Abstract
Purpose
To investigate the utility of attenuation value (Hounsfield unit) of the filled bladder on computed tomography (CT) images and the association of these values with simultaneously obtained urine culture results.
Methods
Between January 2016 and December 2017, retrospective data of 58 patients who were admitted to the emergency department for various symptoms were examined. All patients were evaluated with urine dipstick microscopy, urine culture, and abdominal CT simultaneously. Group 1 consisted of patients with positive urine culture (n = 28) and Group 2 consisted of patients with negative urine culture (n = 30). The attenuation value of urine in the bladder at the level of the bladder trigone was measured inside an elliptical drawing covering all the urine inside the bladder excluding the bladder wall on axial non-contrast CT images. The predictive value of this calculated attenuation value for urine culture positivity was evaluated.
Results
The median attenuation value was − 6 (range − 17.8 to + 11) and 12 (range 0–32) in group 1 and group 2 (p < 0.001). According to cut-off value of − 1 attenuation value, sensitivity for predicting urine culture positivity was 92.9%, whereas specificity was 100% (AUC: 0.977 p < 0.001). Urine culture was positive in all of the 26 patients with attenuation value < − 1, whereas only two of the 32 patients with attenuation value > − 1 had urine culture positivity (p < 0.001 OR 14).
Conclusion
The attenuation value of the urine in the defined area of the bladder may aid in the diagnosis of urinary infection with high sensitivity and specificity and without any additional cost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary tract infections (UTI) are among the most prevalent infectious diseases with a substantial financial burden on society. In the US, UTIs are responsible for more than 7 million physician visits annually [1].
Fifteen percent of antibiotics used in USA were prescribed for UTI [2]. Similar data were reported from European countries [3]. UTI, especially pyelonephritis, is the indication for hospitalization for > 100,000 patients annually in the USA [1].
The urine culture is gold standard for UTI diagnosis; however, it takes some time to get the results. So, clinicians are obliged to rely on some urine and blood tests for diagnosis in the acute phase.
Dipstick tests are easy to perform, give an immediate result, and are relatively cheap. The results of a systematic review showed that a dipstick for leukocyte esterase (LE) and nitrite, where both test results are interpreted in combination, was a good test both for ruling in (both positive) and ruling out (both negative) a UTI. A dipstick positive for either LE or nitrite and negative for the other provides inconclusive diagnostic information and further testing is therefore required for these patients. Microscopy is more time consuming and expensive to perform than a dipstick test, but potentially quicker and cheaper than culture. Together with dipstick tests, a combination of microscopy for pyuria and bacteriuria can be used accurately to rule in and rule out a UTI. An indeterminate test result is again obtained if microscopy is positive for either pyuria or bacteriuria, and negative for the other. Confirmatory culture is required in these patients. In patients considered to have a UTI, further culture to determine antibiotic sensitivities may be an option to inform treatment decisions [4].
The Hounsfield scale of tissue density is based on two values: air with − 1000 HU (minimum HU value) and water with 0 HU. Density of other tissues is related to this range, usually from − 1000 to + 1000 HU (− 1024 to 1024 or 3072 depending on the coding of particular vendor) [5]. HU values are measured and reported in a variety of clinical applications [6,7,8,9].
In this recent study, we aimed to investigate the utility of attenuation value in a unique area. Attenuation value of the filled bladder on the CT images was calculated and the association of this value with simultaneously obtained urine culture results was evaluated.
Materials and methods
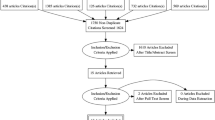
Between January 2016 and December 2017, the retrospective data of 129 patients that presented to the emergency service with complaints of acute abdominal pain and dysuria were evaluated, and all these patients were evaluated with urine dipstick microscopy, urine culture, and abdominal CT simultaneously. Seventy-one of the patients were excluded; 20 had urinary instrumentation (15 urinary catheters, 3 D-J stent, 2 nephrostomy), 30 had intravenous contrast-enhanced CT, 20 had incomplete bladder filling, and 1 had artifacts on imaging due to femur head prosthesis. In the end, 58 patients were included in the recent study that all had urine dipstick microscopy, urine culture, and non-contrast abdominal CT simultaneously.
Imaging technique
All CT scans were carried out using a 64 detector CT scanner (Toshiba®Aquilion64). Raw data obtained on axial plane with 1-mm slices were processed and 5-mm axial and 3-mm slices coronal and sagittal images were achieved on non-contrast CT images. Attenuation value was measured on axial images using Probel®PACS (Picture Archiving and Communication System) system.
The bladder filling rate (bladder volume) was between 150 and 600 cc in all patients. The attenuation value was measured inside an elliptical drawing at the level of the bladder trigone covering all the urine inside the bladder excluding the bladder wall on axial non-contrast CT image and also the distance of the region of interest to the skin was measured (Fig. 1). The measurements were made by one urologist and one radiologist blindly, and the analysis of the data was made using the mean of these two values.
Microbiological diagnosis
Mid-stream urine was sent in a sterile container and cultured in 5% sheep blood agar and eosin-methylene blue agar and incubated at 37° for 18–24 h. The results were quantitatively evaluated [10]. The bacterial growth of ≥ 105cfu/ml was accepted as positive and included in the study.
Biochemical diagnosis
The urine analyses were completed using URISED II&LABUMAT II®(77 ElektronikaKft.Hungarian).
The patients were divided into two groups according to urine culture positivity. Group 1 consisted of patients with positive urine cultures, whereas group 2 consisted of patients with negative urine cultures. The attenuation value of the bladder urine at the level of the defined area was calculated for each patient in the two groups and the association of bacterial growth with attenuation value was assessed. Also, the correlations of attenuation value with WBC in blood, neutrophilia in blood, and urine analysis characteristics were evaluated.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences, version 20.0 (SPSS, Chicago, Ill) software program. According to Urine Culture positivity, patients were divided into two groups as positive Urine Culture (Group 1) and negative Urine Culture (Group 2) groups. Mann–Whitney U test and Chi-square test (Yates’ Chi-square test and Fisher’s exact test) analyses were used for the groups. In addition, to define the cut-off value of the attenuation value for predicting positive urine culture, ROC Curve analysis was used. According to the cut-off value, patients were divided into two groups as under the cut-off value and above the cut-off value. Mann–Whitney U test and Chi-square test (Yates’ Chi-square test and Fischer’s exact test) analyses were also used for these groups. Statistical significance was defined as p < 0.05.
Results
Urine culture was positive in 28 patients (group 1) and urine culture was negative in 30 (group 2). No statistically significant difference was found between the two groups in terms of mean age, sex, WBC count in blood, neutrophil count in blood, urine pH, and urine-specific gravity. Fever was detected in 22 and 2 patients in group 1 and group 2, respectively (p < 0.001). Nitrite positivity was detected in 11 patients in group 1, whereas none of the patients had nitrite positivity in group 2 (p < 0.001). The LE was statistically significantly higher in group 1 compared to group 2 (p < 0.001). The distance of the region of interest to the skin and bladder volume values was similar between the groups. The median attenuation value was − 6 (range − 17.8 to + 11) and 12 (range 0 to + 32) in group 1 and group 2, respectively (Table 1). Also, the attenuation value of the cases was evaluated for each group according to the bladder volumes. An average bladder volume of 300 ml was accepted as cut-off value. Twelve and fifteen of the patients had bladder volume ≤ 300 ml, whereas sixteen and fifteen patients had bladder volume > 300 ml in group 1 and group 2, respectively. The mean attenuation values were − 4.4 ± 6.7 and − 6.7 ± 4.2 for bladder volumes ≤ 300 and > 300 ml, respectively, in group 1 (p = 0.286). The mean attenuation values were 14.4 ± 7.8 and 10.7 ± 7.7 for bladder volumes ≤ 300 and > 300 ml, respectively, in group 2 (p = 0.203).
The calculated cut-off value of the attenuation value for predicting urine culture positivity was − 1 attenuation value based on ROC curve analysis. According to this cut-off value, the sensitivity of attenuation value for predicting urine culture positivity was 92.9% and specificity was 100% (AUC: 0.977 p < 0.001). Urine culture was positive in all of the 26 patients with attenuation value ≤ − 1, whereas only two of the 32 patients with attenuation value > − 1 had urine culture positivity (p < 0.001 OR 14). The correlation of attenuation value with urine culture results is shown in Fig. 2. Also, the attenuation value of ≤ − 1 was correlated with neutrophil count, fever, nitrite positivity, and LE positivity which are known predictors of UTI (Table 2).
Furthermore, three patients in group 1 later had non-contrast CT and simultaneous urine culture after antibiotic treatment. The urine culture was negative in these three patients and also the attenuation value had changed to positive.
Discussion
Although it is a widespread disease, some difficulties still persist in the diagnosis of UTI which poses a problem for physicians. The ideal test should be fast, cheap, and accessible with high accuracy. Some UTI may be asymptomatic or can be manifested with atypical signs and symptoms. For these reasons, there are limited laboratory tests that physicians can trust on, for the diagnosis of UTI.
The chemical analysis of urine is performed with a dipstick test. These dry reactive sticks can detect the specific gravity, urine pH, the presence of nitrites, LE, protein, and peroxidase in urine. The detection of the protein, LE, in urine is indicative of the presence of neutrophils and thus pyuria. LE is detected via WBCs esterase procedure. However, false positivity is common due to contamination with vaginal flora bacteria. Nitrite positivity is a fast indirect indicator of bacteriuria. Bacterial species including E. coli, Klebsiella, Proteus, etc. can convert nitrate to nitrite. However, bacterial species including Pseudomonas, enterococci, and S. Saprophyticus are nitrite-negative organisms and the nitrite test is negative although these bacteria grow in urine cultures [11].
A meta-analysis including 70 studies about the results of dipstick tests revealed a sensitivity of 75%, specificity of 98% for LE and sensitivity as low as 30%, specificity of 90% for nitrite positivity in the diagnosis of UTI. Using a combination of these two parameters, the sensitivity increases above 88% [12].
The results of a systematic review including 12,544 patients revealed that only for nitrite positivity, the likelihood ratio was 29.3 (14.4–59.7), negative likelihood ratio was 0.48 (0.37–0.62). and the diagnostic odds ratio was 61. The same parameters for LE alone were positivity 4.9 (3.3–7.3), 0.31 (0.18–0.51), and 15.8, respectively. The combination of nitrite and LE positivity has likelihood ratio of 9.6 (5.4–17) negative likelihood ratio of 0.54 (0.26–1.1) and diagnostic odds ratio of 17.8 [13].
Bagga et al. reported that LE has sensitivity of 51.2%, specificity of 92.0%, positive predictive value of 60.2%, and negative predictive value of 88.9% in their study evaluating the parameters predicting UTI. In the same study, these parameters were 37.8, 88.1, 42.9, and 85.8%, respectively. The combination of nitrite and LE positivity has sensitivity of 62.2%, specificity of 82.8%, positive predictive value of 45.9%, and negative predictive value of 90.3% [14].
Attenuation values on non-contrast CT can be used to differentiate the type and contents of ascites. In a study by Mizimura et al., the authors evaluated the intraabdominal ascites with non-contrast computed tomography and they tried to differentiate patients with bladder ruptures by calculating the ascites. They found that the value of ascites in bladder rupture (median 5.7; range 3.1–6.1) was significantly lower than in cases of gastrointestinal perforation (median 14.7; range 4.7–25.4) and intestinal ischemia (median 13.3; range 6.0–18.1) (p = 0.004) [15].
In the recent study, we found that the sensitivity of attenuation value was 92.9% and the specificity was 100%, when the cut-off value for attenuation value was accepted as − 1. The measurement of the attenuation value in non-contrast BT can be easily performed as we described. Of course, we do not suggest imaging with CT for diagnosis of UTI. But in cases with an acute event that can be related to urinary tract symptoms and non-contrast CT is ordered for the patient, the reporting of attenuation value may provide valuable information to the physician about the possible diagnosis of UTI. This is because the attenuation value has higher sensitivity and specificity compared to the commonly used urine dipstick test parameters (nitrite, LE).
It is hard to explain the possible mechanism that results in the negative attenuation value for bladder urine with UTI. It may be attributable to disintegration of molecules in urine by bacteria or to the bacterial load itself.
The limitations of this study are its retrospective design, the limited number of patients due to strict inclusion criteria, and the absence of control CT (showing change in attenuation value) and urine culture after treatment. We only have three patients that had non-contrast CT and urine culture after treatment. In all these three patients, we showed that the attenuation value changed to positive values when the urine culture was negative.
Conclusions
The attenuation value of the urine in the defined area of the bladder may aid in the diagnosis of urinary infection with high sensitivity and specificity and without any additional cost. We recommend radiologists to report the attenuation value of bladder urine on non-contrast CT routinely. Further studies are necessary to confirm our results as this is the first study on this topic.
References
Foxman B (2002) Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113:5
Mazzulli T (2002) Resistance trends in urinary tract pathogens and impact on management. J Urol 168:1720
Haslund JM, Dinesen MR, Nielsen ABS et al (2013) Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand J Prim Health Care 31:235
Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J et al (2006) Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model. Health Techno lAssess 10:1
Hounsfield GN. Nobel lecture (1980) 8 December 1979. Computed medical imaging. J Radiol 61:459
Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ (2012) Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. AcadRadiol 19:811
Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N (2013) Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158:588
Bruni SG, Patafio FM, Dufton JA, Nolan RL, Islam O (2013) The assessment of anemia from attenuation values of cranial venous drainage on unenhanced computed tomography of the head. Can Assoc Radiol J 64:46
Ouzaid I, Al-qahtani S, Dominique S, Hupertan V, Fernandez P, Hermieu JF et al (2012) A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): evidence from a prospective study. BJU Int 110:E438
Garcia LS, Isenberg HD (2010) Clinical Microbiology Procedures Handbook, 3rd edn. ASM Press; 2010, Washington, DC
Simerville JA, Maxted WC, Pahira JJ (2005) Urinalysis: a comprehensive review. Am Fam Physician 71:1153
Devillé WL, Yzermans JC, van Duijn NP, Bezemer PD, van der Windt DA, Bouter LM (2004) The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol 4:4
St John A, Boyd JC, Lowes AJ, Price CP (2006) The use of urinary dipstick tests to exclude urinary tract infection: a systematic review of the literature. Am J ClinPathol 126:428
Bagga R, GirotraA (2016) Urinary Dipsticks: Efficacy as Predictor of Urinary Tract Infections. J Bacteriol Mycol 2:15
Mizumura N, Okumura S, Toyoda S, Imagawa A, Ogawa M, Kawasaki M (2016) Non-traumatic bladder rupture showing less than 10 Hounsfield units of ascites. AcuteMedSurg 4:184
Author information
Authors and Affiliations
Contributions
BI: Project development, Manuscriptwriting, Data collection; BIH: Manuscript writing/editing; SE: Data collection, editing; CS: Data analysis; YS: Data collection, Manuscript writing; DT: Protocol/Project development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with theethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Basmaci, I., Bozkurt, I.H., Sefik, E. et al. A novel use of attenuation value (Hounsfield unit) in non-contrast CT: diagnosis of urinary tract infection. Int Urol Nephrol 50, 1557–1562 (2018). https://doi.org/10.1007/s11255-018-1945-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1945-3