Abstract
Crinum brachynema is a bulbous plant belonging to the family Amaryllidaceae which is restricted to Western India. Due to its high rarity and low distribution range, it has been classified as “critically endangered”. Establishing an efficient and unprecedented somatic embryogenesis protocol is necessary for its conservation and large-scale propagation. In this study, regeneration was achieved through somatic embryogenesis using bulb explants on MS media supplemented with various concentrations of 2,4-D alone and in combination with N6-benzyl-adenine. Different advanced phases with maturation of somatic embryo were obtained on MS medium with different ratios of picloram and thidiazuron (TDZ). The highest number of somatic embryos (50.33 ± 1.52) was obtained after eight weeks in the medium supplemented picloram (2.0 mg L−1) in combination with TDZ (0.5 mg L−1). MS medium with reduced concentration of salts in combination with GA3 (1.0 mg L−1) was used for somatic embryo germination. The maximum embryo germination frequency (82.22) was recorded on half-strength MS medium fortified with 1 mg L−1 GA3. The genetic true-to-typeness of regenerated plants was confirmed by ISSR, SCoT and RAPD primers based molecular analyses. This confirmed their genetic homogeneity compared to the mother plant and it also demonstrated the reliability of our somatic embryogenesis system for C. brachynema. The protocol developed may facilitate efforts in reintroduction, restoration, and ex situ conservation of C. brachynema in its natural habitat and its potential commercial utilization.
Key message
We provided the first report on somatic embryogenesis system in C. brachynema. SEM indicated the morphogenesis and several molecular markers revealed genetic homogeneity of the regenerated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Amaryllidaceae includes around 1600 species, distributed in 100 genera, which grow throughout the tropical and warm-temperate regions of the globe (Chahal et al. 2021; Kaur et al. 2022a). Crinum L. is one of the prominent genera of this family with more than 110 species distributed globally (Chahal et al. 2021). In India, a total of 13 Crinum species are found, of which 5 are endemic (Lekhak and Yadav 2011; Patel and Patel 2019). Indian Crinum species have been documented with several potential therapeutic applications such as antidiabetic, anti-inflammatory antiviral, antiaging, antioxidant, antimalarial, antidiarrheal analgesic, antitumour, antimicrobial and anti-Alzheimer due to the presence of valuable active alkaloids (Fennell and Van Staden 2001; Uddin et al. 2012; Refaat et al. 2013; Ghane et al. 2018; Sikder et al. 2021; Kaur et al. 2022b; Patel et al. 2022).
Crinum brachynema, locally known as “Karnaphul”, is a critically endangered herb restricted to Northern region of Western ghats of India (Punekar 2001; Kaur et al. 2022a). Like other Crinum species, C. brachynema is known for its beautiful fragrant flowers and as a potential source of valuable alkaloids (viz. galanthamine and lycorine (Jagtap et al. 2014; Kaur et al. 2022b). This plant is well known for its biological activities as anti-Alzheimer and anticancer, due to the presence of alkaloids skeletons like galanthamine (GAL) and lycorine (Fig. 1E, F) and could be used commercially in pharmaceutical and perfume industries as well (Jagtap et al. 2014; Roy et al. 2018; Santos et al. 2020; Lekhak et al. 2022).
GAL is a reversible, selective isoquinoline alkaloid, which acts as a competitive inhibitor of acetylcholinesterase and modulator of acetylcholine receptors (Heinrich and Teoh 2004; Jagtap et al. 2014; Argade et al. 2022). It is also sold under the product names Nivalin® and Reminyl® as a potential drug for Alzheimer’s disease (Heinrich 2010). C. brachynema is rarely cultivated and over the past few years wild populations of C. brachynema were over-exploited, threatening the very existence of this valuable endemic flora (Kaur et al. 2022a). Furthermore, limited availability, anthropogenic pressure, human activities, over-exploitation of bulb material and forest fires make them difficult to propagate in their natural habitat and it faces the threat of extinction. This plant has been also categorized as “critically endangered” by the International Union for Conservation of Nature (IUCN), under the red list measures (POWO 2019). Its extreme destruction and status underline the need to develop novel biotechnological strategies for in vitro regeneration and conservation, essential to develop rapid propagation and conservation systems as tools to save and expand the populations of C. brachynema.
Plant tissue culture techniques are being used to conserve rare, endangered, and threatened species all over the world. Somatic embryogenesis (SE) has several advantages for increasing the rate of propagation of genetically uniform phenotypes (Chahal et al. 2023; Uma et al. 2023). Plant cells and tissues undergo several genetic changes during in vitro regeneration, including DNA methylation and morpho-physiological changes, chromosomal restructuring and/or doubling (aneuploidy/polyploidy), collectively referred to as “somaclonal variation” (Phillips et al. 1994; Bairu et al. 2011; Takagi 2011).
The somaclonal variations may arise due to several factors such as type of explant, exogenous plant growth regulators (PGRs) and excessive subculturing during in vitro propagation (Bhattacharyya et al. 2018; Chirumamilla et al. 2021). Different genetic markers including random amplified length polymorphism (RAPD) (Sebastiani and Ficcadenti 2016; Bajpai and Chaturvedi 2021), amplified fragment length polymorphism (AFLP) (Yin et al. 2013), direct amplification of minisatellite DNA (DAMD) (Parab et al. 2021; Longchar and Deb 2022), inter-simple sequence repeat (ISSR) (Jaiswal et al. 2021) and start codon targeted polymorphism (SCoT) (Thakur et al. 2016; Bhattacharyya et al. 2018) have been extensively used to discover the genetic variations of in vitro plant species. True-to type plants are vital to preserve the genetic resources of rare, endangered, and threatened species and are crucial for conservation practices and secondary metabolite production.
To date only one recent study (Kaur et al. 2022a) has reported on regeneration and conservation of C. brachynema. A regeneration of in vitro clones through somatic embryogenesis in a shorter period of time is advantageous for rapid propagation and production of bioactive compounds (Kumar et al. 2015, 2017; Lema-Rumińska et al. 2019; Marković et al. 2021; Mishra et al. 2022; Chahal et al. 2023). Therefore, we undertook this aim to establish an efficient protocol to produce true-to-type plantlets via somatic embryogenesis for the first time. The RAPD, ISSR, and SCoT markers were utilized to examine the true-to typeness of in vitro regenerated plants.
Materials and methods
Plant collection, and somatic embryogenesis
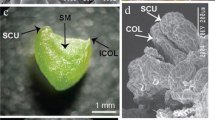
Pant material of C. brachynema were obtained from Satara district (17°56.270′N, 73°41.488′E), Maharashtra, India. (Fig. 1A). The species was identified by a botanist and a voucher was deposited at the herbarium of Shivaji University, Kolhapur, Maharashtra, India.
To obtain bulb explants, plant leaves were taken away, and the outer scale and root were harvested. The bulbs (5–7 cm) (Fig. 1C) were rinsed thoroughly with running tap water and surface sterilized by using our recently published protocol (Kaur et al. 2022a). The disinfected bulb explants were cut longitudinally to give twin scales with basal plate (2.0 cm) inside the laminar air flow under sterile conditions. Sterilized explants were placed in culture flasks containing MS (Murashige and Skoog 1962) media supplemented with sucrose (3%), agar powder (0.8%) and with the plant growth regulators (PGR) 2,4-D, BA, picloram and TDZ, added individually or in mixture for induction of somatic embryogenesis. Bavistin and agar powder, Picloram, TDZ, 2,4-dichlorphenoxyacetic acid (2,4-D), 6-benzyladenine (BA), vitamins, and myo-inositol were procured from Hi-Media®, Pvt. Ltd. India. The pH of the MS medium was adjusted to 5.8 before autoclaving for 20 min at at 121 °C and 1.06 kg cm−2 of pressure. The inoculated culture tubes were maintained and incubated at 25 ± 2 °C in a culture room with a 16/8 h photoperiod from 40 W fluorescent tubes (Philips, India) emitting a photon flux density of 50 µmol m−2 s−1, with a relative humidity of 60% retained by using a humidifier. The medium without any PGR served as control. All cultures were kept under the same conditions as above. The percentage of embryogenic calli was calculated as the total number of embryogenic explants/total number of explants used ×100. Friable embryogenic calli were selected and transferred to MS medium fortified with 0.5–5.0 mg L−1 picloram and 0.5 mg L−1 TDZ. The mean number of somatic embryos was recorded after 8 weeks of culture.
Scanning electron microscopy
During scanning electron microscope (SEM) analysis, different stages of somatic embryos were collected and fixed in 2.5% of glutaraldehyde solution and dehydrated with an ethanolic series (10, 30, 50, 70 and 100%). Dehydrated samples were dried in a critical point dryer and coated with gold in ion coater (D II-29030SCTR). The coated samples were visualized under SEM (FESEM, JSM-7610 F, Oxford Instruments X-Max N) operated at 15–25 kv and images were taken at different magnifications.
Somatic embryo germinations
To study the embryo germination into complete plantlets, modifications were made in MS medium by reducing the sucrose and salt (macro and micronutrients) concentrations. The cotyledonary somatic embryos were transferred to half and full-strength MS medium with 1.0 mg L−1 gibberellic acid (GA3). The inoculated culture tubes were maintained and incubated under the same conditions as above. The embryo germination rate was determined after 8 weeks of time.
Clonal fidelity analysis
Genomic DNA extraction
The genomic DNA was extracted from 4 to 5 cm pieces of fresh leaves of randomly selected seven in vitro regenerated plantlets and from a mother plant to compare the genetic fidelity assessment. Briefly, 500 mg of leaf sample were acquired from plant genomic DNA using the standard method of Doyle and Doyle (1987). CTAB buffer was prepared freshly with a composition of 2% N-cetyl-N,N,N-trimethylammonium bromide (CTAB; w/v), 0.2% β-mercaptomethanol (v/v), 1 M Tris-HCL, (pH 8.0), 20mM ethylene diamine tetra acetic acid (EDTA) and 5.0 M NaCl). The genomic DNA quality and quantity were obtained with a UV–VIS spectrophotometer and confirmed on 1.0% agarose gel electrophoresis.
Polymerase chain reaction
dNTPs were purchased from Takara, Japan, DNA ladder were obtained from Thermofischer, India, whereas primers were ordered from Operon Technologies, USA. The PCR reaction was done with eight primers of RAPD and ten primers of each ISSR and SCoT marker using 96 well plate in a Gradient Thermal Cycler (iGene Labserve or G-storm). The reaction mixture was containing 10× PCR buffer, Taq DNA polymerase (3u/µl), 10mM dNTPS (Takara, Japan) and 2 µL of SCoT, ISSR and RAPD primers (Operon Technologies, USA). The amplification reaction was carried out using a cycling program consisting of initial denaturation at 94 °C for 3 min followed by 32 cycles of each at 94 °C for 30s, extension at 72 °C for 2 min and final extension at 72 °C for 10 min. During PCR reaction analysis, annealing temperature was varied between 50 and 58 °C based on the Tm (melting temperature) of the primer. The amplified PCR products were separated using 2.5% 1× TAE agarose gel, and a 100 bp or 50 bp DNA ladder (Thermo Fisher Scientific, USA) were used to compare the fragmentation pattern of the bands. All the chemicals and reagents used during the study were of analytical grade.
Data analysis
Every experiment was conducted three times with three replicates. The data of embryogenesis (%) and number of somatic embryos in mean values were recorded. All experiments were evaluated by using analysis of variance (ANOVA). Duncan’s multiple range test was used to analyze the significant difference among means with 95% confidence level (P ≤ 0.05).
Results and discussion
Induction of somatic embryogenesis
Somatic embryogenesis is a very efficient tool for large-scale production of regenerated plantlets and has the potential to develop the conservation practices for valuable and endangered species. Categorized as “critically endangered” and an endemic species, the development of a long-term conservation strategy for C. brachynema needs urgent attention. In the present investigation, an efficient plant regeneration system via somatic embryogenesis was established (Figs. 2 and 3). A range of different concentrations of 2,4-D (1.0–10.0 mg L−1) alone and in combination with BA (1.0 mg L−1) were used for somatic embryogenesis induction using bulb explants. Further, combinations of Picloram (1.0–5.0 mg L−1) and TDZ (0.5 mg L−1) were examined to achieve the maturation of somatic embryos. The somatic embryogenesis induction percentage and production of somatic embryos varied with the PGRs tested (Fig. 4). Alone, 2,4-D induced embryogenic calli and had a significant effect on the production of somatic embryos, in agreement with similar results for other Amaryllidaceae species (Mujib et al. 1996; Ptak et al. 2013; Priyadharshini et al. 2020; Chahal et al. 2023). The addition of BA with 2,4-D significantly increased the formation of somatic embryos. A combination of 8.0 mg L−1 2,4-D and 1.0 mg L−1 BA influenced the induction of embryogenic calli (78.5%), and was coupled with a significant impact on the mean number of somatic embryos (35.44) achieved. Our results concur with reports on the embryogenic potential of other species such as Allium schoenoprasum L. (Zdravković-Korać et al. 2010), Chrysanthemum sp. (Naing et al. 2013), Vitis vinifera (Dai et al. 2015)d malabaricum (Chahal et al. 2023). Furthermore, a combination of picloram (2.0 mg L−1) and TDZ (0.5 mg L−1) significantly enhanced the mean number of somatic embryos (50.33) in C. brachynema (Fig. 4F). Although, 2,4-D is the most commonly used PGR during somatic embryogenesis (Jouini et al. 2023), the application of picloram for somatic embryo production has been well-documented in other species (Takamori et al. 2015; Campos-Boza et al. 2022; Ferreira et al. 2022a, b), including several other bulbous plant species such as Lilium longiflorum (Tribulato et al. 1997), Leucojum aestivum (Ptak et al. 2013), Lachenalia virdiflora (Kumar et al. 2016) Crinum malabaricum (Chahal et al. 202320232023).
Somatic embryogenesis showing embryogenic callus induction and formation of somatic embryos in C. brachynema. A, B Induction and proliferation of embryogenic callus. C, D Embryogenic callus with cluster of emerging globular somatic embryos. E Development and maturation of various somatic embryos Scale Bar: A–E = 5 mm
A and B Effect of 2.4-D alone on somatic embryogenesis induction in Crinum brachynema. C and D Showing the effect of 2,4-D in combination with BA on somatic embryogenesis induction in Crinum brachynema. D and E The effect of picloram and thidiazuron on somatic embryo development in Crinum brachynema. Mean ± S.D followed by different letters indicate significant differences analysed by Duncan’s multiple range test at a 95% confidence level (P ≤ 0.05)
The cytokinin TDZ has been widely used in somatic embryo production in different plant species (Baskaran et al. 2012, 2014; Dhekney et al. 2016; Ghahremani et al. 2021). The present investigation revealed that MS medium supplemented with a combination of picloram and TDZ is essential for maximum number of somatic embryo production in C. brachynema. A similar result was found for somatic embryo production in Merwilla plumbea (Baskaran et al. 2012), Drimia robusta (Baskaran et al. 2014), Lachenalia virdiflora (Kumar et al. 2016), Cyrtanthus mackenii (Kumari et al. 2017), Stewartia sp. (Gladfelter et al. 2021) Crinum malabaricum (Chahal et al. 2023).
SEM investigation of C. brachynema samples showed the development of all stages of somatic embryogenesis on the surface of explants, inlcuding globular-shaped somatic embryos (Fig. 5b–d), cotyledonary-shaped somatic embryos (Fig. 5e, f), as well as somatic embryos with well-developed cotyledon with a distinct shoot tip (Fig. 5g, h).
Different stages of C. brachynema somatic embryos viewed under scanning electron microscope. A SEM micrograph showing the appearance of embryogenic calli. B Globular-shaped somatic embryos emerging from the surface of embryogenic calli. C, D Single globular-shaped somatic embryo. E Cluster of globular-shaped somatic embryos E, F SEM micrograph of developing cotyledonary-shaped somatic embryos. G, H Well-developed cotyledonary-shaped embryo with single shoot Scale Bar: A = 1 mm; B–D = 1 μm; E, F = 10 μm; G, H = 1 mm
Embryo germination and plantlet development
Successful conversion of somatic embryos into well-developed plantlets is vital for effective plant regeneration. In this study, embryo germination was 51.11% on half-strength MS medium, whereas full-strength MS medium produced 40% germination. Interestingly, half-strength MS medium supplemented with 1 mg L−1 GA3 gave a maximum frequency of somatic embryo germination (82.22%) with well-rooted plantlets (Fig. 6). The growth and expansion of the root and cotyledonary leaves were more pronounced in half-strength MS medium compared to the full-strength medium. Our results agree with similar findings on several other species (Paul et al. 2011; Raju et al. 2013; Baskaran and Van Staden 2014; Khan et al. 2015; Ferreira et al. 2022a; Lekshmi and Swapna 2022). Similarly, the supplementation of GA3 and its beneficial effect on somatic embryo germination has been reported for several plant species (Cangahuala-Inocente et al. 2007; Siddiqui et al. 2011; Baskaran and Van Staden 2012; Raomai et al. 2014; Fang et al. 20222022).
C. brachynema somatic embryos germination and plantlet conversion. A Germinated cotyledonary-shaped embryos in germination medium. B Well-developed rooted plantlets of (C) brachynema. C The effect of MS medium (full strength and half strength + 1.0 mg L−1 GA3) on the germination of somatic embryos of C. brachynema. All the experiments were repeated thrice with at least three replicates. The significant difference between mean values was obtained via Duncan’s multiple range test at a 95% confidence level (P ≤ 0.05)
Clonal fidelity assessment
The genetic stability studies help in the assessment of the true-to-typeness between the regenerated clones and mother plants (Hou et al. 2022; Chahal et al. 2023; Uma et al. 2023). RAPD and ISSR markers are considered as advanced marker types, yet simple approaches to assess the clonal fidelity of regenerated plantlets (Bhardwaj et al. 2018; Sadhu et al. 2020; Rohela et al. 2020; Bajpai and Chaturvedi 2021; Mood et al. 2022). SCoT markers were proven to be more reliable and highly reproducible (Gorji et al. 2011) that can target the starting codon region of plant genes, and the same primer serves as forward and reverse (Collard and Mackill 2009; Elayaraja et al. 2019; Verma et al. 2022). Moreover, it is always beneficial to utilize more than one marker to assess the clonal fidelity of in vitro regenerated plants (Palombi and Damiano 2002; Rohela et al. 2020; Mishra et al. 2023). Therefore, we undertook to investigate the clonal fidelity assessment of somatic embryo-derived plantlets with the ex vitro mother plant using three (RAPD, ISSR and SCoT) different markers (Fig. 7).
Genetic stability assessment in somatic embryogenesis derived plantlets and mother plant of C. brachynema. A ISSR amplification profile with primer ISSR-3. B SCoT amplification profile with primer SCoT-1. C RAPD amplification profile with primers OPAB-2. Lane 1 represents the 100 bp DNA ladder (M) followed by mother plant (0) in lane 2. Lane 3–9 labelled 1–7 are the in vitro raised plant lines via somatic embryogenesis
A total of ten SCoT and ISSR primers and eight RAPD primers-based fingerprinting was performed by randomly selecting seven regenerants derived from somatic embryos in vitro compared with the ex vitro mother plant (Tables 1, 2 and 3). While validating the clonal fidelity, SCoT amplified primers showed good amplification with the generation of total 18 scorable DNA bands with amplified band sizes of 220 to 2000 bp (Table 1). The ISSR primers scored 22 bands with sizes of 250 to 950 bp (Table 2), while the RAPD primers produced 10 bands in a range of 400 to 1300 bp (Table 3).
The generated bands produced with SCoT, ISSR and RAPD were monomorphic and since true-to-type propagules were obtained via somatic embryogenesis no variation was detected (Fig. 7). SCoT, ISSR and RAPD markers assessments were also performed in several other species such as Eclipta alba (Singh et al. 2012), Angelica glauca (Rawat et al. 2018), Artemisia vulgaris (Jogam et al. 2020), Iris × hollandica (Verma et al. 2022), C. malabaricum (Chahal et al. 2023), Congenesis-Robusta (C×R) cultivar (Mishra et al. 2023). The current report revealed the application of more than one marker, as also confirmed by several researchers (Sadhu et al. 2020; Mood et al. 2022; Uma et al. 2023), which clearly shows that utilization of one single marker is not effective enough to reliably validate the genetic variations of in vitro regenerated plants.
Conclusions
This first report for the development of somatic embryogenesis system of C. brachynema offers an opportunity to ensure germplasm conservation, to control destruction threats, and it also delivers a potential system for the analysis of secondary metabolites. The induction and production of somatic embryos was significantly influenced by the combination of picloram (2.0 mg L−1) and TDZ (0.5 mg L−1). The SEM analyses affirm the developmental differentiation of somatic embryos. The banding pattern from SCoT, ISSR and RAPD markers analyses proved that the in vitro clones highly resembled to their mother plant which ruled out any somaclonal variation induced during somatic embryogenesis. The established model system enables the mass multiplication of genetically stable C. brachynema plants and could be employed as a potential source of active biomolecules in several ailments. In addition to the considerable work carried out, in-depth studies including genetic and metabolic engineering should also be performed in future to maximize the productivity of valuable alkaloids present in this species.
Abbreviations
- ANOVA:
-
Analysis of variance
- BA:
-
6-Benzyladenine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- CTAB:
-
Cetyl trimethyl ammonium bromide
- DMRT:
-
Duncan’s multiple range test
- dNTP:
-
Deoxynucleotide triphosphate
- GAL:
-
Galanthamine
- GA3 :
-
Gibberellic acid
- IUCN:
-
International union for conservation and nature
- ISSR:
-
Inter simple sequence repeat
- LED:
-
Light-emitting diode
- MS:
-
Murashige and Skoog
- PCR:
-
Polymerase chain reaction
- PGRs:
-
Plant growth regulators
- PPFD:
-
Photosynthetic photon flux density
- RAPD:
-
Random amplified polymorphic DNA
- SCoT:
-
Start codon targeted polymorphism
- SE:
-
Somatic embryogenesis
- SEM:
-
Scanning electron microscope
- TAE:
-
Tris-acetate EDTA
- TDZ:
-
Thidiazuron
References
Argade MD, DeCristofano L, Bhattarai N, Schulte MK, Dukat M (2022) Evaluation of galantamine and deconstructed analogs as α7 nAChR and AChE ligands. Res Chem 4:100286. https://doi.org/10.1016/j.rechem.2022.100286
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173. https://doi.org/10.1007/s10725-010-9554-x
Bajpai R, Chaturvedi R (2021) Recurrent secondary embryogenesis in androgenic embryos and clonal fidelity assessment of haploid plants of tea, Camellia assamica ssp. Assamica and Camellia assamica ssp. lasiocaylx. Plant Cell Tiss Org Cult 145:127–141. https://doi.org/10.1007/s11240-020-01997-x
Baskaran P, Van Staden J (2012) Somatic embryogenesis of Merwilla plumbea (Lindl.) Speta. Plant Cell Tiss Org Cult 109:517–524. https://doi.org/10.1007/s11240-012-0118-9
Baskaran P, Van Staden J (2014) Plant regeneration via somatic embryogenesis in Drimia robusta. Plant Cell Tiss Org Cult 119:281–288. https://doi.org/10.1007/s11240-014-0532-2
Bhardwaj AK, Singh B, Kaur K, Roshan P, Sharma A, Dolker D, Naryal A, Saxena S, Pati PK, Chaurasia OP (2018) In vitro propagation, clonal fidelity and phytochemical analysis of Rhodiola imbricate Edgew: a rare trans-himalayan medicinal plant. Plant Cell Tiss Org Cult 135:499–513. https://doi.org/10.1007/s11240-018-1482-x
Bhattacharyya P, Kumar V, Van Staden J (2018) In vitro encapsulation based short term storage and assessment of genetic homogeneity in regenerated Ansellia Africana (Leopard orchid) using gene targeted molecular markers. Plant Cell Tiss Org Cult 133:299–310. https://doi.org/10.1007/s11240-018-1382-0
Campos-Boza S, Vinas M, Solórzano-Cascante P, Holst A, Steinmacher DA, Guerra MP, Jiménez VM (2022) Somatic embryogenesis and plant regeneration from transverse thin cell layers of adult peach palm (Bactris gasipaes) lateral offshoots. Front Plant Sci 13:995307. https://doi.org/10.3389/fpls.2022.995307
Cangahuala-Inocente GC, Dal Vesco LL, Steinmacher D, Torres AC, Guerra MP (2007) Improvements in somatic embryogenesis protocol in Feijoa (Acca sellowiana (Berg) Burret): induction, conversion and synthetic seeds. Sci Hortic 111:228–234. https://doi.org/10.1016/j.scienta.2006.10.030
Chahal S, Lekhak MM, Gupta AP, Ochatt SJ, Kumar V (2023) True-to-typeness and phytomedicinal potential in somatic embryo-derived plants of Crinum malabaricum (Amaryllidaceae): a medicinally important source of pharmaceutical biomolecules. Ind Crop Prod 204:117329. https://doi.org/10.1016/j.indcrop.2023.117329
Chahal S, Lekhak MM, Kaur H, Shekhawat MS, Dey A, Jha P, Pandey DK, Kumar V (2021) Unraveling the medicinal potential and conservation of Indian Crinum (Amaryllidaceae) species. S Afr J Bot 136:7–15. https://doi.org/10.1016/j.sajb.2020.04.029
Chirumamilla P, Gopu C, Jogam P (2021) Highly efficient rapid micropropagation and assessment of genetic fidelity of regenerants by ISSR and SCoT markers of Solanum khasianum Clarke. Plant Cell Tiss Organ Cult 144:397–407. https://doi.org/10.1007/s11240-020-01964-6
Collard BCY, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27:86–93. https://doi.org/10.1007/s11105-008-0060-5
Dai L, Zhou Q, Li R, Du Y, He J, Wang D, Cheng S, Zhang J, Wang Y (2015) Establishment of a picloram-induced somatic embryogenesis system in Vitis vinifera Cv. Chardonnay and genetic transformation of a stilbene synthase gene from wild-growing Vitis species. Plant Cell Tiss Org Cult 121:397–412. https://doi.org/10.1007/s11240-015-0711-9
Dhekney SA, Li ZT, Grant TNL, Gray DJ (2016) Somatic embryogenesis and genetic modification of Vitis. In: Germana MA, Lambardi M (eds) In vitro embryogenesis in higher plants. Springer, New York. https://doi.org/10.1007/978-1-4939-3061-6_11
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Elayaraja D, Subramanyam K, Vasudevan V, Sathish S, Kasthurirengan S, Ganapathi A, Manickavasagam M (2019) Meta-topolin (mT) enhances the in vitro regeneration frequency of Sesamum indicum (L.). Biocatal Agric Biotechnol 21:101320. https://doi.org/10.1016/j.bcab.2019.101320
Fang H, Dong Y, Zhou R, Wang Q, Duan Q, Wang C, Bao Y, Xu S, Lang X, Gai S, Chen R, Yang KQ (2022) Optimization of the induction, germination, and plant regeneration system for somatic embryos in apomictic walnut (Juglans regia L.). Plant Cell Tiss Org Cult 150:289–297. https://doi.org/10.1007/s11240-022-02266-9
Fennell CW, Van Staden J (2001) Crinum species in traditional and modern medicine. J Ethnopharmacol 78:15–26. https://doi.org/10.1016/s0378-8741(01)00305-1
Ferreira JCB, de Araújo Silva-Cardoso IM, Meira RO, da Silca Costa FH, Scherwinski-Pereira JE (2022) Towards development of an efficient somatic embryogenesis protocol for the palm tree Euterpe precatoria (Mart.) From leaf tissues of adult plants. In Vitro Cell Dev Biol Plant 58:750–768. https://doi.org/10.1007/s11627-022-10310-8
Ferreira JCB, de Araújo Silva-Cardoso IM, de Oliveira Meira R, Scherwinski-Pereira JE (2022) Somatic embryogenesis and plant regeneration from zygotic embryos of the palm tree Euterpe precatoria Mart. Plant Cell Tiss Org Cult 148:667–686. https://doi.org/10.1007/s11240-022-02227-2
Ghahremani R, Daylami SD, Mirmasoumi M, Askari N, Vahdati K (2021) Refining a protocol for somatic embryogenesis and plant regeneration of Phalaenopsis amabilis Cv. Jinan from mature tissues. Turk J Agric For 45:11
Ghane SG, Attar UA, Yadav PB, Lekhak MM (2018) Antioxidant, anti-diabetic, acetylcholinesterase inhibitory potential and estimation of alkaloids (lycorine and galanthamine) from Crinum species: an important source of anticancer and anti-Alzheimer drug. Ind Crops Prod 125:168–177
Gladfelter HJ, Johnston J, Wilde HD, Merkle SA (2021) Somatic embryogenesis and cryopreservation of Stewartia species. Plant Cell Tiss Org Cult 144:211–221. https://doi.org/10.1007/s11240-020-01834-1
Gorji AM, Poczai P, Polgar Z, Taller J (2011) Efficiency of arbitrarily amplified dominant markers (SCoT, ISSR and RAPD) for diagnostic fingerprinting in tetraploid potato. Am J Pot Res 88:226–237. https://doi.org/10.1007/s12230-011-9187-2
Heinrich M (2010) Galanthamine from galanthus and other amaryllidaceae—chemistry and biology based on traditional use. In: Cordell GA (ed) The alkaloids. Academic Press, Chennai, pp 157–165
Heinrich M, Teoh HL (2004) Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J Ethnopharmacol 92:147–162. https://doi.org/10.1016/j.jep.2004.02.012
Hou J, Wang D, Su P, Ding S, Wu L (2022) In vitro large-scale propagation and genetic fidelity of Toona sinensis (Juss.) M.Roem., an economically important vegetable tree. Plant Cell Tiss Org Cult 151:275–291. https://doi.org/10.1007/s11240-022-02351-z
Jagtap UB, Lekhak MM, Fulzele DP, Yadav SR, Bapat VA (2014) Analysis of selected Crinum species for galanthamine alkaloid: an anti-Alzheimer drug. Curr Sci 107:2008–2010
Jaiswal P, Kumari N, Kashyap SP, Tiwari SK (2021) Organogenesis from Leaf tissue of Spondias pinnata (L. f.) Kurz, SEM study and genetic fidelity assessment by ISSR and SCoT. Plant Cell Tiss Organ Cult 146:s203-212. https://doi.org/10.1007/s11240-021-02056-9
Jogam P, Sandhya D, Shekhawat MS, Alok A, Manokari M, Abbagani S, Allini VR (2020) Genetic stability analysis using DNA barcoding and molecular markers and foliar micro-morphological analysis of in vitro regenerated and in vivo grown plants of Artemisia vulgaris L. Ind Crops Prod 151:112476. https://doi.org/10.1016/j.indcrop.2020.112476
Jouini N, Yahyaoui E, Tarraf W, İzgü T, Benelli C, Lambardi M, Germaná MA (2023) Somatic embryogenesis in Abies nebrodensis, an endangered sicilian fir. Plant Cell Tiss Org Cult 152:393–404. https://doi.org/10.1007/s11240-022-02415-0
Kaur H, Chahal S, Jha P, Lekhak MM, Shekhawat MS, Naidoo D, Arencibia AD, Ochatt SJ, Kumar V (2022) Harnessing plant biotechnology-based strategies for in vitro galanthamine (GAL) biosynthesis: a potent drug against Alzheimer’s disease. Plant Cell Tiss Org Cult 149:81–103. https://doi.org/10.1007/s11240-022-02229-0
Kaur H, Chahal S, Lekhak MM, Jha P, Ochatt SJ, Kumar V (2022) Meta-topolin induced in vitro regeneration in Crinum brachynema (Amaryllidaceae): a critically endangered and endemic medicinal plant of India. Plant Cell Tiss Org Cult 151:663–672. https://doi.org/10.1007/s11240-022-02380-8
Khan MA, Abbasi BH, Ali H, Ali M, Hussain I (2015) Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Silybum marianum L. Plant Cell Tiss Org Cult 120:127–139. https://doi.org/10.1007/s11240-014-0587-0
Kumar V, Moyo M, Van Staden J (2015) Somatic embryogenesis of Pelargonium sidoides DC. Plant Cell Tiss Org Cult 121:571–577. https://doi.org/10.1007/s11240-015-0726-2
Kumar V, Moyo M, Van Staden J (2016) Enhancing plant regeneration of Lachenalia Viridiflora, a critically endangered ornamental geophyte with high floricultural potential. Sci Hortic 211:263–268. https://doi.org/10.1016/j.scienta.2016.08.024
Kumar V, Moyo M, Van Staden J (2017) Somatic embryogenesis in Hypoxis hemerocallidea: an important African medicinal plant. S Afr J Bot 108:331–336. https://doi.org/10.1016/j.sajb.2016.08.012
Kumari A, Baskaran P, Van Staden J (2017) In vitro propagation via organogenesis and embryogenesis of Cyrtanthus mackenii: a valuable threatened medicinal plant. Plant Cell Tiss Org Cult 131:407–415. https://doi.org/10.1007/s11240-017-1293-5
Lekhak MM, Patel SB, Otari SS, Lekhak UM, Ghane SG (2022) Bioactive potential and RP-HPLC detection of phenolics and alkaloids (lycorine and galanthamine) from ultrasonic-assisted extracts of Crinum roots. S Afr J Bot. https://doi.org/10.1016/j.sajb.2021.07.024
Lekhak MM, Yadav SR (2011) Karyotype studies in two critically endangered and endemic Crinum species (Amaryllidaceae) from Northern-Western ghats of India. Nucleus 54:25–30. https://doi.org/10.1007/s13237-011-0024-2
Lekshmi S, Swapna TS (2022) In vitro plant regeneration through somatic embryogenesis in Anaphyllum Wightii Schott. In Vitro Cell Dev Biol Plant 58:1099–1106. https://doi.org/10.1007/s11627-022-10308-2
Lema-Rumińska J, Kulus D, Tymoszuk A, Varejão JMTB, Bahcevandziev K (2019) Profile of secondary metabolites and genetic stability analysis in new lines of Echinacea purpurea (L.) Moench micropropagated via somatic embryogenesis. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2019.111851
Longchar TB, Deb CR (2022) Optimization of in vitro propagation protocol of Dendrobium Heterocarpum Wall. Ex. Lindl. And clonal genetic fidelity assessment of the regenerates: an orchid of horticultural and medicinal importance. S Afr J Bot 149:67–78. https://doi.org/10.1016/j.sajb.2022.05.058
Marković M, Trifunović Momčilov M, Uzelac B, Jevremović S, Subotić A (2021) Bulb dormancy in vitro—Fritillaria meleagris: initiation, release and physiological parameters. Plants 10:902. https://doi.org/10.3390/plants10050902
Mishra VK, Bajpai R, Chaturvedi R (2022) Androgenic haploid plant development via embryogenesis with simultaneous determination of bioactive metabolites in Cambod tea (Camellia Assamica ssp. lasiocalyx). Plant Cell Tiss Org Cult 148:515–531. https://doi.org/10.1007/s11240-021-02203-2
Mishra MK, Jingade P, Huded AKC, Muniswamy B (2023) Assessment of genetic homogeneity of somatic embryo-derived plants and seed-derived plants of a robusta coffee cultivar using molecular markers and functional genes sequencing. Plant Cell Tiss Organ Cult 153:319–332. https://doi.org/10.1007/s11240-023-02468-9
Mood K, Jogam P, Sirikonda A, Shekhawat MS, Rohela GK, Manokaari M, Allini VR (2022) Micropropagation, morpho-anatomical characterization, and genetic stability studies in Lippia javanica (Burm.f.) Spreng: a multipurpose medicinal plant. Plant Cell Tiss Org Cult 150:427–437. https://doi.org/10.1007/s11240-022-02294-5
Mujib A, Bandyopadhyay S, Jana BK, Ghosh PD (1996) Growth regulator involvement and somatic embryogenesis in Crinum asiaticum. Indian J Plant Physiol 1:84–86
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with Tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Naing AH, Min JS, Park KI, Chung MY, Lim SH, Lim KB, Kim CK (2013) Primary and secondary somatic embryogenesis in Chrysanthemum (Chrysanthemum morifolium) cv. ‘Baeksun’ and assessment of ploidy stability of somatic embryogenesis process by flow cytometry. Acta Physiol Plant 35:2965–2974. https://doi.org/10.1007/s11738-013-1328-4
Palombi M, Damiano C (2002) Comparison between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit (Actinidia Deliciosa A. Chev). Plant Cell Rep 20:1061–1066. https://doi.org/10.1007/s00299-001-0430-z
Parab AR, Lynn CB, Subramaniam S (2021) Assessment of genetic stability on in vitro and ex vitro plants of Ficus carica var. Black jack using ISSR and DAMD markers. Mol Biol Rep 48:7223–7231. https://doi.org/10.1007/s11033-021-06714-1
Patel SB, Otari SS, Kumar V, Rastogi A, Lekhak MM, Ghane SG (2022) Optimization of lycorine using response surface methodology, extraction methods and in vitro antioxidant and anti-diabetic activities from the roots of Giant Spider Lily: a medicinally important bulbous herb. S Afr J Bot 149:816–827. https://doi.org/10.1016/j.sajb.2022.04.022
Patel M, Patel H (2019) Crinum reddyi sp. nov. (Amaryllidaceae) from Gujarat, India. Nordic J Bot 37:1–5. https://doi.org/10.1111/njb.02172
Paul S, Dam A, Bhattacharyya A, Bandyopadhyay TK (2011) An efficient regeneration system via direct and indirect somatic embryogenesis for the medicinal tree Murraya koenigii. Plant Cell Tiss Org Cult 105:271–283. https://doi.org/10.1007/s11240-010-9864-8
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA 91:5222–5226. https://doi.org/10.1073/pnas.91.12.5222
POWO (2019) Plants of the world online. Facilitated by the Royal Botanic Gardens, Kew. https://powo.science.kew.org/
Priyadharshini S, Manokari M, Shekhawat MS (2020) In vitro conservation strategies for the critically endangered Malabar river lily (Crinum malabaricum Lekhak & Yadav) using somatic embryogenesis and synthetic seed production. S Afr J Bot 135:172–180. https://doi.org/10.1016/j.sajb.2020.08.030
Ptak A, Tahchy A, Skrzypek E, Wójtowicz T, Laurain-Mattar D (2013) Influence of auxins on somatic embryogenesis and alkaloid accumulation in Leucojum aestivum callus. Cent Eur J Biol 8:591–599. https://doi.org/10.2478/s11535-013-0160-y
Punekar SA, Mandar DN, Lakshminarasimhan P (2001) Crinum brachynema Herb. (Amaryllidaceae)-An endemic species found again in Mahabaleshwar, Maharashtra State. J Econ Taxon Bot 25:629–630
Raju CS, Kathiravan K, Aslam A, Shajahan A (2013) An efficient regeneration system via somatic embryogenesis in mango ginger (Curcuma amada Roxb). Plant Cell Tiss Org Cult 112:387–393. https://doi.org/10.1007/s11240-012-0244-4
Raomai S, Kumaria S, Tandon P (2014) Plant regeneration through direct somatic embryogenesis from immature zygotic embryos of the medicinal plant, Paris polyphylla sm. Plant Cell Tiss Org Cult 118:445–455. https://doi.org/10.1007/s11240-014-0496-2
Rawat JM, Bhandari A, Mishra S, Rawat B, Dhakad AK, Thakur A, Chandra A (2018) Genetic stability and phytochemical profiling of the in vitro regenerated plants of Angelica Glauca Edgew.: an endangered medicinal plant of Himalaya. Plant Cell Tiss Organ Cult 135:111–118. https://doi.org/10.1007/s11240-018-1448-z
Refaat J, Kamel MS, Ramadan MA, Ali AA (2013) Crinum; an endless source of bioactive principles: a review. Part V. Biological profile. Int J Pharm Sci Res 4:39–1252
Rohela GK, Jogam P, Mir MY, Shabnam AA, Shukla P, Abbagani S, Kamili AN (2020) Indirect regeneration and genetic fidelity analysis of acclimated plantlets through SCoT and ISSR markers in Morus alba L. cv. Chinese White. Biotechnol Rep 25:e00417. https://doi.org/10.1016/j.btre.2020.e00417
Roy M, Liang L, Xiao X, Feng P, Ye M, Liu L (2018) Lycorine: a prospective natural lead for anticancer drug discovery. Biomed Pharmacother 107:615–624. https://doi.org/10.1016/j.biopha.2018.07.147
Sadhu S, Jogam P, Thampu RK, Abbagani S, Penna S, Peddaboina V (2020) High efficiency plant regeneration and genetic fidelity of regenerants by SCoT and ISSR markers in chickpea (Cicer arietinum L.). Plant Cell Tiss Org Cult 141:465–477. https://doi.org/10.1007/s11240-020-01804-7
Santos GS, Sinoti SBP, de Almeida FTC, Silveira D, Simeoni LA, Gomes-Copeland KKP (2020) Use of galantamine in the treatment of Alzheimer’s disease and strategies to optimize its biosynthesis using the in vitro culture technique. Plant Cell Tiss Org Cult 143:13–29. https://doi.org/10.1007/s11240-020-01911-5
Sebastiani MS, Ficcadenti N (2016) In vitro plant regeneration from cotyledonary explants of Cucumis melo L. var. Cantalupensis and genetic stability evaluation using RAPD analysis. Plant Cell Tiss Org Cult 124:69–79. https://doi.org/10.1007/s11240-015-0875-3
Siddiqui Z, Mujib A, Maqsood M (2011) Liquid overlaying improves somatic embryogenesis in Catharanthus roseus. Plant Cell Tiss Organ Cult 104:247–256. https://doi.org/10.1007/s11240-010-9828-z
Sikder L, Alim MI, Saieda B, Islam MT, Martorell M, Sharifi-Rad J, Khan SA (2021) Pharmacological activities of Crinum viviparum: a laboratory-based study. J Herbs Spices Med Plants 27:177–187. https://doi.org/10.1080/10496475.2021.1891177
Singh SK, Rai MK, Sahoo LK (2012) An improved and efficient micropropagation of Eclipta alba through transverse thin cell layer culture and assessment of clonal fidelity using RAPD analysis. Ind Crops Prod 37:328–333. https://doi.org/10.1016/j.indcrop.2011.12.005
Takagi H, Sugawara S, Saito T, Tasaki H, Lu Y, Guan K, Han DS, Good T, Nakano M (2011) Plant regeneration via direct and indirect adventitious shoot formation and chromosome doubled somaclonal variation in Titanotrichum oldhamii (Hemsl.) Solereder. Plant Biotechnol Rep 5:187–195. https://doi.org/10.1007/s11816-011-0172-5
Takamori LM, Neto NBM, Vieira LGE, Ribas AF (2015) Optimization of somatic embryogenesis and in vitro plant regeneration of Urochloa species using picloram. In Vitro Cell Dev Biol 51:554–563. https://doi.org/10.1007/s11627-015-9701-1
Thakur J, Dwivedi MD, Sourabh P, Uniyal PL, Pandey AK (2016) Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle— an endemic and endangered medicinal plant. PLoS ONE 11:e0159050. https://doi.org/10.1371/journal.pone.0159050
Tribulato A, Remotti PC, Löffler HJM, Van Tuyl JM (1997) Somatic embryogenesis and plant regeneration in Lilium longiflorum Thunb. Plant Cell Rep 17:113–118. https://doi.org/10.1007/s002990050362
Uddin MZ, Emran TB, Nath AK, Jenny A, Dutta M, Morshed MM, Kawsar MH (2012) Anti-inflammatory and antioxidant activity of leaf extract of Crinum asiaticum. J Pharm Res 5:5553–5556
Uma S, Karthic R, Kalpana S et al (2023) An efficient embryogenic cell suspension culture system through secondary somatic embryogenesis and regeneration of true-to-type plants in banana cv. Sabri (silk subgroup AAB). Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-023-02570-y
Verma V, Kumar A, Seema Priti, Thakur M, Bhargava B (2022) Meta-Topolin mediated in vitro propagation in an ornamentally important crop Iris × Hollandica Tub. Cv. Professor Blaauw and genetic fidelity studies using SCoT markers. Plant Cell Tiss Org Cult 151:681–694. https://doi.org/10.1007/s11240-022-02383-5
Yin ZF, Zhao B, Bi WL, Chen L, Wang QC (2013) Direct shoot regeneration from basal leaf segments of Lilium and assessment of genetic stability in regenerants by ISSR and AFLP markers. In Vitro Cell Dev Biol Plant 49:333–342. https://doi.org/10.1007/s11627-013-9501-4
Zdravković-Korać S, Milojević J, Tubić L, Ćalić-Dragosavac D, Mitić N, Vinterhalter B (2010) Somatic embryogenesis and plant regeneration from root sections of Allium schoenoprasum L. Plant Cell Tiss Organ Cult 101:237–244. https://doi.org/10.1007/s11240-010-9682-z
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
VK and SO : Conceptualization, Editing and Supervision. HK and MML : Experimentation, Data analysis and Writing All contributors read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest. Two of the authors are members of the editorial board, the editor-in-chief (S. Ochatt) and one of the associate editors (V. Kumar) and, as such, they have not been involved in any stage of the evaluation of the submission. This status had no bearing on the editorial consideration of the manuscript.
Additional information
Communicated by Mohammad Reza Abdollahi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, H., Lekhak, M.M., Ochatt, S.J. et al. Somatic embryogenesis and genetic homogeneity assessment of regenerated plants of Crinum brachynema (Amaryllidaceae): an endemic critically endangered medicinal plant. Plant Cell Tiss Organ Cult 156, 33 (2024). https://doi.org/10.1007/s11240-023-02625-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02625-0