Abstract
Wastewater from municipal and industrial sources is becoming increasingly important in being reused, for example, for irrigation purposes. Wastewater is commonly stored in treatment lagoons in which evaporation is the main cause of water loss. Nonetheless, modeling wastewater evaporation (WWE) has received little attention. Driven by this knowledge gap, this study was performed to explore extent to which impurities affect water evaporation. A dimensional analysis was used to formulate WWE as a function of clear water evaporation (CWE), wastewater properties and climatic variables. We based our modeling approach on experimental data collected from the Neishaboor municipal wastewater treatment plant, Iran. As a result of this analysis, a multiplicative model to formulate WWE as a function of the influencing variables is proposed which indicated a reasonably well accuracy (RMSE = 1.09 mm) for the WWE estimation. Clear water evaporation indicated to be the most correlated variable in the model such that a constant coefficient can also be used to estimate WWE from CWE at the cost of losing accuracy only by 4.6 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to ever-increasing demand for water resources, many regions of the world (e.g. Middle East, North Africa, India, China, Japan and Spain) face critical water resource sustainability issues (Llamas and Custodio 2002). Therefore, water resources supply has become a major concern for development especially in arid and semi-arid areas. Wastewater as an alternative resource is considered for non-potable applications such as irrigating agricultural lands (MacDonald 2003). Wastewater is commonly stored in treatment lagoons in which evaporation is the most important term in estimating water balance components and is the only cause of water loss in most cases (i.e. most lagoons are designed to be impermeable) (Cumba and Hamilton 1998; Parker et al. 1999; Ham 2002). Hence, wastewater evaporation rate which differs from that of clear water, as discussed in the following, needs to be investigated.
There are several factors causing a discrepancy between evaporation rate of wastewater and clear water. Wastewater stored in lagoons is typically dark brown to reddish brown due to the presence of significant amount of purple sulfur bacteria and suspended sediments (Wenke and Vogt 1981; Freedman et al. 1983; Parker et al. 1999). Dark colored wastewater absorbs more solar radiation, enhancing its evaporation compared to clear water. Additionally, increasing vapor pressure of the solution due to the high ammonia concentrations in wastewater, wastewater evaporation may be enhanced. Conversely, the high salinity of the wastewater resists against evaporation by decreasing vapor pressure of the solution (Parker et al. 1999).
Parker et al. (1999) discussed how physical and chemical properties of feedyard effluent may affect evaporation rate. They conducted four experiments to compare evaporation rates at different concentrations of feedyard effluent comprising of 100 % pure effluent, 50 % effluent mixed with 50 % groundwater, 25 % effluent mixed with 75 % groundwater and 100 % groundwater. A fifth experiment was conducted to compare clear water evaporation at different salt concentrations to test for potential vapor pressure effects. They found that evaporation rate for fresh effluent was 8.3 to 10.7 % higher than clear water evaporation rate. As the effluent stayed for about 4 days in the evaporation pans leading to settling and algal growth, differences in evaporation rates between the effluent and clear water diminished.
In spite of its crucial importance, modeling wastewater evaporation has received little attention. To the best of our knowledge, there is no model to specifically relate wastewater evaporation to clear water evaporation occurring in the same environment. This relationship is particularly important for estimating lagoons wastewater evaporation rate using the surrounding environmental variables (e.g. clear water pan evaporation, weather data). Driven by this knowledge gap, this study was performed to explore extent to which impurities affect water evaporation. Dimensional analysis method based on the Buckingham Π theorem (Buckingham 1914) was used to derive a novel model for estimating wastewater evaporation as a function of clear water evaporation and several influencing variables including total suspended solids (TSS), electric conductivity (EC), wastewater temperature (T w ), air temperature (T a ), wind speed (W), and solar radiation (R). We based our modeling approach on experimental data collected from the Neishaboor municipal wastewater treatment plant, Iran.
2 Material and Methods
2.1 Study Area
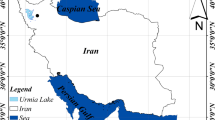
Neishaboor wastewater treatment plant is located at the Neishaboor city between 36° 05׳ N to 36° 06׳ N latitude and 58° 40׳ E to 58° 41׳ E longitude in the northeast of Iran (Fig. 1). The city is characterized by a semi-arid to arid climate, with an average annual precipitation of 265 mm. The mean annual temperature and potential evapotranspiration are 13.8 °C and 2335 mm, respectively (Izady et al. 2012, 2015). The Neishaboor wastewater treatment plant started operation in 2008. It has three anaerobic, primary and secondary facultative lagoons with 1.2, 7.8, and 8.3 hectare (ha) area, respectively (Fig. 1). Wastewater is treated using stabilization lagoons in which the municipal wastewater is collected and after settling, the organic materials are decomposed and stabilized under natural ample sunlight, microorganisms and algal growth. The facultative lagoons are commonly designed to remove most of the remaining biochemical oxygen demand (BOD) through the coordinated activity of algae and heterotrophic bacteria (Tilley et al. 2014). The treated wastewater is used for irrigation of surrounding agricultural lands having an area of 300 ha commonly cultivated with corn silage and winter wheat (Izady 2014).
Location of the study area in north east of Iran along with aerial photo of the Neishaboor wastewater treatment plant. L1, L2, and L3 are acronym for three different anaerobic, primary and secondary facultative lagoons. P0, P1, P2, and P3 show the location of the installed class A pans filled with clear water, wastewater from anaerobic, primary and secondary facultative lagoons, respectively
2.2 Experimental Setup and Methodology
This study was undertaken in three steps namely i) installing class A pans and measuring daily evaporation and temperature of the wastewater and clear water from the pans as well daily wastewater lagoons parameters including T w , EC and TSS, ii) calculating pan coefficients and estimating the evaporation rate from the wastewater lagoons, iii) adopting dimensional analysis to formulate and obtain a mathematical model that relates wastewater evaporation (WWE) to clear water evaporation (CWE) and wastewater and climatic variables.
2.2.1 Class A Pan and Data Measurement
Class A pan (U.S. Department of Commerce 1970), a widely used evaporation pan, was employed for this study. It is made of stainless steel with cylindrical shape of dimensions 12.1 cm diameter and 25.4 cm height. It is installed on a wooden frame so that air circulates easily around and under the pan (Allen et al. 1998). Owing to the fact that the anaerobic, primary and secondary facultative lagoons have different solutions, three class A pans were installed for our experiment which were filled with wastewater from different lagoons (Fig. 2). Evaporation rate as well as daily temperature of the wastewater of each pan were continuously recorded along the study. In addition, another Class A pan filled with clear water was installed for measuring CWE (Fig. 2).
Wastewater T w , TSS and EC were measured daily for the three lagoons. To measure TSS, the wastewater sample was filtered through a pre-weighed filter. The residue retained on the filter was determined by weighing after oven drying at 105 °C. The T w and EC were measured with alcohol thermometer and portable WTW handheld meters (WTW GmbH), respectively. Temperature was measured 10 cm below the wastewater surface within the pans and lagoons. Daily wastewater data (T w , EC and TSS) were recorded over 4 months from April to July 2014. Wind speed and solar radiation data were acquired from the Neishaboor synoptic meteorological station.
2.2.2 Estimation of Wastewater Evaporation
Measurements of pan evaporation can rarely be used directly as estimates of evaporation from a wastewater lagoon because of the scale discrepancy affecting the ambient sensible heat fluxes. A water body contained in the lagoons could have comparatively large thermal inertia leading to slow temperature variations, whereas the pan temperature could vary greatly from day to day with changing environmental conditions. Therefore, the following common correction was applied:
where K pan is pan coefficient and E L and E P are evaporation from wastewater lagoon and pan, respectively. The generally accepted “conversion pan” method (Webb 1966) was used to calculate daily K pan . This method is based on the assumption that evaporation rates from lagoon and pan are a function of vapor pressure difference between the particular water surface and a convenient observation height in the air:
where k is a constant, equal to 0.7 as suggested by Webb (1966), and e l , e p , and e a denote the saturation vapor pressure of lagoon, pan, and air at a reference height above the ground surface (KPa), respectively. The saturation vapor pressure (KPa) was calculated as a function of T (°C) using the equation of Murray (1967):
Wastewater evaporation rate was estimated separately for the three wastewater lagoons based on the pan coefficients calculated using Eqs. (2) and (3). Evaporation rate for the clear water was estimated using the average pan coefficient of 0.7 (Kohler 1954; Kohler et al. 1955; Lapworth 1965; Hounam 1973; Winter 1981).
2.2.3 Dimensional Analysis
Dimensional analysis is commonly used to relate different variables tied to a specific phenomenon especially when the mathematical model of the system is unavailable or complex (Buckingham 1914). Due to the complexity of the relationship between the studied variables here, we applied a dimensional analysis based on the Buckingham Π theorem (Buckingham 1914) to find the effect of wastewater properties and climatic conditions on the wastewater evaporation. The measured daily cumulative wastewater evaporation (WWE), daily cumulative clear water evaporation (CWE), wastewater variables including TSS, EC, and T w and climatic variables including wind speed (W), air temperature (T a ) and daily cumulative solar radiation (R) were considered for this analysis. Dimension of the WWE, CWE, TSS, EC, T w , W, T a , and R respectively is L, L, ML−3, T−1, θ, LT−1, θ, and MT−2 (L: Length, M: Mass, T: Time, θ: Degree). Therefore, the number of variables are 8 and the number of basic dimensions are 4, meaning that all the variables can be combined into 4 (=8 – 4) dimensionless variables (Π terms) to construct the final model.
Because of the key role of the water body temperature in relation with air temperature in evaporation from water bodies (Majidi et al. 2015), we considered T w /T a to be one of the dimensionless variables. Then, after an extensive search for the best model, WWE, CWE and EC were chosen as the best non-repeating variables and hence TSS, W, and R were considered as repeating variables, leading to the following dimensionless variables:
where a1, a2, a3, b1, b2, b3, c1, c2, and c3 are the powers of each variable making the Π terms dimensionless and can be solved by equating the units as follows:
By solving Eq. (5) for the powers, the Π terms are resulted as follows:
Considering the general form of Π terms function, Π1 = f (Π2, Π3, Π4 = T w /T a ), Eq. (6) results in the general formulation for the wastewater evaporation as follows:
A multiplicative form of the function f may be applied, leading Eq. (7) to our final mathematical model for the wastewater evaporation:
where C, α, β and γ (all dimensionless) are constants to fit Eq. (8) to the true physical relationship holding in reality and can be determined using a regression analysis on the experimental data.
Data of all three lagoons were considered together for the dimensional analysis and then randomized to calculate the coefficients C, α, β and γ. Daily measurements from three lagoons were taken into account as sample and therefore total samples were 366 based on 4 months (April to July) and three lagoons data in which 70 and 30 % of the data were respectively considered for the parameter estimation and validation phases. Three different performance criteria consisting of coefficient of determination (R 2), root mean square error (RMSE) and mean absolute relative error (MARE) were used to evaluate the effectiveness and also the accuracy of the proposed method.
where WWE m and WWE e are measured and estimated wastewater evaporation, and N is number of the samples.
3 Results and Discussion
3.1 Wastewater Data Analysis
As stated earlier, wastewater variables consisting of T w (°C), EC (μS/cm) and TSS (mg/l) were measured daily over 4 months from April to July 2014. Figure 3 shows the variation of wastewater average daily temperature for different lagoons along with average daily air temperature. The anaerobic lagoon has the highest temperature in comparison with the other two. Moreover, the difference between air and wastewater temperature generally increases from anaerobic lagoon to secondary facultative lagoon. In other words, wastewater gets cooler when becomes clearer from settling and aerobic activities in the secondary facultative lagoon. This observation is fully understandable, because the anaerobic lagoon has a significantly higher TSS than that of the secondary facultative lagoon (see Fig. 4) leading to higher absorption of the solar radiation and hence the temperature rise in this lagoon. Figure 5 shows the variation of wastewater daily EC for different lagoons. The anaerobic lagoon has the highest EC in comparison with two other lagoons indicating that treatment operation significantly reduces the EC which is an indirect measure of the total dissolved solids.
3.2 Pan Coefficient and Wastewater Evaporation
As mentioned above, the “conversion pan” method, Eqs. (2) and (3), was used for the estimation of pan coefficients based on T of the pan’s wastewater, lagoon’s wastewater and air. Figure 6 exhibits the variation of wastewater temperature of lagoons and their corresponding pans and also air temperature. It is observed that there is no significant discrepancy between the temperatures of different pan wastewater. However, the difference between temperature of lagoon and its corresponding pan is significant and the temperature of all pans are higher than that of the lagoons. As mentioned earlier, this observation reflects the fact that the wastewater in lagoons have large thermal inertia and its temperature varies slowly, whereas the pan wastewater temperature various instantaneously with variation of the environmental conditions.
Table 1 shows monthly calculated pan coefficients for the three evaporation pans with different wastewater. It is observed form Table 1 that the pan coefficient increases as monthly average temperature of the pan’s wastewater increases. The pan coefficient ranges from 0.60 for the pan filled with secondary facultative lagoon’s wastewater up to 0.76 for the pan filled with anaerobic lagoon’s wastewater. Also, these coefficients vary monthly such that they generally increase from April to July as a result of changing weather conditions. In fact, pan coefficient variations are attributed to the effects of wastewater type that controls the gain and loss of solar radiation to different evaporation pans.
The calculated pan coefficients were used along with Eq. (1) to estimate the evaporation rate for each wastewater lagoon (Fig. 7). Daily evaporation rates generally increase from April to July for all three lagoons. In addition, dark brown colored anaerobic wastewater lagoon exhibits the highest evaporation rate. This result arises from the fact that wastewater often includes a substantial portion of solid materials that are not biodegradable. A part of such materials are very light tending to rise and float on the surface of the suspension and form a so-called “scum layer” (Schofield and Rees 1988). This phenomenon is evident in the anaerobic lagoon with the highest TSS in which a reddish brown scum layer is formed (see Fig. 8) and leads to more radiation absorption in this lagoon.
The evaporation rate of clear water was estimated considering average pan coefficient of 0.7 (Kohler 1954; Kohler et al. 1955; Lapworth 1965; Hounam 1973; Winter 1981) and compared with the wastewater evaporation rate. The results show that the clear water evaporation rate is lesser than evaporation rate of all three wastewater lagoons (Table 2). Average evaporation rates for the wastewater of the three lagoons are 40.5, 24, and 8.5 % higher than clear water evaporation rates for the study period.
3.3 Dimensional Analysis Results
Based on the regression analysis, the constants of Eq. (8) were obtained as C = 2.608, α = 0.895, β = −0.076, and γ = 0.256 and the final mathematical model as follows:
where K is defined as K = W 2 .TSS/R, WWE and CWE are respectively cumulative daily wastewater and clear water evaporation (mm), W is wind speed (m/s), TSS is total suspended solids (mg/l), R is cumulative daily solar radiation (MJ/m2) and T w and T a are respectively wastewater and air temperature (° C).
Table 3 shows performance statistics of Eq. (10) for the calibration phase in which R 2, RMSE and MARE are 73.2 %, 1.23 mm, and 20.6 %, respectively. The derived equation was afterward validated using independent dataset. The comparison between measured and estimated wastewater evaporation is shown in Fig. 9 which indicates that the dynamic fluctuations of the measured and estimated wastewater evaporation match very well verifying the efficiency and accuracy of the proposed model. According to the performance indicators (R 2, RMSE and MARE) the derived equation fairly predicts the wastewater evaporation, as demonstrated by Table 3. The RMSE statistic, which is a measure of the global goodness of fit between the estimated and measured wastewater evaporation is 1.09 mm that is considered quite satisfactory. The MARE statistic, which is used to quantify the prediction accuracy of the proposed method, is 22.0 %.
The power α in Eq. (10) is substantially higher than β and γ, apparently indicating that the dimensionless variable K.CWE has the most significant influence on the wastewater evaporation. Therefore, we also evaluated Eq. (10) by assigning α = 1 and β = γ = 0, and recalculating the constant C. As a result, Eq. (10) was reduced to a simple linear relationship:
This simple relationship comes as a substantial finding of this study after evaluating the effect of all the influencing variables through the dimensional analysis, verifying that a constant coefficient can be used to estimate wastewater evaporation from clear water evaporation at the cost of losing accuracy only by 4.6 % (validation RMSE = 1.14 mm for Eq. (11) compared to 1.09 mm for Eq. (10)).
4 Conclusions
The problem of estimating wastewater evaporation either in municipal wastewater treatment plants or in animal waste ponds and lagoons is that there is no specific model to relate wastewater evaporation to clear water evaporation and some wastewater properties and climatic variables. Several studies applied a percentage of clear water-filled class A pan evaporation to estimate evaporation form wastewater ponds or lagoons. Robinson (1973) used 70 % of the clear water-filled class A pan evaporation rate to estimate evaporation from a beef cattle holding pond in California. Davis et al. (1973) utilized 100 % of the clear water to estimate evaporation from a newly constructed dairy waste pond in California. Cumba and Hamilton (1998) used 70 % of the evaporation rate estimated using the Modified Penman Combination Method and they stated that evaporation was the most sensitive parameter in their model. On the contrary, Parker et al. (1999) found that evaporation rate for fresh effluent was 8.3 to 10.7 % higher than clear water evaporation rate. Hence, a question arises as to the accuracy of estimating wastewater evaporation using clear water evaporation. To cope with this issue, Eq. (10) based on dimensional analysis was developed here for estimating wastewater evaporation and was validated using experiments conducted in the Neishaboor municipal wastewater treatment plant, Iran. The derived equation advances the estimation by involving several other variables besides clear water evaporation. This approach also verifies that wastewater evaporation relative to clear water evaporation fairly remains a constant over time. The relationship can be approximately expressed by Eq. (11) which can significantly reduce time and cost needed for the field measurements using class A pan evaporation measurements.
References
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration – guidelines for computing crop water requirements. FAO Irrigation and Drainage Paper 56
Buckingham E (1914) On physically similar systems; illustrations of the use of dimensional equations. Phys Rev 4(4):345–376
Cumba HJ, Hamilton DW (1998) Anaerobic lagoon liquid balance. ASAE Paper No. 98–4115. St. Joseph, Mich.: ASAE. CWQCC. 1997. Confined animal feeding operations control regulation No. 81 (5CCR 1002–81). Denver, Colorado: Colorado Department of Public Health and Environment, Water Quality Control Commission.
Davis S, Fairbank W, Weisheit H (1973) Dairy waste ponds effectively self-sealing. Trans ASAE 16:69–71
Freedman D, Koopman B, Lincoln EP (1983) Chemical and biological flocculation of purple sulfur bacteria in anaerobic lagoon effluent. J Agric Eng Res 28(2):115–125
Ham JM (2002) Seepage losses from animal waste lagoons: a summary of a four-years investigation in Kansas. Trans ASAE 45(4):983–992
Hounam CE (1973) Comparison between pan and lake evaporation. WMO Technical Note no. 126. Geneva, Switzerland, 52 pp
Izady A (2014) Seepage losses estimation from Neishaboor wastewater treatment plant - a water balance study. Report for Khorasan-Razavi Water and Wastewater Company (In Persian)
Izady A, Davary K, Alizadeh A, Ghahraman B, Sadeghi M, Moghaddamnia A (2012) Application of panel-data modeling to predict groundwater levels in the Neishaboor Plain, Iran. Hydrogeol J 20(3):435–447. doi:10.1007/s10040-011-0814-2
Izady A, Davary K, Alizadeh A, Ziaei AN, Akhavan S, Alipoor A, Joodavi A, Brusseau ML (2015) Groundwater conceptualization and modeling using distributed SWAT-based recharge for the semi-arid agricultural Neishaboor Plain, Iran. Hydrogeol J 23(1):47–68. doi:10.1007/s10040-014-1219-9
Kohler MA (1954) Lake and pan evaporation. Water-loss investigations: lake Hefner studies, technical report. US Geol Surv Prof Pap 269:127–149
Kohler MA, Nordenson TJ, Fox WE (1955) Evaporation from Pans and Lakes. US Department of Commerce, Weather Bureau, Research Paper 38
Lapworth CF (1965) Evaporation from a reservoir near London. J Inst Water Environ Manag 19:163–181
Llamas MR, Custodio E (Eds.) (2002) Intensive use of groundwater: challenges and Opportunities. CRC Press
MacDonald DH (2003) The economics of water: taking full account of first use, reuse and return to the environment. CSIRO Report for the Australian Water Conservation and Reuse Research Program
Majidi M, Alizadeh A, Farid A, Vazifedoust M (2015) Estimating evaporation from lakes and reservoirs under limited data condition in a semi-arid region. Water Resour Manag 29(10):3711–3733
Murray FW (1967) On the computation of saturation vapor pressure. J Appl Meteorol 6(1):203–204
Parker DB, Auvermann BW, Williams DL (1999) Comparison of evaporation rates from feedyard pond effluent and clear water as applied to seepage predictions. Trans ASAE 42(4):981–986
Robinson FE (1973) Changes in seepage rate from an unlined cattle waste digestion pond. Trans ASAE 16:95–96
Schofield CP, Rees YJ (1988) Passive separation and the efficiency of anaerobic digestion of cattle slurry. J Agric Eng Res 40(3):175–186
Tilley E, Ulrich L, Luethi C, Reymond P, Zurbruegg C (2014) Compendium of sanitation systems and technologies. 2nd Revised edn. Duebendorf, Switzerland: Swiss Federal Institute of Aquatic Science and Technology (Eawag)
U.S. Department of Commerce (1970) Climatological Summary. Brownsville, Texas, p 46
Webb EK (1966) A pan-lake evaporation relationship. J Hydrol 4:1–11
Wenke TL, Vogt JC (1981) Temporal changes in a pink feedlot lagoon. Appl Environ Microbiol 41(2):381–385
Winter TC (1981) Uncertainties in estimating the water balance of lakes. Water Resour Bull 17:82–115
WTW GmbH, Portable meters for the determination of the parameters pH, ORP, dissolved oxygen, EC. http://www.wtw.com/en/home.html
Acknowledgments
Authors would like to thank the “Khorasan-Razavi Water and Wastewater Company” for the financial support. We also wish to acknowledge the “Neishaboor Water and Wastewater Company” and “Neishaboor municipal wastewater treatment plant” to set-up class A pans and measuring daily wastewater parameters.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Izady, A., Abdalla, O., Sadeghi, M. et al. A Novel Approach to Modeling Wastewater Evaporation Based on Dimensional Analysis. Water Resour Manage 30, 2801–2814 (2016). https://doi.org/10.1007/s11269-016-1324-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11269-016-1324-8