Abstract
The low efficiency of somatic embryo induction, proliferation, germination, and conversion to plantlets is a major problem in walnut. In this study, we used the cotyledons of immature embryos of apomictic seeds in walnut plus trees ‘Y17’ as explants. The optimum medium and culture conditions of somatic embryo induction, proliferation, and germination were tested through comparative experiments. The results showed that the optimum formula for somatic embryo induction, proliferation, and germination medium was DKW medium with 1.0 mg L−1 6-BA, 2.0 mg L−1 KT, and 0.01 mg L−1 IBA, DKW medium with 30–40 g L−1 sucrose and 7–8 g L−1 agar, and DKW medium with 2.0 mg L−1 concentrations of GA3, respectively. Meanwhile, the optimum time of seedling training was 36 h, and the substrate ratio was perlite: peat soil: vermiculite (1: 2: 1) after plant transplanting. The inter simple sequence repeat marker banding patterns of the primary embryo, secondary embryo and regenerated plants were all identical to that of the mother plant and proved the genetic stability of regenerated plants. An excellent walnut tissue culture system from walnut somatic embryo to the whole plant was formed, which can accelerate the speed of walnut genetic improvement.
Key message
An excellent walnut tissue culture system from walnut somatic embryo to the whole plant was formed, which can accelerate the speed of walnut genetic improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Walnut (Juglans regia L.) is a deciduous tree, which is an ecologically important ‘woody oil’ tree species grown worldwide for both fruit and wood (Bernard et al. 2018; Feng et al. 2021). The high content of oil, proteins, antioxidants, minerals, and vitamins in walnut kernels is very beneficial to the human body (Jariteh et al. 2015; Al-Snafi 2018). At present, walnut propagation occurs mainly through seedlings and grafting, but it is difficult to stabilize the inheritance of excellent variety characteristics through natural pollination and seedling propagation. Apomictic somatic embryogenesis can produce genetically identical copies of an original plant and provides the means to multiply and regenerate novel plants from genetically engineered cells, which has wide application prospects in the reproduction of germplasm resources (Arnold et al. 2002; Oh et al. 2010).
Apomixis is a form of asexual reproduction without the nuclear fusion of male and female gametes that leads to clonal progeny genetically identical to the mother plant (Koltunow and Grossniklaus 2003; Chen et al. 2018). Based upon these characteristics, the introduction of apomixis into sexual crops can fix a given genotype or heterosis via the clonal production of seeds, transform the current plant breeding paradigm by apomixis breeding, and dramatically reduce the time and costs for breeding and seed production, which has been considered a revolutionary technology and will have a huge impact on agriculture (Rodriguez-Leal and Vielle-Calzada 2012; Van Dijk et al. 2016). Studies have shown that apomixis exists in the walnut family; although the apomixis rate is generally lower than 17.3%, in some genotypes, the proportion of apomixis is 23.5 to 81.2% (Solar et al. 1995; Wu et al. 2010).
Somatic embryogenesis refers to the process in which somatic or non-sexual cells are induced to form bipolar embryos, which go through four stages: globular, heart-shaped, torpedo, and cotyledon stages (Quiroz-Figueroa et al. 2006). Somatic embryos are important not only as a tool for mass clonal propagation but also as explants for tissue culture and plant regeneration (McGranahan et al. 1990; Jalali et al. 2017). Somatic embryo production in walnut was first reported by Tulecke and McGranahan (1985). Although the efficiency of somatic embryo induction and proliferation has since improved, they still exhibit very low rates of germination and plant regeneration (Deng and Cornu 1992; Vahdati et al. 2006). Research has shown that embryos without the shoot and root apex and those that are translucent are unable to germinate to generate whole plants (Jariteh et al. 2015).
To improve the maturation and germination of somatic embryos in walnut, culturing technologies, including cold storage, desiccation, gibberellic acid (GA3), and liquid germination medium, have been tested (Tulecke and McGranahan 1985; Deng and Cornu 1992; Tang et al. 2001). Vahdati et al. (2008) found that 2 mg L−1 ABA in the maturation medium produced normal somatic embryos in walnut. Jalali et al. (2017) found that 7.5% PEG-4000 and 3.0% sucrose produced the highest rate (50.0%) of normal shooting embryos. In this study, we used somatic embryo cotyledons of apomictic walnut as explants and optimized somatic embryo induction, proliferation medium, and somatic embryo germination medium through comparative experiments.
The bottle opening time of seedling training and the substrate ratios after plant transplantation were determined. An excellent walnut tissue culture system, from walnut somatic embryos to complete plants, was formed, which can accelerate the speed of walnut breeding combining the characteristics of apomixis and somatic embryo induction.
Materials and methods
Plant material
The cotyledons of immature embryos of apomictic seeds were obtained from walnut plus trees ‘Y17’ as explants. The plus trees Y17 were conserved by patch budding onto walnut seedling rootstock at the Forestry Experimental Station of Shandong Agricultural University, Tai’an, Shandong Province, China (36° 10′ 19.2″ N, 117° 09′ 1.3″ E) in late May 2009. In the middle of April 2018, the female flower stigmas of Y17 were bagged when walnut female flowers were exposed but not pollinated. After removing the bags in early June, young walnut fruits were picked and washed with clean water for 20–30 min, then disinfected with 75% alcohol for 30 s and soaked in 3% sodium hypochlorite solution for 25 min. Finally, the walnut fruits were washed 4–5 times with deionized water, and the surface water was removed with sterile absorbent filter paper. The young embryos were taken out in an ultra-clean workbench, and the cotyledons were cut into 1.0 cm2 square pieces for subsequent somatic embryo induction.
Walnut somatic embryo induction
DKW, WPM, MS, 1/2 MS plus 30 g L−1 sucrose, and 7 g L−1 agar were used as the basic media (pH 5.7). Cultivation was conducted with different plant growth regulators, namely, 6-BA, KT, and IBA, at different concentration gradients. In total, there were 9 treatments, and 6-BA (0.5, 1.0, 2.0 mg L−1), KT (0.5, 2.0, 4.0 mg L−1), and IBA (0.001, 0.01, 0.1 mg L−1) were selected for the three-factor and three-level orthogonal experimental design. Each medium was inoculated with 4–8 immature embryos, repeated 3 times. In the first week after inoculation, they were transferred to fresh induction media every day and then transferred to fresh media once a week. The growth of somatic embryos was observed every week, and the induction rate of somatic embryos was counted after 4 weeks. The cultures were kept in the dark at 22 ± 1 °C. The induced primary somatic embryos were cultured for light intensity about 30 days and transferred to the proliferation medium.
Walnut primary somatic embryo proliferation
Primary somatic embryos with good growth were selected to carry out the proliferation experiment on the subculture medium. The media used in the experiment were DKW, WPM, and MS medium plus 30 g L−1 sucrose and 7 g L−1 agar. Each subculture medium was inoculated with 4–8 primary somatic embryos, which were transferred to fresh media once a week. The experiment was repeated three times. The culture was carried out in the dark at 22 ± 1 °C. The proliferation of somatic embryos was observed every week, and the proliferation rates were counted after four weeks. In addition, to verify the effect of different sucrose concentrations on the somatic embryo proliferation rate, DKW plus 7 g L−1 agar and different concentrations of sucrose were used as the basic media (pH value = 5.7). The sucrose concentration gradient was 0, 10, 20, 30, 40, and 50 g L−1. Each group had five petri dishes. Each petri dish was inoculated with 4–8 primary somatic embryos, repeated three times. Culture was carried out in the dark at 22 ± 1 °C. After two weeks, the effect of sucrose concentration on walnut somatic embryo proliferation was determined by counting the number of embryos.
Histocytological observation of walnut somatic embryos
The well-growing secondary somatic embryos were fixed with 1 mL FAA (Formaldehyde-acetic acid) overnight. After discarding the fixing solution, the secondary somatic embryos were treated with 50, 70, 85, and 95% alcohol (30 min each) successively and soaked twice with absolute ethanol to ensure complete dehydration. Then, xylene was used for transparent treatment, which can be miscible with paraffin. After paraffin embedding, the wax blocks were divided according to the placement position of the materials, and the divided wax blocks were trimmed into trapezoids. The wax edges were aligned as far up, down, left, and right as possible, and 2–3 mm of the wax edges were left around the sample. The wax block was fixed on the Lycra rotary slicer, and the cutting thickness was set to a 4–8 μm wax band. The cut flake wax was placed into the water pan of a TK-218 constant temperature spreader to spread the flake. The temperature was set to 38–40 °C. After the wax belt was fully unfolded, it was removed from the water with a glass slide and spread flat. The slide was dried in a slide dryer at 37 °C. Then, after dewaxing, alcohol rehydration, safranine solid green staining, and neutral gum sealing, the slide was observed under an optical microscope.
Somatic embryo germination and seedling formation
Walnut secondary somatic embryos with good growth were used as experimental materials. DKW, WPM, MS, 1/2 MS plus 30 g L−1 sucrose, and 7 g L−1 agar were used as the basic media. Cultivation was conducted with GA3 at different concentrations (0, 1.0, 2.0, 3.0, 4.0, and 5.0 mg L−1). Each culture bottle was inoculated with 2–3 germinated somatic embryos. Each treatment included 10 culture bottles and was repeated three times. Day and night light cycle culture was carried out. The light intensity was 37.5 umol m−2 s−1, and the light time was 16 h d−1. The temperature was 25 ± 1 °C, and light culture was carried out for 2 weeks. The germination rate of somatic embryos was calculated, and the growth status of somatic embryos was observed.
Seedling training and transplanting
Rooting walnut seedlings were bottled for 0, 12, 24, 36, 48, 60, and 72 h for seedling training. After seedling training, bottled seedlings with normal development, 3–4 true leaves, and a well-developed root system were transplanted to the mixed substrate with perlite, peat soil, and vermiculite. The tested substrate ratios were 1: 1: 1, 1: 2: 1, 1: 3: 1, 1: 4: 1, and 1: 4: 2 (perlite: peat soil: vermiculite). Thirty seedlings from each treatment were divided into three groups. After planting, they were placed in a ventilation shed with shading net and watered with clean water to maintain good humidity conditions (relative humidity 70–80%). The survival rate of transplantation was calculated after three weeks.
Genetic fidelity analysis of the regenerated plants with ISSR markers
The genetic fidelity of the primary embryo, secondary embryo and regenerated plants, compared with the mother plant, was analyzed using ISSR marker (Potter et al., 2002). The ISSR primers (ISSR7: AGAGAGAGAGAGAGAGTC and ISSR22: ACTCACAC ACACACACAT) were synthesized by Shanghai Sangon Biological Company (698 Xiangmin Rd., Chedun Industrial Park, Songjiang, Shanghai, China).The PCRs were performed in a total reaction volume of 25 μL containing 2 × Rapid Taq Master Mix (12.5 μL, TransGen Biotech, Peking, China), genomic DNA (1 μL, 20 ng μL−1), primer (2 μL, 10 μM) and sterilized double distilled water (9.5 μL). PCR amplification was performed in a thermal cycler (Biometra, T-Gradient Thermoblock, Germany) with an initial denaturation of DNA at 95 °C for 3 min, followed by 30 s denaturation at 94 °C, 45 s annealing at 50 °C and 60 s extension at 72 °C followed by 38 repeated cycles. The final extension was 10 min at 72 °C and a holding temperature of 4 °C. The amplified products were separated on 1.5% agarose gel using1 × TAE buffer. The sizes of amplicons were estimated by comparison with a 2 kb DNA ladder (TransGen Biotech, Peking, China). The gels were photographed by a gel-recording system (Bio-Rad, USA).
Data analyses
All analyses were conducted using Excel and the Statistical Program for Social Sciences 19.0 software (SPSS Inc., Chicago, IL, USA). In all analyses, significant differences were indicated by values of P < 0.05.
Results
Walnut somatic embryo induction
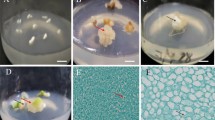
Walnut immature embryo cotyledons (Fig. 1a) were used to inoculate the basic media of DKW, WPM, MS, and 1/2 MS supplemented with different hormones. After 6 weeks, the callus induction rate was 35.59% and the somatic embryo induction rate was 14.12% on DKW medium. On this media, embryos developed to the cotyledon stage and existed in clusters on the surface of immature embryos (Fig. 1b). The somatic embryo induction rates on WPM, MS, and 1/2 MS medium were 7.67, 6.14, and 3.6%, respectively. The results of the analysis of variance showed that the effects of different media on somatic embryo induction rate were significantly different (Table 1). Therefore, the DKW medium was the best medium for walnut somatic embryo induction.
Regeneration process from somatic embryo to whole plant of walnut. a Walnut embryo cotyledon was used as explant. b Somatic embryo induction of walnut in optimum medium (DKW + 1.0 mg L−1 6-BA, 2.0 mg L−1 KT, and 0.01 mg L−1 IBA). c Walnut somatic embryo proliferation in optimum medium (DKW + 30–40 g L−1 sucrose and 7–8 g L−1 agar). d–f Walnut somatic embryo germination in optimum medium (DKW + 2.0 mg L−1 GA3). g Walnut seedling formation. h Walnut seedling training and transplanting in the optimum substrate ratio (1:2:1 perlite: peat soil: vermiculite). Scale bar = 5 mm
The effects of plant growth regulators on walnut somatic embryos were different. The induction rate of somatic embryos increased at first and then decreased as the 6-BA concentration increased. The average induction rate (10.48%) was the highest at 1.0 mg L−1. When KT was used at a 2.0 mg L−1 concentration, the average induction rate of somatic embryos was highest at around 8.60%. In the media supplemented with IBA, the average induction rate also increased at first, then decreased as the IBA concentration increased. The highest induction rate, 8.02%, was reached when the IBA concentration was 0.01 mg L−1. Multiple comparisons and analysis of variance revealed that the optimum formula for somatic embryo induction using immature embryo cotyledons was 1.0 mg L−1 6-BA, 2.0 mg L−1 KT, and 0.01 mg L−1 IBA (Table 2).
Walnut somatic embryo proliferation
Secondary embryos were produced by somatic embryo proliferation (Fig. 1c). The proliferation coefficient of somatic embryos was the highest in DKW medium, up to 48.24%, followed by WPM and MS medium, but secondary embryos were not produced in 1/2 MS medium (Table 1). The proliferation coefficient increased at first and then decreased as the sucrose concentration increased. Analysis of variance showed that when the sucrose concentration was 30–40 g L−1, the highest secondary embryo production rate and the highest average number of secondary embryos were obtained, which was significantly different from other concentrations (Table 3). Collectively, these results indicated that the optimum medium formula for proliferating somatic embryos was DKW medium with 30–40 g L−1 sucrose and 7–8 g L−1 agar.
Morphological and histocytological observation of walnut somatic embryos
Walnut secondary embryos were produced by epidermal single-cell origin. Through morphological and histocytological observation, the globular (Fig. 2a, e), heart-shaped (Fig. 2b, f), torpedo-shaped (Fig. 2c, g), and cotyledon stages (Fig. 2d, h) of somatic embryos were observed. Walnut somatic embryos were polar in development. The inner cells are divided into basal vascular tissue in the globular phase (Fig. 2e). The prototype layer developed, and the gradual establishment of polarity was observed in the torpedo-shaped stage (Fig. 2g). Y-shaped vascular bundles were clearly observed at the cotyledon stage (Fig. 2h). The formation of cotyledon somatic embryos was found to be conducive to subsequent somatic embryo germination.
Morphological and histocytological observation of walnut somatic embryos. a globular, b heart-shaped, c torpedo-shaped, d cotyledon stages of somatic embryos through morphological observation. Scale bar = 1000 μm. e globular, f heart-shaped, g torpedo-shaped, h cotyledon stages of somatic embryos through histocytological observation. Scale bar = 100 μm
Somatic embryo germination and seedling formation
The secondary embryos refrigerated at 4 °C for 2–3 weeks were placed in a normal light incubator. The epicotyl and hypocotyl began to grow at almost the same time, forming young leaves, developing the taproot, and growing normally with both branches and roots (Fig. 1d–f). When DKW was used as the basic medium, the root system grew well, and the plant grew strongly. The rooting rate was the highest (43.43%) in DKW, which was significantly higher than that in other media. Furthermore, these seedlings also had the longest taproots (5.47 cm) and the most taproot branches (4.46; Table 4). With the addition of GA3, the germination rate of somatic embryos increased at first and then decreased. When the GA3 concentration was 2.0 mg L−1, the germination rate of somatic embryos was 35.69%. At this concentration, the germinated somatic embryos had both branches and roots and could form complete plants, which was significantly different from other concentrations (Table 5). Based on these results, the DKW medium with a 2.0 mg L−1 concentration of GA3 was the most beneficial for the germination of secondary somatic embryos.
Seedling training and transplanting
The cultured somatic embryo seedlings with 3–4 leaves and well-developed roots (Fig. 1g) were used for seedling training. The survival rate of transplantation after seedling training was closely related to the bottle opening time. With the increase of bottle opening time, the survival rate of transplantation increased at first and then decreased. When the bottle opening time was 36 h, the seedlings grew well and the survival rate of transplantation was 56.67%, which was significantly higher than that of other bottle opening times (Table 6). The seedlings were transplanted after training, in which the substrate ratios had significant effects on the survival rate of walnut somatic embryo seedlings. When the substrate ratio was 1: 2: 1 (perlite: peat soil: vermiculite), the plants grew well (Fig. 1h) and transplanting had the highest survival rate (57.69%), which was significantly higher than that of other substrate ratios (Table 7).
Genetic fidelity analysis using ISSR markers
Genetic fidelity is one of the most essential requisites for the success of any in vitro propagation procedure. Two primers (ISSR7 and ISSR22) amplified monomorphic bands in the primary embryo, secondary embryo, regenerated plants, and mother plant. As shown in Fig. 3, the ISSR marker banding patterns of the primary embryo, secondary embryo and regenerated plants were all identical to that of the mother plant, indicating the primary embryo, secondary embryo, regenerated plants were genetically stable.
Genetic fidelity analysis of the mother plant and regenerated plants of walnut by ISSR molecular markers. Profiles were obtained from primers ISSR-7 (a) and ISSR-22 (b). Lane MP: Mother plant. Lane 1–3: primary embryo induced by cotyledon. Lane 4–6: secondary embryo. Lane 7–9: regenerated plants. Lane M: Molecular ruler (2 kb DNA ladder)
Discussion
Somatic embryogenesis is the developmental process in which somatic embryogenic cells undergo a series of morphological and biochemical changes, resulting in the formation of a somatic embryo capable of regenerating plants (Yang and Zhang 2010). The development of the embryo included the morphogenetic stage characterized by the basic structure of the embryo and the metabolic stage accompanied by biochemical activities that prepare the embryo for quiescence (Lopes and Larkins 1993; Harada 1999). Embryo development can be divided into globular-shaped, heart-shaped, torpedo-shaped, and cotyledonal stages during the morphogenetic stage (Yang and Zhang 2010). Similar to the results of other studies on somatic embryogenesis and embryo maturation (Tulecke and McGranahan 1985; Deng and Cornu 1992; Vahdati et al. 2008), the histological investigation revealed that globular-shaped, heart-shaped, torpedo-shaped, and cotyledon stage embryos were routinely observed in this study. Additionally, the normal mature somatic embryos, which had a white appearance, enlarged cotyledons, and shoot and root apices, could germinate (Deng and Cornu 1992). During the experiment, translucent or vitreous embryos which is difficult to quantify in the statistical process were found not to germinate in this study. This result is consistent with previous studies. This may also explain the low induction and proliferation rate of somatic embryos.
With the continuous improvement and development of tissue culture technology and methods, progress in the micropropagation of walnut has been achieved using somatic embryos. DKW medium is the most commonly used basic medium in walnut tissue culture. Jariteh et al. (2015) studied the developmental changes in protein, proline, and some antioxidants in somatic and zygotic embryos of walnut induced on a DKW-based medium. Tang et al. (2001) found that DW (1/2 DKW + 1/2 WPM) and MW (1/2 MS + 1/2 WPM) media were more effective than DKW, MS, WPM, and MD (1/2 MS + 1/2 DKW) in the improvement of secondary somatic embryo production. In this study, by comparing the induction rate and proliferation rate of walnut somatic embryos on DKW, WPM, MS, and 1/2 MS basic medium, DKW medium was found to be the best basic medium during the process of somatic embryo induction and proliferation.
As a general rule, plant growth regulators and endogenous hormones are the most critical factors in the induction and development of somatic embryos, and the most commonly used are 2,4-D, IBA, ABA, GA3, and 6-BA (Stasolla and Yeung 2003). Long et al. (1995) found that the best treatment for the induction of somatic embryos from immature cotyledon explants was agar-solidified WPM with 0.1 µM 2, 4-D, and 5.0 μM TDZ (Thidiazuron), with an induction rate up to 78–80%. Bekir and Hatice (2007) showed that the percentage of germination (69.1%) was highest with desiccated embryos originating from open-pollinated seeds of the ‘Bilecik’ genotype on DKW basal medium containing 8.6 µM GA3. In the present study, the optimum formula for somatic embryo induction and germination was a DKW medium with 1.0 mg L−1 6-BA, 2.0 mg L−1 KT, and 0.01 mg L−1 IBA and a DKW medium with 2.0 mg L−1 GA3, respectively. Additionally, other factors of culture conditions, such as pH, agar, and nitrogen level, can affect the ratio of embryogenic tissue (Tautorus et al. 1991). Similar to the above conclusion, the optimum media formula for proliferating somatic embryos in this study was DKW medium with 30–40 g L−1 sucrose and 7–8 g L−1 agar.
Abbreviations
- DKW:
-
Driver and kuniyuki walnut
- 6-BA:
-
6-Benzylaminopruine
- KT:
-
Kinetin
- IBA:
-
Indole-3-butyric acid
- GA3 :
-
Gibberellic acid (3)
- WPM:
-
Wood plant medium
- MS:
-
Murashige and skoog medium
- ISSR:
-
Inter simple sequence repeats
References
Al-Snafi AE (2018) Chemical constituents, nutritional, pharmacological and therapeutic importance of Juglans regia-a review. IOSR J Pharm 8(11):1–21
Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tiss Org Cult 69:233–249
Bernard A, Lheureux F, Dirlewanger E (2018) Walnut: past and future of geneticim provement. Tree Genet Genomes 14(1):1
Chen X, Lai HG, Sun Q, Liu JP, Chen SB, Zhu WL (2018) Induction of apomixis by dimethyl sulfoxide (DMSO) and genetic identification of apomictic plants in cassava. Breed Sci 68(2):227–232
Deng MD, Cornu D (1992) Maturation and germination of walnut somatic emryos. Plant Cell Tissue Organ Cult 28:195–202
Feng S, Fang H, Liu X, Dong Y, Yang KQ (2021) Genome-wide identification and characterization of long non-coding RNAs conferring resistance to Colletotrichum gloeosporioides in walnut (Juglans regia). BMC Genomics 22(1):15
Harada JJ (1999) Signaling in plant embryogenesis. Curr Opin Plant Biol 2:23–27
Jalali MA, Sirmandi HB, Hatamzadeh A (2017) Effects of carbohydrate source and polyethylene glycol on maturation and germination of somatic embryos in walnut (Juglans regia L.). J Crop Sci Biotechnol 20(1):29–35
Jariteh M, Ebrahimzadeh H, Niknam V, Mirmasoumi M, Vahdati K (2015) Developmental changes of protein, proline and some antioxidant enzymes activities in somatic and zygotic embryos of Persian walnut (Juglans regia L.). Plant Cell, Tissue Organ Culture 122(1):101–115
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annu Rev Plant Biol 54:547–574
Long LM, Preece JE, Sambeek JWV (1995) Adventitious regeneration of Juglans nigra L (eastern black walnut). Plant Cell Rep 14(12):799–803
Lopes MA, Larkins BA (1993) Endosperm origin, development, and function. Plant Cell 5:1383–1399
McGranahan G, Leslie C, Uratsu S, Dandekar A (1990) Improve defficiency of the walnut somatic embryo gene transfer system. Plant Cell Rep 8:512–516
Oh MJ, Na HR, Choi HK, Liu JR, Kim SW (2010) High frequency plant regeneration system for Nymphoides coreana via somatic embryogenesis from zygotic embryoderived embryogenic cell suspension cultures. Plant Biotech Rep 4:125–128
Potter D, Gao F, Aiello G, Leslie C, McGranahan G (2002) Intersimple sequence repeat markers for fingerprinting and determining genetic relationships of walnut (Juglans regia) cultivars. J Am Soc Hortic Sci 127(1):75–81
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tiss Org Cult 86:285–301
Rodriguez-Leal D, Vielle-Calzada JP (2012) Regulation of apomixis: learning from sexual experience. Curr Opin Plant Biol 15:549–555
Solar A, Smole J, Simoncic S (1995) The ability of apomictic fruit setting in five walnut cultivars (Juglands regia L.). Kmetijstvo
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tiss Org Cult 74:15–35
Tang H, Ren Z, Reustle G, Krczal G, Germaine E (2001) Optimizing secondary somatic embryo production in English walnut (Juglans regia L.). Acta Hort 544:187–194
Tautorus TE, Fowke LC, Dunstan DI (1991) Somatic embryogenesis in conifers. Can J Bot 69:1873–1899
Tulecke W, McGranahan G (1985) Somatic embryogenesis and plant regeneration from cotyledons of walnut, Juglans regia L. Plant Sci 40:57–63
Vahdati K, Jariteh M, Niknam V, Mirmasoumi M, Ebrahimzadeh H (2006) Somatic embryogenesis and embryo maturation in Persian walnut. Acta Hortic 705:199–205
Vahdati K, Bayat SH, Ebrahimzadeh H, Jariteh M, Mirmasoumi M (2008) Effect of exogenous ABA on somatic embryo maturationand germination in Persian walnut (Juglans regia L.). Plant Cell Tiss Organ Cult 93:163–171
van Dijk PJ, Rigola D, Schauer SE (2016) Plant breeding: surprisingly, less sex is better. Curr Biol 26:R122-124
Wu G, Yang J, Zhang P, Liu Q, Song Y (2010) Study on the apomixis in Shanxi Mianhetao walnut. J Fruit Sci 27(2):221–226
Yang X, Zhang X (2010) Regulation of somatic embryogenesis in higher plants. Crit Rev Plant Sci 29(1):36–57
Funding
This research was supported by the National Natural Science Foundation of China (32001340), the Improved Variety Program of Shandong Province of China (2020LZGC090102), and the Natural Science Foundation of Shandong Province (ZR2020QC169).
Author information
Authors and Affiliations
Contributions
RC and KY contributed the conception, design, and final revision. HF and YD contributed to design and drafting the manuscript. YB, SX, XL, SG, QW and QD analyzed and interpreted the data. RZ and CW contributed to supervise and revise the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Communicated by Francisco de Assis Alves Mourão Filho.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, H., Dong, Y., Zhou, R. et al. Optimization of the induction, germination, and plant regeneration system for somatic embryos in apomictic walnut (Juglans regia L.). Plant Cell Tiss Organ Cult 150, 289–297 (2022). https://doi.org/10.1007/s11240-022-02266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02266-9