Abstract
Leucine-rich repeat-receptor-like kinases (LRR-RLKs) are major gene families that play an important role in in many aspects of plant growth and development particularly in the process of signal transmission. RLK XYLEM INTERMIXED WITH PHLOEM 1 (XIP1)/C-TERMINALLY ENCODED PEPTIDE (CEP) RECEPTOR 1 (CEPR1) has been identified as a leucine-rich repeat (LRR) receptor kinase. In this study, the MdCEPR1 gene (GenBank ID: DQ221207) from apple (Malus × domestica), was isolated and characterized. MdCEPR1 transcripts were highly accumulated in roots and leaves, and MdCEPR1 was significantly induced under low nitrate conditions. In addition, suppressing the MdCEPR1 gene in apple calli increased anthocyanin content. Overexpression of MdCEPR1 promoted growth of apple calli and Arabidopsis thaliana under low nitrate condition by increasing nitrate assimilation and up regulating the expression of genes involved in nitrate assimilation. Ectopic expression of MdCEPR1 also promoted lateral root development in transgenic Arabidopsis. Taken together, our results indicated that MdCEPR1 acts as a positive regulator of plant nitrate utilization and lateral root development.
Key message
In this study, the MdCEPR1 gene from apple, was isolated and characterized, and our results indicated that MdCEPR1 acts as a positive regulator of plant nitrate utilization and lateral root development. MdCEPR1 may be a useful target for marker-assisted breeding to improve crop yield and reduce the use of chemical fertilizer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen is the key nutrient that determines fruit yield and quality (Crawford and Glass 1998; Williams and Miller 2001), and is one of the essential mineral elements for fruit trees. It is an important component of cell protoplasts, nucleic acids, hormones, alkaloids, and enzymes, so a supply of nitrogen is necessary for normal plant growth (Pallardy 2008). The supply of nitrogen is directly related to organ differentiation and formation of plant structures. Nitrogen is mainly taken up as ammonium nitrogen and nitrate nitrogen for utilization by plants (Lee et al. 1992). Nitrate is the main source of nitrogen for most plants and acts as a signaling molecule to regulate gene expression associated with metabolism and development (Gutiérrez et al. 2008).
Plant receptor-like protein kinases (RLKs) belong to a major gene family, with more than 610 and 1,132 members in Arabidopsis and rice, respectively (Shiu et al. 2004). Typical RLKs of plants contain three domains: an extracellular ligand-binding domain, a transmembrane domain, and a C-terminal intracellular kinase domain (Christiaan et al. 2012). Leucine-rich repeat-receptor-like kinases (LRR-RLKs) are the largest branch of the plant RLK family, including over 200 members that contain many LRRs in the extracellular domain (Shiu and Bleecker 2003). In plants, a number of LRR-RLKs have been functionally identified and play important roles in hormone receptors and disease resistance. For example, BRASSINOSTEROID-INSENSITIVE 1 (BRI1) is a component of the brassinosteroid receptor, which interacts with ligands to participate in the stress response in plants (Li et al. 2002). FLAGELLIN-SENSITIVE 2 (FLS2) is a component of the flagellin receptor in Arabidopsis, which is involved in defense responses to pathogens (Gómez-Gómez and Boller 2000). Some LRR-RLKs are also involved in plant developmental processes. CLAVATA1 (CLV1) receptor kinase controls meristem size and stem cell number (Nimchuk et al. 2011). ERECTA participated in the regulation of organ growth, stomatal differentiation, and inflorescence structure (Shpak et al. 2004). AtSERK1 responds to biotic and abiotic stress and enhances embryogenic competence in culture (Salaj et al. 2008). RLK XYLEM INTERMIXED WITH PHLOEM 1 (XIP1)/C-TERMINALLY ENCODED PEPTIDE (CEP) RECEPTOR 1 (CEPR1) has been identified as a LRR receptor kinase that is necessary for development of the vascular system (Bryan et al. 2012). More recently, CEPR1 and CEPR2 have been shown to be the receptor for CEP1 and other members of the CEP family. CEPR1 contains a short secretory signal peptide sequence, an N-terminal extracellular LRR receptor domain, a C-terminal cytoplasmic serine/threonine kinase domain, and a single helical transmembrane region (Roberts et al. 2016). CEPR1 belongs to the LRR XI family and loss-of-function mutants despite their general morphology and normal fertility show ectopic lignification of the phloem and accumulation of leaf anthocyanins (Bryan et al. 2012). Interestingly, the cepr1 cepr2 double mutant has a variety of phenotypes, including smaller light green leaves and reduced expression of the nitrate transporter. Other studies have shown that CEPR1 regulate the nitrogen-dependent responses in the long-distance systemic signaling pathway (Tabata et al. 2014). CEP5 and CEPR1 are involved in the regulation of lateral root initiation in Arabidopsis and cepr1 has a phenotype with reduced lateral root density compared to the wild-type (WT) (Roberts et al. 2016).
In this study, a CEPR1 gene (MdCEPR1) was cloned from apple (Malus × domestica), followed by an expression analysis and functional characterization. Our results suggest that MdCEPR1 regulate the nitrogen response and construction of root morphology. MdCEPR1 plays a critical role in optimal root system deployment of apple for more effective access to nutrition.
Materials and methods
Plant materials and growth conditions
The apple calli used in this study were obtained from the young embryos of ‘Orin’ apple, and subcultured on modified ‘Orin’ calli medium (MS medium supplemented with 1.3 mg L− 1 2,4-dichlorophenoxy (2,4-D) and 0.6 mg L− 1 6-benzylaminopurine (6-BA) at 25 ℃ in the dark (An et al. 2017). The in vitro shoot cultures of the apple variety ‘Gala’ were grown on MS medium containing 0.5 mg L − 1 6-benzylaminopurine (6-BA), 0.1 mg L − 1 gibberellins (GA3), and 0.2 mg L − 1 1-naphthaleneacetic acid (NAA) and rooted in MS medium containing 0.15 mg L− 1 NAA at 16 h light/8 h darkness (You et al. 2014). One-month-old uniform ‘Gala’ seedlings were selected and transplanted to PVC pots (25 cm long, 20 cm wide and 15 cm high) containing 6L nutrient solution. Floating plates were used to support seedling growing in the solution. The composition of modified Hoagland’s nutrient solution (Hoagland and Arnon 1950) was as follows: 10 mM KNO3, 2 mM MgSO4, 1 mM KH2PO4, 0.045 mM H3BO3, 0.01 mM MnCl2, 0.8 µM ZnSO4, 0.3 µM CuSO4, 0.4 µM Na2MoO4, and 0.02 µM Fe-EDTA. The solution for the low nitrate treatment contained 0.1 mM KNO3, and K was compensated by KCl. Arabidopsis thaliana (Col-0) was used in this study for genetic transformation. The seeds were surface-sterilized with 4% NaClO. The sterilized seeds were stored at 4 °C for 2 days and sown onto plates containing MS medium with 0.6% agar at 22 °C.
Sequence alignment and phylogenetic analysis
A BLASTP search (http://www.ncbi.nlm.nih.gov/BLAST/) was performed to obtain the MdCEPR1 homologs. Multiple sequence alignments of the CEPR1 sequences were performed using ClustalX (version 2.1). Phylogenetic analysis of CEPR1 was performed according to the neighbor-joining method in the MEGA5 program (http://www.megasoftware.net/) (Hall 2013). The MdCEPR1 secondary protein structure was predicted using Simple Modular Architecture Research Tool (SMART) software (http://smart.embl-heidelberg.de/).
Generation of the MdCEPR1 transgenic calli and transgenic Arabidopsis
The open reading frame (ORF) and the reverse complement DNA fragment of MdCEPR1 were obtained by real time-polymerase chain reaction (RT-PCR). The ORF region of the MdCEPR1 cDNA was used for sense overexpression (MdCEPR1-OX), and the DNA fragment of MdCEPR1 reverse complement were used for antisense suppression (MdCEPR1-anti). The PCR products were inserted into the transformed vector pRI101, and then the plasmids were introduced into Agrobacterium strain LBA4404 for obtaining transgenic apple calli according to the method as described by An et al. (2015). To create MdCEPR1 transgenic Arabidopsis, the plant transformation vectors described above (pRI101- MdCEPR1) were transformed into Arabidopsis plants (Columbia) by the floral dip method (Clough and Bent 1998). Transcription levels of MdCEPR1 in related lines were monitored by qRT-PCR. The T1 generations of transgenic plants were used for phenotypic analysis, and the phenotypes were confirmed in the following 2 to 3 generations.
Gene expression analysis
All samples were stored at − 80 °C. RNA was extracted from triplicate biological replicates of the above samples using the Trizol kit. Two micrograms of total RNA were used to synthesise first-strand cDNA by reverse transcription. RT-qPCR analysis was performed using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) on a Bio-Rad iQ5 instrument (USA). The reactions were performed using the following cycling parameters: 5 min initial denaturation at 94 °C, 30 cycles of denaturation at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s. MdActin was used as the control and the 2−ΔΔCt calculation method was used for data analysis. All of the primers used are listed in Supplementary Table S1. All of the samples were repeated at least three times. The results were based on the average of three times biological replicates.
Determination of anthocyanin content
The ‘Orin’ apple calli were cultured on medium and were place in a phytotron (temperature: 22 °C, photon flux density: 70 µmol m−2s−1) for 1 week. Then, total anthocyanin content was extracted from both transgenic and WT calli using a methanol-HCl method as described by An et al. (2015).
Nitrogen deficiency treatment and determination of nitrate content and nitrate reductase activity
To investigate different growth rates under nitrogen deficiency conditions, the 15-day-old individual genotype apple calli were treated with different concentrations of nitrate medium (0.1 and 10 mM). Subsequently, these apple calli were grown for 2 weeks under continuous dark conditions. The 10-day-old Arabidopsis seedlings were grown on vermiculite for 3 weeks, and watered with a nutrient solution containing 10 mM NO3− and 0.1 mM NO3− respectively. Then, the plant material was collected for determination of nitrate content and nitrate reductase activity.
Nitrate content was measured using the salicylic acid method (Cataldo et al. 1975; Li et al. 2017). First, samples (1 g) were frozen in liquid N2 and milled into powder. Then, samples were boiled at 100 °C for 20 min with 10 mL of deionized water, centrifuged at 15,000×g for 10 min. Next, 0.1 mL of the supernatant and 0.4 mL of 5% salicylic acid-sulfuric acid solution were added to a new tube for reaction. Twenty minutes later, 9.5 mL of 8% NaOH solution was added slowly to the tubes and cooled to room temperature. The optical density of the reaction solution at 410 nm was measured and the deionized water served as the control. The nitrate content were normalized according to the following formula: N = C·V/W (N, nitrate content; C, nitrate concentration calculated using OD410 in the regression equation; V, total volume of extracted sample; W, weight of sample). The standard curve was made using 10–120 mg L− 1 KNO3, and the regression equation was calculated according to the standard curve.
Nitrate reductase activity (NRA) was measured as described by Freschi et al. (2010) and Li et al. (2017). First, samples (0.5 g) were washed with distilled water and placed in tubes. Next, 9 ml of 0.1 M phosphate buffer (pH7.5) with 3% propanol and 0.1 M KNO3 were added to the tubes for reaction at 30 °C in the dark. Thirty minutes later, 1 mL of trichloroacetic acid was added to stop the reaction. Then, the 2 mL of supernatant was transferred into a new tube, and 4 mL of 0.2% N-1-naphthyl-ethylene-diamine and 4 mL of sulfanilamide mixed with 3 M HCl were added. The optical density of the reaction solution at 540 nm was measured after 30 min. The standard curve was made using 0–2 g NaNO2, and the regression equation was calculated according to the standard curve. The nitrate reductase activity (NRA) was normalized according to the of nitrite content produced per hour per gram of fresh weight (nmol nitrite− 1 g− 1 FW).
Nitrate uptake assay using 15NO3−
The 15NO3− was used to analyze nitrate uptake activity as described by Han et al. (2016). Ten-day-old A. thaliana seedlings were transferred to 0.1 mM CaSO4 for 1 min, then to their respective hydroponic nutrient solutions with 15NO3− ( 99% atom) replacing unlabeled NO3− for 30 min. Finally, the A. thaliana seedlings were transferred to 0.1 mM CaSO4 for 1 min, after which the roots were washed with deionized water. Then, the seedlings were dried at 70 ℃ to constant weight and ground. 15N content was analyzed using a continuous-flow isotope ratio mass spectrometer coupled with an elemental analyzer.
Statistical analysis
All experiments were performed at least three times and the results were based on the average of three replicates. The data were analyzed with appropriate methods using DPS (7.05) software (http://www.dpsw.cn/dps_eng/). A p-value < 0.05 was considered significant.
Results
MdCEPR1 protein structure and phylogenetic tree analysis
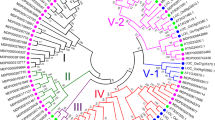
To investigate the functions of CEPR1 in apple, the AtCEPR1 (AT5G49660) sequence was used as a query to search similar sequences in apple genome database (https://www.rosaceae.org/blast) with the BLASTP program. As shown in Supplementary Fig. S1, we identified the apple AtCEPR1 homologous gene and named it MdCEPR1 (MDP0000122376). A sequence analysis revealed that the full-length MdCEPR1 cDNA sequence consisted of 3392 nucleotides with an ORF of 2892 nucleotides, which encoded a 963 amino acid peptide. Alignment with the CEPR1 protein sequences from Arabidopsis showed that MdCEPR1 had 63% identity to AtCEPR1. Then the MdCEPR1 amino acid sequence was analyzed, and five LRR regions were predicted, with two conserved regions, including a transmembrane region and a serine/threonine protein kinase catalytic domain (Fig. 1a). To explore the evolutionary relationships among the CEPR1 proteins in plants, 22 CEPR1 family members were aligned and a phylogenetic tree was constructed using the neighbor-joining method. The results clearly indicate that MdCEPR1 has a close genetic relationship with PbCEPR1 and shares clades with PmCEPR1 and PpCEPR1 (Fig. 1b).
Protein structure and phylogenetic tree analysis of MdCEPR1. Functional domains analysis of MdCEPR1. a The amino acid sequence of MdCEPR1 was analyzed using SMART (http://smart.embl-heidelberg.de/). Red represents the five LRR repeat domains (100–128 aa, 171–197 aa, 245–269 aa, 293–317 aa, and 485–533 aa). Blue represents the transmembrane region (593–612 aa). Green represents the serine/threonine protein kinase catalytic domain (654–931 aa). b Phylogenetic analysis of MdCEPR1 and CEPR1 from 16 other plants using the neighbor joining method. MdCEPR1 is denoted by an asterisk, and the distance indicated by “0.2” refers to the percent sequence divergence.TcCEPR1: Theobroma cacao, XP_007018366.1; GrCEPR1: Gossypium raimondii, XP_012446740.1; CoCEPR1: Corchorus olitorius, ACI42311.1; AtCEPR1: Arabidopsis thaliana, AT5G49660.1; PbCEPR1: Pyrus bretschneideri, XP_009357235.1; PmCEPR1: Prunus mume, XP_008219158.1; PpCEPR1: Prunus persica, XP_007227013.1; ZjCEPR1: Ziziphus jujube, XP_015887774.1; CmCEPR1: Cucumis melo, XP_008466101.1; CsCEPR1: Cucumis sativus, XP_004136411.1; PtCEPR1: Populus trichocarpa, XP_002301126.1; PeCEPR1: Populus euphratica, XP_011017021.1; VfCEPR1: Vernicia fordii, AMM42884.1; JcCEPR1: Jatropha curcas, XP_012068114.1; RcCEPR1: Ricinus communis, XP_002510008.1; CcCEPR1: Citrus clementine, XP_006442751.1. (color figure online)
MdCEPR1 expression patterns
The spatial expression pattern of MdCEPR1 was investigated by qRT-PCR to elucidate the function of MdCEPR1 in plants. The results show that the MdCEPR1 transcript accumulated in five apple tissues (roots, stems, leaves, flowers, and fruit). The amount of the transcript was significantly higher in roots and leaves than in stems, flowers, or fruit (Fig. 2a). To investigate whether MdCEPR1 expression was induced by nitrogen deficiency, the expression of MdCEPR1 was examined under a 0.1 mM L− 1 nitrate condition. Transcript analysis revealed that the transcript levels of MdCEPR1 were significantly increased in response to nitrogen deficiency treatments in roots and shoots compared with the control treatment (Fig. 2b). The MdCEPR1 expression level in the root reached the maximum 8 h after treatment, which was earlier than that in the shoot. This finding indicates that the root system first senses nitrogen deficiency stress and then signals the shoot, causing the MdCEPR1 response in the shoot. These results suggest that MdCEPR1 is probably involved in the response to a nitrogen deficiency.
MdCEPR1expression patterns. a Transcript levels of MdCEPR1 in different organs. The value for fruit was set to 1. b Time-course expression levels of MdCEPR1 at different periods in the roots and shoots of ‘Gala’ seedlings under 10 mM NO3− and 0.1 mM NO3− conditions. These data were analyzed by the 2−ΔΔCT method
MdCEPR1 negatively regulates anthocyanin accumulation and inhibits expression levels of flavonoid structural genes in apple calli
AtCEPR1 loss-of-function mutants regulate leaf anthocyanin accumulation (Bryan et al. 2012). To investigate whether MdCEPR1 also regulates the accumulation of anthocyanins in apple, MdCEPR1 overexpression and antisense expression vectors were constructed, and were transformed into ‘Orin’ apple calli. Then, total DNA of the transgenic materials were extracted, and PCR were used to determine gene transformation (Supplementary Fig. S2A) and qRT-PCR were used to detect the expression level of MdCEPR1 (Supplementary Fig. S2B). As a result, overexpressing calli (MdCEPR1-OX) and antisense suppressing calli (MdCEPR1-anti) were obtained (Fig. 3a). The accumulation of anthocyanins in MdCEPR1-anti was significantly higher than in WT, and MdCEPR1-OX accumulated less anthocyanin compared to the WT under light conditions (Fig. 3b–d). The expression levels of flavonoid structural genes were analyzed by qRT-PCR in the WT and transgenic calli (MdCEPR1-OX and MdCEPR1-anti). The results show that the expression of major genes related to the anthocyanin biosynthetic pathway were slightly suppressed in MdCEPR1-OX compared with WT, including MdMYB1, MdPAL, MdCHI, MdF3H, MdDFR, MdANS1, MdANR1, and MdUFGT (Fig. 4). In contrast, expression of these genes was significantly upregulated in MdCEPR1-anti compared with WT and MdCEPR1-OX. These results indicate that MdCEPR1 and AtCEPR1 share similar functions in regulating anthocyanin accumulation.
Overexpression of MdCEPR1 enhances tolerance to nitrogen deficiency by increasing nitrate assimilation in apple calli
The 15-day-old individual genotype calli were transferred to different concentrations of nitrate medium (0.1 and 10 mM) for 2 week to assess the role of MdCEPR1 in nitrate assimilation in apple. As shown in Fig. 5a, the fresh weight of MdCEPR1-anti was slightly lower than that of WT and MdCEPR1-OX when grown on nitrogen-rich medium (10 mM NO3−). However, growth of MdCEPR1-OX was significantly superior to the WT and MdCEPR1-anti, and MdCEPR1-anti did not grow well on nitrogen-deficient medium (0.1 mM NO3−) compared with WT and MdCEPR1-OX (Fig. 5b). This result demonstrates that MdCEPR1 rescued the phenotypic defects of the apple calli under nitrogen-deficient conditions. To further analyze the actual role of MdCEPR1 in nitrate absorption, nitrate content and NRA of WT and transgenic calli were determined under N-rich and N-limited conditions. Statistical analyses indicated that overexpressing MdCEPR1 increased nitrate content and NRA (Fig. 4c, d). However, the nitrate contents and NRA were significantly lower compared with the WT in MdCEPR1-anti (Fig. 5c, d). Then, the transcript levels of genes related to nitrate uptake, transport, and assimilation were analyzed by qRT-PCR in transgenic calli. The expression levels of MdNRT1.1, MdNRT2.1, and MdNIA1 in WT and transgenic calli were assayed. As shown in Fig. 5, all tested nitrate-related genes had significantly higher expression levels in MdCEPR1-OX compared with the WT control, and the expression levels were significantly downregulated in MdCEPR1-anti (Fig. 5e–g). These results demonstrated that MdCEPR1 plays a vital role in nitrate assimilation and signaling in apple.
MdCEPR1promotes growth of apple calli under a nitrogen-deficient condition by increasing nitrate assimilation and upregulating the expression of genes involved in nitrate assimilation in apple (a) Wild type (WT) and transgenic calli (MdCEPR1-OX and MdCEPR1-anti) were grown in a medium containing different concentrations of nitrate (10 and 0.1 mM) for 10 days. b Fresh weight, c nitrate content, d nitrate reductase activity, and relative expression levels of (e) MdNRT1.1, f MdNRT2.1 and g MdNIA1 of WT and transgenic calli (MdCEPR1-OX and MdCEPR1-anti). Values are mean ± SD of three replicate experiments.
Ectopic expression of MdCEPR1 enhances tolerance to nitrogen deficiency in Arabidopsis thaliana
To investigate whether MdCEPR1 modulates nitrate assimilation in different plant species, MdCEPR1 transgenic Arabidopsis plants were generated after repeated selection through kanamycin resistance assays and PCR detection. Then, the expression levels of MdCEPR1 were measured by qRT-PCR in the transgenic lines, and three independent ectopic transgenic lines (MdCEPR1-L1, L2, and L3) were selected for the subsequent analysis (Supplementary Fig. S3). As shown in Fig. 6a, 10-day-old wild-type (Col) and transgenic Arabidopsis seedlings were grown on vermiculite, and watered with a nutrient solution containing 10 mM NO3− and 0.1 mM NO3− for 3 weeks, respectively. Phenotypic observations showed that the MdCEPR1 overexpressing plants developed dark blue leaves compared with WT under the 10 mM NO3− condition, and had a higher fresh weight (Fig. 6b) and chlorophyll content than the WT under both nitrogen concentrations (Fig. 6c). In addition, the root phenotype of the MdCEPR1 transgenic lines was analyzed under the 0.1 mM NO3− condition. As shown in Figs. S4 and S5, the MdCEPR1 transgenic lines had more root biomass than the WT.
Ectopically expressedMdCEPR1enhances tolerance to nitrogen deficiency in Arabidopsis thaliana. a Phenotypes of 4 weeks old MdCEPR1 transgenic Arabidopsis seedlings (L1, L2, and L3) and the wild-type (Col-0) growing in vermiculite and watered with a nutrient solution containing 10 mM NO3− and 0.1 mM NO3−, respectively. b Fresh weight and c chlorophyll contents of the wild type (Col) and transgenic Arabidopsis (L1, L2 and L3). Values are mean ± SD of three replicate experiments.
Ectopic expression of MdCEPR1 increases nitrate uptake and utilization in Arabidopsis thaliana
Nitrate content and NRA were examined to further analyze the function of MdCEPR1 in nitrate absorption by Arabidopsis. The results show that the MdCEPR1 transgenic plants exhibited a much higher nitrate content and NRA under the two nitrogen concentrations (Fig. 7a, b). Then, 10-day-old transgenic Arabidopsis seedlings were treated with different concentrations of 15NO3− (0.1 mM and 10 mM), and 15NO3− content was determined. The results show that MdCEPR1-overexpressing plants exhibited much higher nitrate uptake activity at both the 0.1 mM and 10 mM 15NO3− external concentrations, and the effect was more pronounced at the lower concentration (Fig. 7c). The expression levels of the genes involved in nitrate uptake and assimilation in WT and transgenic Arabidopsis plants were analyzed by qRT-PCR. The results show that the transcript levels of AtNRT1.1, AtNRT2.1, and AtNIA1 increased remarkably in the transgenic lines (Fig. 7d–f). These results further confirm the function of MdCEPR1 in promoting nitrate uptake and assimilation.
Ectopically expressedMdCEPR1increases nitrate uptake and utilization in Arabidopsis thaliana. a The 15N concentration, b nitrate content, c nitrate reductase activity, and relative expression levels of d AtNRT1.1, e AtNRT2.1, and f AtNIA1 of wild type (Col) and MdCEPR1 transgenic Arabidopsis (L1, L2, and L3). Values are mean ± SD of three replicate experiments.
Ectopic expression of MdCEPR1 increases lateral root density under different nitrate concentrations
Previous studies have demonstrated that root development is regulated by nitrate availability (Zhang and Forde 2000), and that AtCEPR1 plays an important role in lateral root initiation (Roberts et al. 2016). Therefore, we analyzed the root phenotype of MdCEPR1 transgenic Arabidopsis seedlings under different concentrations of nitrate medium (0.1 and 10 mM) to examine the function of MdCEPR1 in root development (Fig. 8a). A detailed analysis of the root showed that overexpressing MdCEPR1 significantly increased the number of lateral roots on Arabidopsis seedlings, but no obvious difference in the length of the primary root was detected when the seedlings were grown in 10 mM nitrate medium. In addition, the MdCEPR1 transgenic lines had a significantly longer primary root than the WT on 0.1 mM nitrate medium, verifying the function of MdCEPR1 to enhance tolerance to a nitrogen deficiency.
Ectopically expressedMdCEPR1increases lateral root density under different nitrate concentrations (10 and 0.1 mM). a The root-growth phenotypes, b primary root (PR) length, and c lateral root (LR) number of wild type (Col) and MdCEPR1 transgenic Arabidopsis seedlings (L1, L2, and L3) growing in a medium with different concentrations of nitrate (10 and 0.1 mM). Values are mean ± SD of three replicate experiments.
Discussion
MdCEPR1 may play an important role in the adaptation to nitrogen-limiting conditions
Nitrogen is one of the most basic elements of organisms and has an irreplaceable role in fruit metabolism, morphogenesis, biochemical processes, stage development, and fruit yield and quality (Miner et al. 1997; Sánchez et al. 2002). However, plants are constantly challenged by different nutrient conditions, and the uneven distribution of nitrogen in the soil always affects plant growth (Tabatabaie et al. 2004). Therefore, plants need to carry out long-distance transduction of nitrogen-sensing signals and promote nitrogen absorption in nitrogen-rich areas. Plant RLKs play an important role sensing environmental changes at the cell surface. Environmental signals are transduced by RLKs via activated signaling pathways to trigger adaptive responses (Kim et al. 2009). Recent studies have provided evidence that HYPERNODULATION ABERRANT ROOT FORMATION1 (HAR1) is involved in the autoregulation and nitrate inhibition of nodulation (Okamoto and Kawaguchi 2015). The CLE-CLAVATA signaling pathway plays key roles in the regulation of systemic auto-regulation of nodulation integrated with nitrogen signaling mechanisms and lateral root development (Araya et al. 2014). CEPR1 is another LRR-RK that affects N uptake and assimilation in Arabidopsis via cell-to-cell communication (Tabata et al. 2014).
Here, we cloned MdCEPR1, the apple homolog of AtCEPR1. The protein sequence alignment analysis revealed that MdCEPR1 had five LRR regions and two conserved regions, including a transmembrane region and a serine/threonine protein kinase catalytic domain. The hydrophobic LRR domain forms a helix that easily interacts with the ligand (Bell et al. 2005). After interacting with the ligand, the signal is transferred from the extracellular to the intracellular space via the intracellular kinase domain, so the MdCEPR1 protein is used as a signaling receptor to transfer the signal from the extracellular to the intracellular space. In this study, MdCEPR1 was expressed in all tissues, including the roots, stems, leaves, flowers, and fruit. A particularly high MdCEPR1 transcript level was observed in the roots and leaves (Fig. 2a). This result, as explained by (Bryan et al. 2012), indicates the MdCEPR1 may play a role in the vasculature of all organs. Moreover, the MdCEPR1-anti calli exhibited much less growth than the control and MdCEPR1-OX under nitrogen-deficient conditions, and had lower nitrate content and NRA compared with the WT. The qRT-PCR results show that the genes related to nitrate uptake, transport and assimilation in MdCEPR1-overexpressing (MdCEPR1-OX) calli were significantly upregulated compared with the WT. These results suggest that MdCEPR1 may play an important role in adaptation to nitrogen-limiting conditions.
Nitrate content was not the only factor affecting anthocyanin accumulation in MdCEPR1
Anthocyanin is an important plant pigment that accumulates in the vacuoles of various cells and tissues. Evidence suggests that low temperature and UV wavelengths induce anthocyanin synthesis (Bryan et al. 2012). Deficiencies in phosphorus or nitrogen can cause anthocyanin to increase (Henry et al. 2012; Ji et al. 2015). In this study, the MdCEPR1-anti calli showed the same phenotype of anthocyanin accumulation, and the anthocyanin-related genes in MdCEPR1-anti were significantly upregulated. Therefore, the accumulation of anthocyanin from MdCEPR1-anti calli may be caused by a nitrogen deficiency. Interestingly, some LRR-RLK mutants also affect anthocyanin accumulation. For example, the sucrose stress-induced anthocyanin accumulation phenotype is inhibited in A. thaliana flg22 or elf18 (Saijo 2010). PXY mutants exhibit a red cotyledon (Fiume and Fletcher 2012). It can be speculated that the effect of MdCEPR1 on the accumulation of anthocyanin may be present in other pathways.
MdCEPR1 affects plant root development as a CEP receptor
Plants absorb soil moisture and nutrients through continuous production of lateral roots. Previous research has shown that lateral root development and nutrient uptake are controlled by local and systemic responses in plants (Michael et al. 2016; Mounier et al. 2013; Ruffel and Coruzzi 2011). The CEP family is a class of long-distance signaling molecules that participates in signal transmission triggered by N-starvation from roots to shoots in Arabidopsis (Tabata et al. 2014). AtCEP1 has been reported to negatively regulate plant root growth (Ohyama et al. 2008). Recent research has shown that CEP genes are induced by limited nitrogen, and CEP family peptides act on the downstream receptor kinase CEP Receptor 1 (CEPR1), which induces upregulation of nitrate transporter genes to compensate for local nitrogen starvation (Tabata et al. 2014). Roberts et al. 2016 showed that CEP5 participates in lateral root initiation locally through XIP1/CEPR1. The present study showed that MdCEPR1 may also affect root development. However, the MdCEP gene in apple has not been reported. Further experiments are needed to demonstrate that MdCEPR1 is also affected by MdCEP during root development.
References
An JP, Liu X, Li HH et al (2017) Apple RING E3 ligase MdMIEL1 inhibits anthocyanin accumulation by ubiquitinating and degrading MdMYB1 protein. Plant Cell Physiol 58(11):1953–1962
An XH, Tian Y, Chen KQ, Liu XJ, Liu DD, Xie XB et al (2015) MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol 56:650–662
Araya T, Miyamoto M, Wibowo J et al (2014) CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proc Natl Acad Sci USA 111:2029–2034
Bell JK, Botos I, Hall PR, Askins J, Shiloach J, Segal DM, Davies DR (2005) The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc Natl Acad Sci USA 102:10976–10980
Bryan AC, Obaidi A, Wierzba M, Tax FE (2012) XYLEM INTERMIXED WITH PHLOEM1, a leucine-rich repeat receptor-like kinase required for stem growth and vascular development in Arabidopsis thaliana. Planta 235:111–122
Cataldo DA, Maroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80
Christiaan G, Milena R, John M, Morten P (2012) Receptor-like kinase complexes in plant innate immunity. Front Plant Sci 3:209
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 10:389–395
Fiume E, Fletcher JC (2012) Regulation of Arabidopsis embryo and endosperm development by the polypeptide signaling molecule CLE8. Plant Cell 24:1000–1012
Freschi L, Rodrigues MA, Tiné MA, Mercier H (2010) Correlation between citric acid and nitrate metabolisms during CAM cycle in the atmospheric bromeliad Tillandsia pohliana. J Plant Physiol 167:1577–1583
Gómez-Gómez L, Boller T (2000) FLS2: a LRR receptor-like kinase involved in recognition of the flagellin elicitor in Arabidopsis. Mol Cell 5(5):1003–1011 1003–1011
Gutiérrez RA, Stokes TL, Thum K, Xu X, Obertello M, Katari MS, Coruzzi GM (2008) Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc Natl Acad Sci USA 105:4939–4944
Hall BG (2013) Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 30:1229
Han YL, Song HX, Liao Q, Yu Y, Jian SF, Lepo JE, Guan CY (2016) Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol 170:1684
Henry A, Chopra S, Clark DG, Lynch JP (2012) Responses to low phosphorus in high and low foliar anthocyanin coleus (Solenostemon scutellarioides) and maize (Zea mays). Funct Plant Biol 39:255–265
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station 347
Ji XH, Wang YT, Zhang R, Wu SJ, An MM, Li M, Chen XS (2015) Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple&nbsp;(Malus sieversii f. niedzwetzkyana). Plant Cell, Tissue and Organ Culture 120:325–337
Kim HS, Jung MS, Lee SM, Kim KE, Byun H, Choi MS, Chung WS (2009) An S-locus receptor-like kinase plays a role as a negative regulator in plant defense responses. Biochem Biophys Res Commun 381:424–428
Lee RB, Purves JV, Ratcliffe RG, Saker LR (1992) Nitrogen assimilation and the control of ammonium and nitrate absorption by maize roots. J Exp Bot 43:1385–1396
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110:213–222
Li HH, Liu X, An JP, Hao YJ, Wang XF, You CX (2017) Cloning and elucidation of the functional role of apple MdLBD13 in anthocyanin biosynthesis and nitrate assimilation. Plant Cell Tissue Organ Cult 130:47–59
Michael T, Nijat I, Djordjevic MA (2016) New role for a CEP peptide and its receptor: complex control of lateral roots. J Exp Bot 67:4797–4799
Miner GS, Poling EB, Carroll DE, Nelson LA, Campbell CR (1997) Influence of fall nitrogen and spring nitrogen—potassium applications on yield and fruit quality of ‘Chandler’ strawberry. J Am Soc Hortic Sci 122:290–295
Mounier E, Pervent M, Ljung K, Gojon A, Nacry P (2013) Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environment 37:162–174
Nimchuk ZL, Tarr PT, Ohno C, Qu X, Meyerowitz EM (2011) Plant stem cell signaling involves ligand-dependent trafficking of the CLAVATA1 receptor kinase. Curr Biol 21:345–352
Ohyama K, Ogawa M, Matsubayashi Y (2008) Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. Plant J 55:152–160
Okamoto S, Kawaguchi M (2015) Shoot HAR1 mediates nitrate inhibition of nodulation in Lotus japonicus. Plant Signaling Behavior 10:e1000138
Pallardy SG (2008) Nitrogen metabolism. Physiology of woody plants. Academic Press, New York, pp 233–254
Roberts I et al (2016) CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. J Exp Bot 67:4889–4899
Ruffel S, Coruzzi GM (2011) Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc Natl Acad Sci USA 108:18524–18529
Saijo Y (2010) ER quality control of immune receptors and regulators in plants. Cellular microbiology 12(6):716–724
Salaj J, Recklinghausen IRV, Hecht V, Vries SCD, Schel JHN, Lammeren AAMV (2008) AtSERK1 expression precedes and coincides with early somatic embryogenesis in Arabidopsis thaliana. Plant Physiol Biochem 46:709–714
Sánchez E, Ruiz JM, Romero L (2002) Proline metabolism in response to nitrogen toxicity in fruit of French Bean plants ( Phaseolus vulgaris L. cv Strike). Plant Growth Regul 36:261–265
Shiu SH, Bleecker AB (2003) Expansion of the receptor-like kinase/pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132:530–543
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16:1220–1234
Shpak ED, Berthiaume CT, Hill EJ, Torii KU (2004) Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131:1491–1501
Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y (2014) Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346:343–346
Tabatabaie SJ, Gregory PJ, Hadley P (2004) Uneven distribution of nutrients in the root zone affects the incidence of blossom end rot and concentration of calcium and potassium in fruits of tomato. Plant Soil 258:169–178
Williams L, Miller A (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu Rev Plant Physiol Plant Mol Biol 52:659–688
You CX, Zhao Q, Wang XF et al (2014) A dsRNA-binding protein MdDRB1 associated with miRNA biogenesis modifies adventitious rooting and tree architecture in apple. Plant Biotechnol J 12(2):183–192
Zhang H, Forde BG (2000) Regulation of Arabidopsis root development by nitrate availability. J Exp Bot 51(342):51–59
Acknowledgements
We thank Ministry of Agriculture (CARS-27), Shandong Province (SDAIT-06-03, J18KA174), Natural Science Foundation of Shandong Province (ZR2011CQ007) and NSFC (31471854, 31601742) for funding to support this work.
Author information
Authors and Affiliations
Contributions
Y-JH, X-FW and RL designed the research. RL, J-PA, and C-XY performed the experiments and analyzed the data. Y-JH, RL and X-FW wrote the manuscript text.
Corresponding authors
Additional information
Coomunicated by Henryk Flachowsky.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11240_2019_1745_MOESM2_ESM.tif
Supplementary material 2—Identifcation of transgenic apple calli. (A) PCR for transgenic apple calli. (B) Relative expression levels of MdCEPR1 in transgenic calli (MdCEPR1-OX and MdCEPR1-anti) and the wild-type control (TIF 637.3 kb)
11240_2019_1745_MOESM3_ESM.tif
Supplementary material 3—Identifcation of MdCEPR1 transgenic Arabidopsis thaliana. (A) PCR for MdCEPR1 transgenic Arabidopsis thaliana. (B) Relative expression levels of MdCEPR1 in wild-type (Col) and MdCEPR1 transgenic Arabidopsis (L1, L2, and L3) (TIF 1058.7 kb)
11240_2019_1745_MOESM4_ESM.tif
Supplementary material 4—Root-growth phenotypes of wild type (Col) and MdCEPR1 transgenic Arabidopsis (L1, L2, and L3) growing in vermiculite and watered with a nutrient solution containing 0.1 mM NO3- (TIF 9256.1 kb)
11240_2019_1745_MOESM5_ESM.tif
Supplementary material 5— Root dry weight of wild-type (Col) and MdCEPR1 transgenic Arabidopsis (L1, L2, and L3) (TIF 109.3 kb)
Rights and permissions
About this article
Cite this article
Li, R., An, JP., You, CX. et al. Molecular cloning and functional characterization of the CEP RECEPTOR 1 gene MdCEPR1 of Apple (Malus × domestica). Plant Cell Tiss Organ Cult 140, 539–550 (2020). https://doi.org/10.1007/s11240-019-01745-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01745-w