized double distilled water (DDDW) was used. All acids were purchased from Merck® (analytical grade). The cleaning of the material was performed with concentrated nitric acid as described (Tschöpel et al. 1980).
Sediment samples for metals determination were oven dried at 65°C on glass dishes, homogenized by using a pestle and mortar and each of the weighted samples (about, 5.0 g) was taken to a 100 mL beaker, to which 5.0 mL of HNO3 was added and digested near dryness at 90°C on a hot plate. The digested samples were cooled at room temperature and filtered by using filter papers and collected in 100 mL beaker. The filter papers were washed with ca 20 mL of water and the contents of the beaker were transferred to 100 mL volumetric flasks. The solutions were analyzed for metals in a Pye Unicam flame atomic absorption spectrophotometer. Analyses were undertaken in triplicate (De Paula and Mozeto 2001).
Frozen Odonata larvae were thawed at room temperature. Larvae were pooled to obtain at least 0.10 g of dry weight. Digestion was performed in a similar used for the sediment analyses. The cooled digested samples were transferred to 50 mL volumetric flasks. Pooled samples were analyzed by an inductively coupled plasma atomic emission spectrometer. Analyses were undertaken in triplicate (Pourang 1996).
Organic matter content, was determined by mass loss after ignition (550°C, 4 h) in dry fractions of sediments (dried in stove at 60°C for 12 h), in accordance to the techniques described before (Maitland 1979).
2.4 Statistical Analysis
For each data set (metals concentration in the sediments and in the larvae of Odonata of the ten streams), a multivariate analysis of Cluster, with UPGMA using the Bray–Curtis similarity measure was applied to calculate the similarity between the sites. The sediments and larvae of Odonata, which showed a close correlation, were identified and grouped to a further test of similarity (ANOSIM). The test of similarity (ANOSIM) using Bray–Curtis similarity measure was used to detect significant differences between the two groups. For this analysis, 5% as p-level was considered. The Cluster analysis was calculated by using the computer software STATISTICA (Version 5.1, StatSoft 1995) and in order to calculate the ANOSIM, the PAST Program (Version 1.68) was used (Hammer et al. 2001).
3 Results
3.1 Organic Matter Contents
The organic matter content was relatively low for the ten streams analyzed, with exception of the Fazzari one (S9). Values varied from 0.58% to 31.80%. A high value was detected for the Fazzari (S9) stream, with riparian vegetation, and the lowest one was observed for the São Vicente stream (S6), with sugar cane cultivation, without riparian vegetation (Fig. 2).
Mean values and standard deviations of the organic matter content of sediments from the ten sampling sites. Legends as Table 1
3.2 Metal Concentration in the Sediments
Cadmium was not detected in the sediment of the streams. Copper was detected with very low values for the streams located in preserved areas. The highest values for copper were detected for the Água Sumida stream (S1) and Andes stream (S4), both located in a sugar cane cultivation area (concentration about 60 mg kg−1) (Fig. 3). Iron and zinc exhibited major concentrations for the streams sediments in opened areas (without riparian vegetation) with sugar cane cultivation. Iron concentrations varied from 6,000 mg kg−1 in the Água Preta stream (S5), located in a sugar cane cultivation area, to 900 mg.kg−1 for the Fazzari stream (S9), located in a preserved area (Fig. 3). Chromium was detected in high concentrations for the sites S2, S3 and S6, located in sugar cane cultivation areas. The São João (S2) and Bela Vista (S3) streams present similar chromium values of 0.80 mg.kg−1 (Fig. 3). Chromium was not detected for the S4, S5 and S8 sites.
Mean values and standard deviation of metal concentrations determined in sediments from the ten sampling sites. Legends as Table 1
3.3 Metal Concentration in the Larvae of Odonata
The larvae of Odonata presented the same pattern of metal concentrations observed for the sediments (Fig. 4). The streams located in sugar cane cultivation areas presented higher values of the metals in the larvae of Odonata than those sites located in control areas. Copper presented the highest values for the São Vicente stream (S6), with sugar cane cultivation, reaching 0.20 mg.kg−1. The lowest value was detected for the Espraiado stream (S10), located in a preserved area. Simultaneously, zinc varied from 0.14 mg kg−1 for the São Vicente stream to 0.04 mg kg−1 for the Espraiado stream. Cadmium presented the highest value for the São João stream (S2), located in an impacted area (0.0040 mg kg−1) and the lowest value for the Espraiado stream. Lead was detected in all streams (Fig. 4), with minor values for the larvae located in preserved streams. Iron presented the highest values for the Bela Vista stream (S3), located in an impacted area. Aluminum appears in all streams, but the lowest values were observed for the forested streams (Fig. 4).
Mean values and standard deviations of metal concentrations detected in the dragonflies from the ten sampling sites. Legends as Table 1
3.4 Statistical Analysis
The Cluster Analysis applied to the values of the metal concentrations of the aquatic sediments cluster one group, located in preserved areas (streams S7, S8, S9 and S10) and other group, located in an adjacent area with sugar cane cultivation (S1, S2, S3, S5 and S6). The test of similarity (ANOSIM) applied to the two groups, pointed to significant differences (p = 0.005) between the situations (Fig. 5a).
Dendrograms of the Cluster analysis (using UPGMA with Bray–Curtis similarity measure) applied for the metal concentrations. a Applied to the sediments of the ten streams. b Applied to the dragonflies of the ten streams. Legends as Table 1
At the same time, the Cluster Analysis applied to the values of metal concentrations for the Odonata larvae, cluster one group, located in preserved areas (streams S7, S8, S9 and S10) and an other group, located in an adjacent area with sugar cane cultivation (S1 to S6).The test of similarity (ANOSIM) applied to the two groups, showed significative differences (p = 0.006) between the two situations (Fig. 5b).
4 Discussion
In the present study, metal concentrations in stream sediments and in larvae of Odonata showed differences in accordance with the land use in the adjacent areas (sugar cane cultivation or preserved area). Streams located in areas with sugar cane activity presented the highest values of the metals in the sediments and in larvae of Odonata. Except by the Fazzari stream (S9), the low values of organic matter content detected in the sediments of the ten streams suggest that this variable was not influence determination of the concentrations of the metals of the streams. The granulometric fraction of the sediments was similar in all streams, with the predominance of sand substrates (fine and coarse).
Metals are considered one of the most common contaminants in waters and their origin can be natural or anthropic. The anthropic origin may come from the industrial effluents and agricultural areas through the leaching processes (Mertz 1986). As the studied streams are located at the same hydrographic river basin with the same geology land formation, the highest values for metal concentrations detected for streams located in areas with sugar cane cultivation confirms the influence of the agriculture activity in the streams of the adjacent areas. In Brazil, there are no maximum or minimum standard values for metal concentrations in sediments and for the aquatic fauna. Nevertheless, in the present study, the concentration for copper, zinc and lead in the sediments located in areas with sugar cane activity were higher than the standard values permitted for soils in the State of São Paulo (CETESB 2005). According to Santos (1999) and Lima (1990), metal concentrations in the sediments of preserved areas in the State of São Paulo were lower than those found in this study.
Cadmium was not detected in the aquatic sediment. However, this potentially toxic metal was detected in small concentrations for the Odonata larvae. Characterization of cadmium inputs in aquatic systems is incomplete (Mertz 1986) but the manufacture of cadmium-containing products (like batteries) accounts for its largest discharge, followed by phosphate fertilizers (Kostial 1986). Cadmium is a heavy metal, which occurs in the nature generally associated with other metals as zinc and lead and its extraction, production and application in the industry and agriculture were increased in the last years (Massabni et al. 2002). This metal is widely used in several fertilizers in sugar cane cultivation, added as NPK fertilizers and as source of micronutrients (Angelotti-Netto et al. 2004). Some studies have demonstrated that this metal, in high concentrations in the aquatic environment, can cause alterations in the growth of some species of insects, as larvae of Chironomus riparius (Postma et al. 1995).
Copper was detected in large amounts in the sediments of the streams of extensive sugar cane cultivation. Contamination by copper may have origin from domestic and industrial sewages or from leaching of agricultural products by rain (Mertz 1986). Cooper was largely used in fertilizers as a micronutrient (Angelotti-Netto et al. 2004). In the same way, the high values of zinc found in areas with sugar cane activity can be attributed to its large use in fertilizers. Major concentrations of zinc in the streams located in areas with sugar cane cultivation may also be related to burning action, a common process in this agricultural activity. The low concentration values for copper and zinc in the sediments of streams protected by riparian vegetation are in accordance with the results described in the literature (Santos 1999; Lima 1990), for Ribeirão Anhumas and Cafundó streams (Jataí Ecological Station), both situated in preserved areas.
Chromium was found in the sediments of all the streams. Chromium is rarely found in natural waters (Mertz 1986), but it can occur as a contaminant of waters from leather industries and is also used as micronutrient in fertilizers for the sugar cane cultivation, being added to NPK fertilizers (Angelotti-Netto et al. 2004).
Iron concentration is generally related to the geologic formation of the region. In the present work, the results confirm that the region considered is rich in iron (Barreto 1999). The high concentration for iron observed in the streams located in opened areas, indicate that deforestation of riparian vegetation can contribute to increase concentration of this metal in the streams.
Because of technical problems, lead concentrations were not analyzed in the sediments of the streams, but they were analyzed and detected for Odonata larvae. Lead in water may be originated from soils, rocks, fallout, dust or vehicular exhausts (Quarteman 1986). It is a heavy metal widely used in some fertilizers for sugar cane cultivation, being added in NPK fertilizers (Angelotti-Netto et al. 2004). Pb is generally presented in higher concentrations in fertilizers than the other metals cited here (see Angelotti-Netto et al. 2004). Although not analyzed in the sediments, the detection of lead in Odonata larvae is an indicative of the sediment contamination, because these aquatic organisms live and feed in this aquatic compartment. High concentrations of Pb can cause severe damages to aquatic and terrestrial animals due to bioaccumulation processes (Quarteman 1986). Its concentration values found in the present work can be considered high, especially in the case of the São Vicente stream (S6) where concentration are higher than the permitted values for soils in Brazil.
5 Conclusion
The results show the problems caused in streams, without riparian vegetation, due to the use of great quantities of fertilizers in areas of sugar cane agriculture. Every year, sugar cane cultivation, with fertilizers application, is repeated. This process may cause in a near future severe damage to the aquatic communities. The results also claim to the importance of the riparian vegetation preservation in the process of filtering and barrage of the leached products from areas with sugar cane agriculture. In Brazil there are no reference values for metal concentrations in the sediments and for the aquatic fauna. However, the results presented in this work points to high values of Cu and Zn, for the impacted areas, when compared with the reference values for soils. This work also tries to show the importance of the elaboration of a monitoring system that uses the sediment as a compartment in the environmental studies. Moreover, contamination of aquatic insects with a terrestrial adult life cycle, as Odonata organisms, represents an important link for the transference of metals to upper trophic levels, as fishes, reptiles, birds and mammals.
References
Angelotti-Netto, A., Crestana, S., De Oliveira, S. C., & Barbosa, R. V. R. (2004). Metais pesados provenientes de atividade agrícola: formas, prevenção e controle. In: E. L. G. Espíndola, & E. Wendland (Eds.), Bacia Hidrográfica Rima Editora, São Carlos, pp. 1–14.
Armas, E. D., Monteiro, R. T. R., Amâncio, A. V., Correa, R. C. L., & Guercio, M. A. (2005). The use of pesticides in sugar cane at the Corumbataí River Basin and the risk of water pollution. Quimica Nova, 28, 975–982.
Barreto, A. S. (1999). Estudo da distribuição de metais em ambiente lótico, com ênfase da assimilação pelas comunidades biológicas e na sua quantificação no sedimento e na água. PhD Thesis, Universidade de São Paulo (USP). 276p. (in Portuguese with English abstract). São Paulo.
Carvalho Filho, S. M. (2000). Colheita mecanizada: desempenho operacional e econômico em cana sem queima prévia. PhD Thesis, ESALQ (USP), Piracicaba–SP. 108 pp. (in Portuguese with English abstract). São Paulo.
Cetesb. (2005). Companhia de Tecnologia de Saneamento Ambiental–. Relatório de esteblecimento de valores orientadoresz para solos e águas subterrâneas no Estado de São Paulo. 4p. São Paulo
Clements, W. H. (1991). Community responses of stream organisms to heavy metals. Colorado: Univ. Press.
Corbi, J. J., & Trivinho-Strixino, S. (2008). Relationship between sugar cane cultivation and stream macroinvertebrate communities: A study developed in the southeast of Brazil. Brazilian Archives of Biology and Technology, 51(1) (in press).
Corbi, J. J., Trivinho-Strixino, S., Dos Santos, A., & Del Grande, M. (2006). Environmental diagnostic of metals and organochlorated compounds in streams near sugar cane plantations activity (State of São Paulo, Brazil). Quimica Nova, 29, 61–65.
De Paula, F. C. F., & Mozeto, A. A. (2001). Biogeochemical evolution of trace elements in a pristine watershed at the Brazilian southeastern coast. Applied Geochemistry, 16, 1139–1151.
Du, J. Z., Mu, H. D., Song, H. Q., Yan, S. P., Gu, Y. J., & Zhang, J. (2007). 100 years of sediment history of heavy metals in Daya Bay, China. Water, Air, and Soil Pollution, 190, 343–351.
Dudgeon, D. (1989). The influence of riparian vegetation on the functional organization of four Hong Kong stream communities. Hydrobiologia, 179, 183–194.
Hammer, O., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological Statistics software package for education and data analysis, Paleontologia eletronica.
Haus, N., Zimmermann, S., Wiegand, J., & Sures, B. (2007). Occurrence of platinum and additional traffic related heavy metals in sediments and biota. Chemosphere, 66, 619–629.
Institute of Agro-industrial Development, (1998). Indicadores de desempenho da agroindústria canavieira-safra/97/98. Ribeirão Preto, IDEA, 1998, 116p.
Ip, C. C. M., Li, X. D., Zhang, G., Wai, O. W. H., & Li, Y. S. (2007). Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environmental Pollution, 147, 311–323.
Kostial, K. (1986). Cadmium. In: Mertz, W. (Ed.), Trace elements in human and animal nutrition. Academic Press, Inc. pp. 319–345.
Lima, N. R. W. (1990). Analysis of heavy metals in hydric systems of the Jataí Ecological Station. Acta Limnologica Brasiliensia, 3, 1001–1021 in Portuguese with English abstract.
Maitland, P. S. (1979). The distribution of zoobenthos and sediments in Loch Leven, Kinross, Scotland. Archives Hydrobiology, 5, 98–125.
Martins, S. J. (2001). Recuperação de matas ciliares, primeira ed. Editora Conceito, Viçosa, MG.
Massabni, A. C., Melnikov, P., Cuin, A., Corbi, P. P., & Corbi, J. J. (2002). O Cádmio, seus efeitos no homem e no meio ambiente. Jornal de Bioquímica Médica, 11, 5–7 in Portuguese with English abstract.
Merrit, R. W., & Cummins, K. W. (1996). An introduction to aquatic insects of North America. Dubuque, Iowa, USA: Kendall-hunt.
Mertz, W. (1986). Trace elements in human and animal nutrition (5th ed.). London: Academic.
Nery, M. S. (2000). Desempenhos operacional e econômico de uma colhedora em cana crua. PhD Thesis, Universidade de São Paulo (USP). 108 pp. (in Portuguese with English abstract).
Notten, M. J. M., Oosthoek, A. J. P., Rozema, J., & Aerts, R. (2005). Heavy metal concentrations in a soil–plant–snail food chain along a terrestrial soil pollution gradient. Environmental Pollution, 138, 178–190.
Ometo, J. P. H. B., Martinelli, L. A., Ballister, M. V., Gessner, A., Krische, A. V., & Victoria, R. L. (2000). The effects of land use on water chemistry and macroinvertebrates rates in two streams of the Piracicaba river basin South-east Brazil. Freshwater Biology, 44, 327–337.
Postma, J. F., Kyed, M., & Admiraal, W. (1995). Site specific differentiation in metal tolerance in the midge Chironomus riparius (Diptera, Chironomiae). Hydrobiologia, 315, 159–165.
Pourang, N. (1996). Heavy metal concentrations in surficial sediments and benthic macroinvertebrates from Anzali wetland, Iran. Hydrobiologia, 331, 53–61.
Primavesi, O., Freitas, A. R., Primavesi, A. C., & Oliveira, H. T. (2002). Water quality of the Camchim’s Creek watershed in São Carlos, SP, Brazil, occupied by beef and dairy cattle activities. Brazilian Archives of Biology and Technology, 45, 209–217.
Quarteman, J. (1986). Lead. In W. Mertz, (Ed.), Trace elements in human and animal nutrition (pp. 281–317). Academic.
Rocha, J. C., & Rosa, A. H. (2003). Substâncias húmicas aquáticas: interação com espécies metálicas. São Paulo. Editora Unesp.
Saiki, M. K., Martin, B. A., Thompson, L. D., & Welsh, D. (2001). Copper, cadmium, and zinc concentrations in juvenile Chinook salmon and selected fish-forage organisms (aquatic insects) in the upper Sacramento River, California. Water, Air, and Soil Pollution, 132, 127–139.
Santos, A. (1999). Distribuição de metais no reservatório de captação de água superficial Anhumas Américo Brasiliense–SP. USP. PhD Thesis, Universidade de São Paulo (USP). (in Portuguese with English abstract).
Schipper, A. M., Wijnhoven, S., Leuven, R. S., Ragas, A. M., & Hendricks, A. J. (2008). Spatial distribution and internal metal concentrations of terrestrial arthropods in a moderately contaminated lowland floodplain along the Rhine River. Environmental Pollution, 151(1), 17–26.
Schroder, T. (2005). Solid solution partitioning of heavy metals in floodplain soils of the rivers Rhine and Meuse: Field sampling and geochemical modelling. PhD thesis, Wageningen University, Wageningen.
StatSoft, Inc. (1995). Statistica for the windows operating system. Release 5. StatSoft, Inc., Tulsa OK, USA.
Tschöpel, P., Kotz, L., Shulz, W., Veber, M., & Tölg, G. (1980). Zur Ursache und vemeirdung systematischer fehler bei elementbestimmungen in wäbrigen lösungen im ng/ml- und pg/ml. Fresenius Journal Analytical Chemistry, 302, 1–14.
Vliet, P. C. J., Zee, S. E. A. T. M., & Ma, W. C. (2005). Heavy metal concentrations in soil and earthworms in a floodplain grassland. Environmental Pollution, 138, 505–516.
Warren, L. A., Tessier, A., & Hare, L. (1998). Modelling cadmium accumulation by benthic invertebrates in situ: The relative contribution of sediment and overlying water reservoirs to organism cadmium concentrations. Limnology and Oceanography, 43(7), 1442–1454.
Wayland, M., & Crosley, R. (2006). Selenium and other trace elements in aquatic insects in coal mine-affected streams in the rocky mountains of Alberta, Canada. Archives of Environmental Contamination and Toxicology, 50, 511–522.
Acknowledgements
We would like to thank Dr. Pedro P. Corbi, Dr. Vanessa Colombo and Dr. Fábio Roque for their fruitful suggestions. We also like to thank Lucas Lecci for the map configuration. Financial support: CNPq (Brazilian National Scientific Council) and BIOTA-FAPESP (São Paulo State Research Foundation, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corbi, J.J., Trivinho-Strixino, S. & dos Santos, A. Environmental Evaluation of Metals in Sediments and Dragonflies Due to Sugar Cane Cultivation in Neotropical Streams. Water Air Soil Pollut 195, 325–333 (2008). https://doi.org/10.1007/s11270-008-9749-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9749-1