Abstract
Posterior reversible encephalopathy syndrome (PRES) has been described as a neurological condition observed in a variety of clinical settings and is characterized by focal neurological deficits, seizures, headaches, altered mental status, and visual impairment, associated with transient typical lesions on neuroimaging, predominantly in the posterior part of the brain. The most common risk factors for PRES are hypertension, renal diseases, and the use of calcineurin inhibitors. The incidence of PRES in children with renal disorders varies between 4 and 9%, according to different reports. Vasogenic cerebral edema is considered the major pathophysiological mechanism of PRES. There are two main theories regarding the genesis of this edema: (1) hyperperfusion, due to autoregulatory failure of the cerebral vasculature, and (2) hypoperfusion, due to vasoconstriction of the cerebral arteries. In addition, PRES might also be the result of a systemic inflammatory state causing endothelial dysfunction. The management of PRES includes BP control, treatment of seizures, and removal of or reduction in calcineurin inhibitors. Intravenous administration of antihypertensive therapy is preferred, and various drugs have been used in this regard, including nicardipine, labetalol, sodium nitroprusside, and hydralazine. The prognosis of PRES is usually benign, except for rare cases with intracranial hemorrhage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterior reversible encephalopathy syndrome (PRES) has been first described by Hinchey et al. [1] as a neurological condition observed in a variety of clinical settings and is characterized by seizures, headaches, altered mental status, and visual impairment, associated with transient typical lesions on neuroimaging, predominantly in the posterior part of the brain. Renal patients are at particular risk of PRES, because of the frequent association of kidney diseases with hypertension. The incidence of PRES in children with renal disorders varies between 4 and 9%, according to different reports [2,3,4,5,6]. However, this incidence might be underestimated, because some patients may develop PRES without seizures [4, 5].
Terminology
“Reversible posterior leukoencephalopathy syndrome” was the original term used for this clinico-radiological entity, as described in patients with renal insufficiency, hypertension, or under immunosuppressive therapy. This terminology intended to emphasize the reversible nature and the limited distribution of the brain lesions. However, this term is inaccurate, because morphological abnormalities of PRES are not strictly confined to the white matter, and they are not always reversible [7, 8]. Several other terms have been subsequently advocated, such as “posterior reversible encephalopathy syndrome” [9], “immunosuppressive-associated leukoencephalopathy” [10], “hyperperfusion encephalopathies” [11], “reversible posterior cerebral edema syndrome” [12], or “reversible occipito-parietal encephalopathy” [13]. Although there is still some debate about its accuracy, PRES is currently the most widely accepted [4].
Etiopathogenesis
Most of the PRES reported cases in children are secondary to immunosuppressive therapy used in hemato-oncological diseases, such as aplastic anemia, acute leukemias, thalassemia, non-Hodgkin lymphoma, lymphohistiocytosis, autoimmune lymphoproliferative syndrome, and Evans syndrome [14,15,16]. There are also case reports of PRES associated with Henoch–Schönlein purpura [17], systemic lupus erythematosus [18], Guillain–Barré syndrome, and preeclampsia [11]. PRES in children with various kidney diseases was reported in several studies [4, 6, 19,20,21,22,23,24,25].
Vasogenic cerebral edema is considered the major pathophysiological event in PRES. There are two main theories regarding the genesis of this vasogenic edema: (1) hyperperfusion due to autoregulatory failure of the cerebral vasculature and (2) hypoperfusion due to vasoconstriction of the cerebral arteries (which has been proved in positron emission tomography studies). These mechanisms may coexist or occur alternatively [1, 4, 11, 26].
The most popular theory is that severe hypertension causes a breakdown in the autoregulation of the cerebral circulation. The cerebral blood flow is usually regulated by dilatation or constriction of vessels to maintain adequate tissue perfusion and to simultaneously avoid excessive intracerebral hypertension. Autoregulation breakdown occurs above a mean arterial blood pressure (BP) of 150–160 mmHg; in chronic hypertension, it occurs at higher BP. Uncontrolled hypertension leads to hyperperfusion and cerebral vessel damage, resulting in interstitial extravasation of proteins and fluids, causing vasogenic edema. Irreversible damage is seen at a mean BP above 200 mmHg [26]. Conditions commonly associated with PRES, such as chronic hypertension, are known to reduce the effectiveness of vascular autoregulation. However, this theory does not explain why BP in PRES does not usually exceed the upper limit of autoregulation, why PRES may also occur in the absence of hypertension, and why the extent of cerebral edema is not directly related to the severity of hypertension [27,28,29].
According to the second theory, hypertension causes activation of the autoregulatory system, which results in vasoconstriction of the brain vessels, with subsequent hypoperfusion, ischemia, and fluid leakage [4].
The preferential involvement in the posterior cerebral circulation has been postulated to be due to a sympathetic innervation (which protects the brain from sudden increases in BP) relatively less developed in the arterioles supplied by the vertebro-basilar system than in the anterior circulation [29].
However, PRES can develop even in the absence of hypertension [13, 26]. Another theory suggests that PRES is the result of a systemic inflammatory state causing endothelial dysfunction. This theory is supported by the observation that PRES is often associated with inflammatory conditions like autoimmune diseases, sepsis, eclampsia, and renal transplantation [26]. When BP is high, the vasoconstriction that occurs by autoregulation could exacerbate inflammatory endothelial dysfunction, causing hypoxia, and subsequent vasogenic edema. This would explain why the control of hypertension favors the recovery of PRES [28, 30]. Calcineurin inhibitors are known to injure the vascular endothelium, damage endothelial cells of the cerebral arteries, and subsequently cause a release of vasoactive agents that may induce vasogenic edema [10, 31, 32].
Risk factors
Vasogenic edema, as the common underlying mechanism of PRES, can be associated with several risk factors, including hypertension, use of calcineurin inhibitors, and renal diseases.
Acute hypertension results in vasoconstriction, followed by vasodilatation, increased vascular permeability, blood–brain barrier dysfunction, and cerebral edema. Hypertensive crisis is probably the most common risk factor for PRES, and the re-occurence of the initial insult or a new trigger may cause the recurrence of PRES. Many patients with PRES caused by acute hypertension showed clinical resolution after BP reduction and recurrence of PRES after new hypertensive attacks [1, 2, 5, 11].
Yamanda et al. [2, 5] found no correlation between the duration or severity of hypertension and the neurological symptoms and neuroimaging findings in PRES patients, suggesting that acute rise in BP, rather than duration or severity of hypertension, is the trigger factor of PRES. Discrepancies between the severity of the lesions and that of hypertension have been reported in other studies as well [34, 35].
It has also been suggested that children are prone to develop PRES at lower BP levels than adults, probably because the BP threshold of cerebral blood flow autoregulation is lower in children as compared to adults [36].
The use of calcineurin inhibitors, cyclosporine and tacrolimus, has been demonstrated as an important predisposing factor for the development of PRES in patients with kidney disease [4, 10, 25, 34, 37, 38]. Although high blood concentrations of calcineurin inhibitors may increase the risk, some patients develop PRES within the therapeutic range of these concentrations. The relationship between the trough level of cyclosporine and the development of PRES remains controversial. In Ishikura’s case series [39], patients with cyclosporine trough levels <80 ng/ml developed PRES. Other studies demonstrated that immunosuppressive and cytotoxic drugs may induce and exacerbate hypertension and may lower the seizure threshold [33, 41]. However, most of the liver transplant patients do not develop PRES even with very high trough levels of calcineurin inhibitors [39, 40]. Thus, it is impossible to define a trough level of serum cyclosporine that is absolutely safe. Instead, other concurrent risk factors for PRES need to be considered and searched for.
Since steroids can also induce PRES [6] and hypertension [42], the combination of steroids and calcineurin inhibitors can increase the risk of PRES.
Other immunosuppressant agents, such as rapamycin, have also been reported to induce PRES in adult kidney transplant recipients [43].
Nephrotic syndrome (NS) is a condition that may predispose to the development of PRES. Patients with NS are at risk of PRES because they often receive calcineurin inhibitors and steroids and they often have hypertension and/or renal insufficiency. However, even mild hypertension may be detrimental in NS patients treated with cyclosporine. Furthermore, in NS vasogenic edema could be induced by decreased intravascular oncotic pressure, increased permeability of intracerebral capillaries, and fluid overload [39]. T cell activation and inflammatory cytokine production have been suggested as additional predisposing factors for PRES in children with NS, particularly during relapses [4, 6, 23].
Other kidney diseases in children that may predispose to the development of PRES include acute glomerulonephritis [24, 44], hemolytic uremic syndrome [45], lupus nephritis [46, 47], Wegener’s granulomatosis [25], Henoch–Schönlein purpura nephritis [48], renal insufficiency or end-stage renal disease (ESRD) [6, 39], renal artery stenosis [49], and grade IV vesico-ureteric reflux [23, 50]. In the setting of ESRD, both the rise in BP and uremia itself may serve as triggers for PRES [51].
Yamanda et al. [2] found that younger PRES patients were more predisposed to recurrent seizures. This is consistent with previous studies in pediatric renal transplant recipients [52]. An experimental study suggested that exposure to calcineurin inhibitors at a very young age results in severe neurotoxicity, due to a more permeable blood–brain barrier [53]. Thus, cytokines involved in the PRES process may more readily cross the blood–brain barrier in younger children. Autoregulatory response improves with increasing age and brain maturation, whereas immature brain is more susceptible to vasoconstriction during hypertension. These factors may be responsible for recurrent seizures in younger PRES patients [32, 54].
It has also been noticed that PRES developed more often in boys than in girls [20, 33, 39, 41, 55]. In adult series, females were affected more frequently than males, even when eclampsia cases were excluded [1, 7, 56]. On the other hand, severe neurological symptoms (e.g., recurrent seizures) are also more common in female patients [2]. This may be explained by the fact that girls have fewer interneuronal connections and higher diffusivity in the parieto-occipital regions than boys [57].
Hypomagnesemia, hypocholesterolemia, hypercalcemia, aluminum overload, high-dose methylprednisolone, acute hepatic failure, nonsteroidal anti-inflammatory drugs, blood transfusions, erythropoietin therapy, human immunodeficiency virus infection, and intravenous gamma globulin therapy might also act as contributory factors in the etiology of PRES [12, 17, 58].
Clinical manifestations
There are no consensual guidelines for validation of PRES diagnosis [15, 33]. Seizures are the most common clinical manifestation of PRES the majority presenting as generalized tonic–clonic seizures. They usually occur at the onset of PRES, but can also develop later in the course of the disease [41]. Other frequent symptoms include altered consciousness, coma, stupor, lethargy, confusion, severe headache, nausea/vomiting, or vision impairment. Ophthalmological symptoms are quite specific and may include visual blurring, hemianopsia, scotomas, visual hallucinations, and cortical blindness [4, 17]. Development of Anton’s syndrome (denial of visual loss) has also been reported [1]. Focal neurological deficit is an uncommon abnormality in PRES [18].
Most of these symptoms usually develop abruptly and resolve within a few weeks, with proper management. Although the reversible nature of the symptoms is characteristic of PRES, permanent neurological damage may occur, in the absence of early recognition and treatment. It is also possible that, due to structural and developmental differences between children’s central nervous system and that of adults, PRES may be more aggressive in children [5].
Neuroimaging
The diagnostic of PRES is mainly based on magnetic resonance imaging (MRI), which is currently considered the gold standard in this regard [4, 39].
Conventional cranial computed tomography (CT) has been widely used, showing PRES lesions as low-density areas. The advantages of CT reside in its ability to detect intracranial hemorrhage and in its applicability in severely ill patients [59]. However, it has limited sensitivity for the detection of PRES, particularly in the acute phase [10].
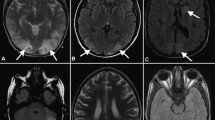
Classical MRI typically shows edema involving the white matter in the posterior portions of the cerebral hemispheres, usually bilateral, in the parieto-occipital regions, which are the most commonly affected [1] (Figs. 1, 2). T2-weighted images and fluid-attenuated inversion recovery (FLAIR) images have been frequently employed [7], with both of the sequences showing lesions as hyperintensities. For detection of subtle peripheral lesions, FLAIR is superior to conventional MRI techniques, as this sequence suppresses the signal of the adjacent cerebrospinal fluid and can, therefore, render the lesions of PRES more conspicuous [9, 60].
Although PRES commonly involves the parietal–occipital region, three different imaging patterns have been described. The first is the holo-hemispheric watershed pattern, with a linear involvement in the frontal, parietal, and occipital lobes predominantly, along a watershed distribution. The second is the superior frontal sulcus pattern, with predominant involvement in the frontal lobes, and the third was the dominant parietal–occipital pattern, in which the typical predominance of lesions in the posterior lobes is seen [26, 32]. Asymmetrical and/or partial manifestations of these three primary patterns were also described [26, 32]. Atypical sites of involvement include the brain stem, cerebellum, basal ganglia, thalami, internal capsule, and splenium of corpus callosum [8, 26, 51, 61].
The “diffusion-weighted imaging” (DWI) with quantification by “apparent diffusion coefficient” (ADC) mapping may provide more accurate and specific images [4, 7, 59]. DWI and ADC denote small movements of water molecules, known as Brownian motion. Taken together, DWI and ADC mapping can differentiate between PRES and cerebral infarction, as well as predict the outcome of PRES, by detecting severe or advanced lesions that can result in irreversible damage [62]. A useful approach is to first detect the lesions by DWI and then evaluate the diffusion state by ADC mapping. Several authors strongly advocate the use of DWI and ADC maps for accurate diagnosis and prediction of PRES outcomes [5, 63].
DWI usually demonstrates hypo- to isointense areas, which suggest increased water diffusion due to vasogenic edema. In contrast, high intensities on DWI were seen when cytotoxic edema was present [9, 17]. DWI and ADC have been found to be also helpful in differentiating atypical presentations of PRES from conditions like central pontine/extrapontine myelinolysis, non-hemorrhagic infarcts, and hypoglycemic or hypoxic encephalopathy. Due to vasogenic edema in PRES, ADC shows increased values with slightly increased signal intensity on DWI, whereas the other conditions show reduced ADC values due to cytotoxic edema [64, 65].
Magnetic resonance angiography, MR spectroscopy, and MR perfusion scans are rarely performed in patients with PRES [59, 66], and the experience with these methods in the pediatric population is limited. Proton MR spectroscopy studies showed that PRES causes diffuse metabolic abnormalities in the brain, even with normal MRI findings [67].
Angiography in PRES demonstrates reversible focal and diffuse abnormalities, which are thought to reflect endothelial dysfunction [65]. MR angiography has shown moderate-to-severe vessel irregularity, consistent with vasoconstriction and vasodilation, in the majority of the patients, and MR perfusion imaging has shown significantly reduced relative cerebral blood volume in one case series [68]. These might be interesting approaches to explore the mechanism of PRES, but are not readily applicable in clinical practice [4].
Other investigations
Other investigations do not usually provide specific information. Electroencephalography occasionally shows non-specific slow waves and spikes [69] and is only valuable for evaluating seizure activity and for ruling out subclinical status epilepticus [66]. Cerebrospinal fluid examination is only useful for ruling out infective or inflammatory diseases [4].
Differential diagnosis
The clinical manifestations and neuroradiological findings are typical for PRES, regardless of its etiology [4]. The differential diagnosis must mainly rule out cerebral infarction and venous thrombosis.
Progressive multiple leukoencephalopathy (PML) is an opportunistic infection of the brain caused by the JC virus, with variable clinical presentation and lethal outcome. To exclude PML, a search for JC virus DNA in the cerebrospinal fluid is required. The increased use of strong immunosuppressive medication in kidney transplantation and in the treatment of autoimmune diseases, including calcineurin inhibitors, mycophenolate mofetil, and rituximab, is likely to result in increased incidence of PML. Sometimes, T2-weighted and FLAIR images of PML mimic those of PRES, and DWI findings of patients with PML differ in asynchronous lesions and are dependent on their stage [70, 71].
Pseudotumor cerebri, an idiopathic condition of elevated intracranial pressure lesion, shows similar symptoms, including headache, nausea, and visual disturbance [72]. It can be distinguished from PRES by using normal cranial MRI. Furthermore, PRES has not been associated with elevated intracranial pressure [4].
Other differential diagnoses of PRES include acute disseminated encephalomyelitis, infectious encephalitis, and meningitis. Particularly, herpes simplex encephalitis should be considered and, when suspected, rapid treatment with intravenous acyclovir and antibiotics may be lifesaving, while the diagnostic workup is still being pursued [4, 30].
Venous sinus thrombosis and subdural, intracerebral, or subarachnoid hemorrhage can all present with headache, seizures, reduced consciousness, and focal neurological signs.
It is important to also consider the diagnosis of posterior circulation stroke, because its treatment (which may include urgent thrombolysis) and prognosis both differ from those of PRES. Basilar artery thrombosis can present with progressive neurological deficits and can result in tetraparesis, coma, or locked-in syndrome [30].
Central nervous system vasculitis can present with symptoms similar to those of PRES, but the MRI findings are usually more diffuse, and many of the clinical and imaging features are irreversible. The diagnosis may be difficult because systemic signs of inflammation are often absent [27, 32] and rapid treatment is vital to prevent further complications.
Autoimmune encephalitis and metabolic encephalopathies (including central pontine myelinolysis), uremia, or drug toxicity (e.g., with cyclosporine) can also have similar symptoms [30].
Other differential diagnoses of PRES may include intracranial progressive multifocal leukoencephalopathy and X-linked adrenoleukodystrophy [73, 74].
Treatment
The management of PRES includes control of BP, treatment of seizures, and removal of or reduction in all possible causative factors [11, 66]. After ruling out cerebral infarction, BP should be lowered to near the 99th ‰ level for the patient’s age and sex. The mean BP should be reduced by 25% within the first hour, followed by a much slower reduction thereafter [66]. Intravenous administration of antihypertensive therapy is preferred, and various drugs have been used, including nicardipine, labetalol, sodium nitroprusside, and hydralazine [9]. Nicardipine is very effective in rapid lowering of BP. The infusion rate of this agent should start at 0.5 μg/kg/min and can be increased up to 5 μg/kg/min.
Seizures may progress to status epilepticus and should be treated with intravenous anticonvulsants. Diazepam and lorazepam are often used as the first-line agents, with phenytoin and phenobarbital as the second line. Midazolam is also a useful treatment [4].
Elimination of other predisposing factors must be considered. Discontinuation of calcineurin inhibitors and avoiding their re-administration after an episode of PRES are issues of controversy [4]. One particularly controversial issue concerns the recipients of organ transplants, as the withdrawal of such drugs can cause acute graft rejection. Dose reduction has resulted in good outcomes in some case series [59, 75], while others prefer complete withdrawal, at least for a certain period [66].
Correction of fluid overload and/or electrolyte disturbances is required, if necessary [4].
Prognosis
Prompt diagnosis and treatment, as well as good and rapid communication among caregivers [76], are the keys to achieving a good outcome in children with PRES [77]. The prognosis of this condition is generally benign, except for rare patients with intracranial hemorrhage [3, 4, 59]. There is some debate whether PRES is truly a reversible disease, since prolonged seizures, hypertension, or both may result in permanent neurological deficits and cerebral infarction [78].
In a case series [4], 2 out of 20 children with PRES and kidney disease developed long-term brain damage (with developmental delay). Other case series of similar patients also reported some chronic neurological impairment or imaging abnormalities [8], chronic epilepsy [4, 27], recurrent PRES [5, 51, 79], or subtle neurological deficits, including subclinical developmental delay and learning disabilities [4].
These cases suggest the necessity of long-term follow-up of PRES patients, including those who show complete clinical and radiological recovery in the short term.
Conclusions
PRES should be suspected in all children with kidney disease hypertension and/or immunosuppressive treatment (such as cyclosporine), who develop sudden neurological symptoms, even if imaging abnormalities are not restricted to the subcortical white matter of the occipital lobe. Severe neurological complications may develop if left untreated. Therefore, early recognition of PRES and optimal therapy are important to prevent serious neurological sequelae in these patients.
Rigourous control of hypertension and blood concentration of calcineurin inhibitors are important strategies in managing children with kidney disease, in order to prevent the development of PRES.
Further advances with MRI, including DWI with ADC mapping, are required to improve diagnostic accuracy and the ability to predict outcomes in patients with early-stage PRES, as well as to better understand the complex pathophysiology of this disorder. Further research is needed to set up guidelines for PRES diagnosis and treatment.
References
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR (1996) A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334:494–500
Yamada A, Ueda N (2012) Age and gender may affect posterior reversible encephalopathy syndrome in renal disease. Pediatr Nephrol 27(2):277–283
Ishikura K, Ikeda M, Hamasaki Y, Hataya H, Shishido S, Asanuma H, Nishimura G, Hiramoto R, Honda M (2006) Posterior reversible encephalopathy syndrome in children: its high prevalence and more extensive imaging findings. Am J Kidney Dis 48:231–238
Ishikura K, Hamasaki Y, Sakai T, Hataya H, Mak RH, Honda M (2012) Posterior reversible encephalopathy syndrome in children with kidney diseases. Pediatr Nephrol 27(3):375–384
Onder AM, Lopez R, Teomete U, Francoeur D, Bhatia R, Knowbi O, Hizaji R, Chandar J, Abitbol C, Zilleruelo G (2007) Posterior reversible encephalopathy syndrome in the pediatric renal population. Pediatr Nephrol 22:1921–1929
Ikeda M, Ito S, Hataya H, Honda M, Anbo K (2001) Reversible posterior leukoencephalopathy in a patient with minimal-change nephrotic syndrome. Am J Kidney Dis 37:E30
Stott VL, Hurrell MA, Anderson TJ (2005) Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J 35:83–90
Prasad N, Gulati S, Gupta RK, Kumar R, Sharma K, Sharma RK (2003) Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol 18:1161–1166
Casey SO, Sampaio RC, Michel E, Truwit CL (2000) Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol 21:1199–1206
Singh N, Bonham A, Fukui M (2000) Immuno suppressive associated leukoencephalopathy in organ transplant recipients. Transplantation 69:467–472
Schwartz RB (2002) Hyperperfusion encephalopathies: hypertensive encephalopathy and related conditions. Neurologist 8:22–34
Dillon WP, Rowley H (1998) The reversible posterior cerebral edema syndrome. AJNR Am J Neuroradiol 19:591
Pavlakis SG, Frank Y, Chusid R (1999) Hypertensive encephalopathy, reversible occipitoparietal encephalopathy, or reversible posterior leukoencephalopathy: three names for an old syndrome. J Child Neurol 14:277–281
Chandramohan V, Nagarajan VP, Sathyamoorthi MS et al (2012) Posterior reversible encephalopathy syndrome in a child with autoimmune lymphoproliferative syndrome: case report and review of literature. J Pediatr Neurosci 7(3):221–224
Arzanian MT, Shamsian BS, Karimzadeh P, Kajiyazdi M, Malek F, Hammoud M (2014) Posterior reversible encephalopathy syndrome in pediatric hematologic-oncologic disease: literature review and case presentation. Iran J Child Neurol 8(2):1–10
Gafton B, Porumb V, Ungurianu S, Marinca MV, Cocea C, Croitoru A, Balan G, Miron N, Eliade Ciuleanu T, Miron L (2014) Hepatocellular carcinoma: insights in the biological treatment beyond sorafenib. J BUON 19(4):858–866
Endo A, Fuchigami T, Hasegawa M, Hashimoto K, Fujita Y, Inamo Y, Mugishima H (2012) Posterior reversible encephalopathy syndrome in childhood: report of four cases and review of the literature. Pediatr Emerg Care 28(2):153–157
El-Naggari MA, Al-Nabhani D, El-Nour I, El-Manzalawy A, Abdelmogheth A-AA (2015) Posterior reversible encephalopathy syndrome in two Omani children with underlying renal diseases. Sult Qaboos Univ Med J 15(3):424–428
Saeed B, Abou-Zor N, Amer Z, Kanani I, Hilal M (2008) Cyclosporin-A induced posterior reversible encephalopathy syndrome. Saudi J Kidney Dis Transpl 19(3):439–442
Gera DN, Patil SB, Iyer A, Kute VB, Gandhi S, Kumar D, Trivedi HL (2014) Posterior reversible encephalopathy syndrome in children with kidney disease. Indian J Nephrol 24(1):28–34
Tenta M, Uchida HA, Nunoue T, Umebayashi R, Okuyama Y, Kitagawa M, Maeshima Y, Sugiyama H, Wada J (2015) Successful treatment by mycophenolate mofetil in a patient with focal segmental glomerulosclerosis associated with posterior reversible encephalopathy syndrome. CEN Case Rep 4(2):190–195
Sakai N, Kawasaki Y, Imaizumi T, Kanno S, Go H, Mitomo M, Ushijima Y, Suyama K, Ito M, Hashimoto K, Hosoya M (2010) Two patients with focal segmental glomerulosclerosis complicated by cyclosporine-induced reversible posterior leukoencephalopathy syndrome. Clin Nephrol 73:482–486
Nakahara C, Hasegawa N, Izumi I, Kanemoto K, Iwasaki N (2005) The use of cyclosporine in a boy with a prior episode of posterior encephalopathy. Pediatr Nephrol 20:657–661
Soylu A, Kavukcu S, Turkmen M, Akbas Y (2001) Posterior leukoencephalopathy syndrome in poststreptococcal acute glomerulonephritis. Pediatr Nephrol 16:601–603
Ohta T, Sakano T, Shiotsu M, Furue T, Ohtani H, Kinoshita Y, Mizoue T, Kiya K, Tanaka I (2004) Reversible posterior leukoencephalopathy in a patient with Wegener granulomatosis. Pediatr Nephrol 19:442–444
Bartynski WS (2008) Posterior reversible encephalopathy syndrome. I. Fundamental imaging and clinical features. AJNR Am J Neuroradiol 29:1036–1042
Roth C, Ferbert A (2011) The posterior reversible encephalopathy syndrome: What’s certain, what’s new? Pract Neurol 11:136–144
Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA (2010) Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 85:427–432
McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS et al (2007) Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 189:904–912
Hobson EV, Craven I, Blank SC (2012) Posterior reversible encephalopathy syndrome: a truly treatable neurologic illness. Peritoneal Dial Int J Int Soc Peritoneal Dial 32(6):590–594
Bechstein WO (2000) Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 13:313–326
Bartynski WS (2008) Posterior reversible encephalopathy syndrome. II. Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 29:1043–1049
De Laat P, te Winkel ML, Devos AS, Catsman-Berrevoets CE, Pieters R, van den Heuvel-Eibrink MM (2011) Posterior reversible encephalopathy syndrome in childhood cancer. Ann Oncol 22:472–478
Magnasco A, Rossi A, Catarsi P, Gusmano R, Ginevri F, Perfumo F, Ghiggeri GM (2008) Cyclosporin and organ specific toxicity: clinical aspects, pharmacogenetics and perspectives. Curr Clin Pharmacol 3:166–173
Mueller-Mang C, Mang T, Pirker A, Klein K, Prchla C, Prayer D (2009) Posterior reversible encephalopathy syndrome: Do predisposing factors make a difference in MRI appearance? Neuroradiology 51:373–383
Jones BV, Egelhoff JC, Patterson RJ (1997) Hypertensive encephalopathy in children. AJNR Am J Neuroradiol 18:101–106
de Oliveira RA, Fechine LM, Neto FC, Nicodemus JM, Silva GB Jr, Silva LS (2008) Posterior reversible encephalopathy syndrome (PRES) induced by cyclosporine use in a patient with collapsing focal glomeruloesclerosis. Int Urol Nephrol 40(4):1095–1098
Akutsu N, Iwashita C, Maruyama M, Ootsuki K, Ito T, Saigo K, Kenmochi T (2008) Two cases of calcineurin inhibitor-associated reversible posterior leukoencephalopathy syndrome in renal transplant recipients. Transpl Proc 40:2416–2418
Ishikura K, Ikeda M, Hamasaki Y, Hataya H, Nishimura G, Hiramoto R, Honda M (2008) Nephrotic state as a risk factor for developing posterior reversible encephalopathy syndrome in paediatric patients with nephrotic syndrome. Nephrol Dial Transpl 23(8):2531–2536
Mueller AR, Platz KP, Bechstein WO et al (1994) Neurotoxicity after orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation 58:155–170
Incecik F, Herguner MO, Altunbasak S, Erbey F, Leblebisatan G (2009) Evaluation of nine children with reversible posterior encephalopathy syndrome. Neurol India 57:475–477
Bai JPF, Lesko LJ, Burckart GJ (2010) Understanding the genetic basis for adverse effects: the calcineurin inhibitors. Pharmacotherapy 30:195–209
Qin W, Tan CY, Huang X, Huang Z, Tao Y, Fu P (2011) Rapamycin-induced posterior reversible encephalopathy in a kidney transplantation patient. Int Urol Nephrol 43(3):913–916
Becquet O, Pasche J, Gatti H, Chenel C, Abely M, Morville P, Pietrement C (2010) Acute post-streptococcal glomerulonephritis in children of French Polynesia: a 3-year retrospective study. Pediatr Nephrol 25:275–280
Gomez-Lado C, Martinon-Torres F, Alvarez-Moreno A, Eiris- Punal J, Carreira-Sande N, Rodriguez-Nunez A, Castro-Gago M (2007) Reversible posterior leukoencephalopathy syndrome: an infrequent complication in the course of haemolytic-uremic syndrome. Rev Neurol 44:475–478
Punaro M, Abou-Jaoude P, Cimaz R, Ranchin B (2007) Unusual neurologic manifestations (II): posterior reversible encephalopathy syndrome (PRES) in the context of juvenile systemic lupus erythematosus. Lupus 16:576–579
Zhang YX, Liu JR, Ding MP, Huang J, Zhang M, Jansen O, Deuschl G, Eschenfelder CC (2008) Reversible posterior encephalopathy syndrome in systemic lupus erythematosus and lupus nephritis. Intern Med 47:867–875
Ozcakar ZB, Ekim M, Fitoz S, Teber S, Hizel S, Acar B, Yuksel S, Yalcinkaya F (2004) Hypertension induced reversible posterior leukoencephalopathy syndrome: a report of two cases. Eur J Pediatr 163:728–730
Benoist G, Dossier C, Elmaleh M, Dauger S (2013) Posterior reversible encephalopathy syndrome revealing renal artery stenosis in a child. BMJ Case Rep 23:2013
Sharma S, Gupta R, Sehgal R, Aggarwal KC (2014) Atypical presentation of posterior reversible encephalopathy: in a child with bilateral grade IV vesicoureteric reflux. J Trop Pediatr 60:331–333
Girişgen İ, Tosun A, Sönmez F, Özsunar Y (2010) Recurrent and atypical posterior reversible encephalopathy syndrome in a child with peritoneal dialysis. Turk J Pediatr 52:416–419
Awan AQ, Lewis MA, Postlethwaite RJ, Webb NJA (1999) Seizures following renal transplantation in childhood. Pediatr Nephrol 13:275–277
Zheng W (2001) Neurotoxicology of the brain barrier system: new implications. Clin Toxicol 39:711–719
Tuor UI, Grewal D (1994) Autoregulation of cerebral blood flow: influence of local brain development and postnatal age. Am J Physiol 267:H2220–H2228
Kwon S, Koo J, Lee S (2001) Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol 24:361–364
Lee VH, Wijdicks EF, Manno EM, Rabinstein AA (2008) Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 65:205–210
Schmithorst VJ, Holland SK, Dardzinski BJ (2008) Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp 29:696–710
Hughes RL (1990) Cyclosporine-related central nervous system toxicity in cardiac transplantation. N Engl J Med 323:420–421
Schwartz RB, Bravo SM, Klufas RA, Hsu L, Barnes PD, Robson CD, Antin JH (1995) Cyclosporine neurotoxicity and its relationship to hypertensive encephalopathy: CT and MR findings in 16 cases. AJR Am J Roentgenol 165:627–631
Ong B, Bergin P, Heffernan T, Stuckey S (2009) Transient seizure-related MRI abnormalities. J Neuroimaging 19:301–310
Peter P, George A (2012) Posterior reversible encephalopathy syndrome and the pediatric population. J Pediatr Neurosci 7(2):136–138
Covarrubias DJ, Luetmer PH, Campeau NG (2002) Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol 23:1038–1048
Pande AR, Ando K, Ishikura R, Nagami Y, Takada Y, Wada A, Watanabe Y, Miki Y, Uchino A, Nakao N (2006) Clinicoradiological factors influencing the reversibility of posterior reversible encephalopathy syndrome: a multicenter study. Radiat Med 24:659–668
Ahn KJ, You WJ, Jeong SL, Lee JW, Kim BS, Lee JH et al (2004) Atypical manifestations of reversible posterior leukoencephalopathy syndrome: findings on diffusion imaging and ADC mapping. Neuroradiology 46:978–983
Bartynski WS, Boardman JF (2007) Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 28:1320–1327
Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, Striano S, Tufano R (2007) Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med 33:230–236
Eichler FS, Wang P, Wityk RJ (2002) Diffuse metabolic abnormalities in reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol 23:833–837
Bartynski WS, Boardman JF (2008) Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 29:447–455
Wright RR, Mathews KD (1996) Hypertensive encephalopathy in childhood. J Child Neurol 11:193–196
Paues J, Vrethem M (2010) Fatal progressive multifocal leukoencephalopathy in a patient with non-Hodgkin lymphoma treated with rituximab. J Clin Virol 48:291–293
Kumar D (2010) Emerging viruses in transplantation. Curr Opin Infect Dis 23:374–378
Ko MW, Liu GT (2010) Pediatric idiopathic intracranial hypertension (pseudotumor cerebri). Horm Res Paediatr 74:381–389
Lamy C, Oppenheim C, Meder JF, Mas JL (2004) Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging 14:89–96
llner A (2003) In: Blaser SI (ed) Pocket radiologist PedsNeuro. Salt Lake City, UT, medical reference. PRES, New York, pp 220–222
Parvex P, Pinsk M, Bell LE, O’Gorman AM, Patenaude YG, Gupta IR (2001) Reversible encephalopathy associated with tacrolimus in pediatric renal transplants. Pediatr Nephrol 16:537–542
Gavrilovici C, Oprea L (2013) Clinical ethics, research ethics and community ethics-the moral triad of nowadays society. Revista Romana De Bioetica 11(3):3–5
Gavrilovici C, Goldsmith DJ, Reid C, Gubeth-Tatomir P, Covic A (2004) What is the role of ambulatory BP monitoring in pediatric nephrology? J Nephrol 17(5):642–652
Abe K (2004) Reversible posterior leukoencephalopathy syndrome. Intern Med 43:900–901
Nishida M, Sato H, Kobayashi N, Morimoto M, Hamaoka K (2009) Wernicke’s encephalopathy in a patient with nephrotic syndrome. Eur J Pediatr 168:731–734
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gavrilovici, C., Miron, I., Voroneanu, L. et al. Posterior reversible encephalopathy syndrome in children with kidney disease. Int Urol Nephrol 49, 1793–1800 (2017). https://doi.org/10.1007/s11255-017-1684-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1684-x