Abstract
The plant specific lateral organ boundaries domain (LBD) family of transcription factors (TFs) is involved in many aspects of plant growth and development. In this study, MdLBD13, a nitrate-induced LBD family gene from the apple (Malus × domestica), was isolated and characterized. Overexpression of MdLBD13 repressed anthocyanin biosynthesis by reducing expression of the structural genes associated with the flavonoid pathway. Overexpression ofMdLBD13 also repressed expression of the N–responsive genes that are required for nitrate uptake, transport and assimilation, resulting in reduced nitrate content and nitrate reductase activity in apple calli, as well as in Arabidopsis. Ectopic expression of MdLBD13 also promoted lateral root development in transgenic Arabidopsis. These results demonstrate that MdLBD13acts as a negative regulator in anthocyanin biosynthesis and nitrate utilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is an indispensable nutrient element for plant growth. It is well-known that nitrate (NO3 −) is the main source of nitrogen that is absorbed by plants. Nitrate is taken up by the roots and subsequently transported to the shoots by several NRT family transporters, such as CHL1 (NRT1.1) (Tsay 1993), NRT1.2 (Huang et al. 1999), NRT1.5 (Lin et al. 2008), NRT2.1 (Little et al. 2005), NRT2.2 (Li et al. 2007), NRT2.4 and NRT2.5 (Kiba et al. 2012; Lezhneva et al. 2014), which is subsequently assimilated by the NIAs or the nitrate reductases (NRs).
In addition to being a major nutrient, nitrate also acts as an important signaling molecule that regulates many aspects of plant growth and development, such as seed germination (Alboresiet al. 2005), root architecture (Zhang et al. 1999), shoot development (Scheible et al. 1997), leaf expansion (Walch-Liu et al. 2000), stomatal opening (Guo et al. 2003), flowering (Bernier et al. 1993), and senescence (Crawford and Forde 2002).In recent years, many molecular components that are involved in regulating the nitrate response have been characterized in Arabidopsis. ANR1, a transcription factor of the MADS box family, is a well-characterized component that is involved in nitrate-stimulated lateral root elongation (Gan et al. 2005). Nin-like proteins (NLPs) bind the nitrate-responsive cis-element (NRE) and activate the expression of nitrate–responsive genes (Konishi and Yanagisawa 2013). In Arabidopsis, three LBD transcription factors, AtLBD37/38/39, have been reported as negative regulators in the nitrate-response (Rubin et al. 2009). In recent years, SPL9, TGA1/TGA4, AFB3, NAC4 and NRG2 have been identified as important nitrate regulators involved in nitrate signaling (Krouk et al. 2010; Vidal et al. 2010, 2013; Alvarez et al. 2014; Xu et al. 2016).However, additional molecular components that are associated with N/nitrate signaling transduction need to be further explored.
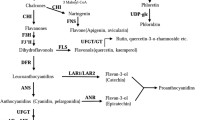
In addition to plant growth and development, nitrate also affected many secondary metabolic pathways (e.g., anthocyanin accumulation) (Scheible et al. 2004). Anthocyanins not only provide color to fruits and flowers but are also important antioxidants for both plant growth and human health (Nagata et al. 2003). The accumulation of anthocyanin is stimulated by multiple environmental factors, such as high sugar, light, low temperature, drought, phytohormones, and phosphate and nitrate depletion (Nakabayashi and Saito et al. 2015; Ji et al. 2015). The biosynthesis of anthocyanin occurs via the flavonoid pathway, which is regulated by a series of structural genes involved in anthocyanin biosynthesis (F3H, DFR, CHS, CHI, UFGT, etc.) that are, in turn, regulated by the MBW complex (R2R3 MYB TFs, basic helix–loop–helix TFs and WD40 proteins) (Ramsay and Glover 2005).
The research on MBW complex-mediated anthocyanin synthesis has covered many plant species, such as Arabidopsis, petunia, apple, maize, snapdragon, and tomato (Allan et al. 2008; Li 2014; Albert et al. 2011). MdMYB1, a R2R3 MYB TF, which is an allele of MdMYB10 and MdMYBA, has been characterized as a key regulatory gene for anthocyanin accumulation (Ban et al. 2007; Espley et al. 2007). MdMYB1 is degraded by MdCOP1 via the 26S proteasome pathway to influence anthocyanin accumulation (Li et al. 2012). MdMYB1 also interacts with MdbHLH3 to activate the expression of structural genes in anthocyanin biosynthesis leading to anthocyanin accumulation (Espley et al. 2007; Xie et al. 2012). Even so, several other regulators upstream of the MBW complex remain uncharacterized.
LBD genes encode zinc-finger DNA binding transcription factors, which are divided into two classes according to the structure of the LOB domain at their N termini (Matsumura et al. 2009). In Arabidopsis and apple, 43 and 58 members, respectively, of the LBD gene family have been identified (Shuai et al. 2002; Wang et al. 2013). Recently, several LBD genes involved in growth and development in different plants have been functionally characterized (Majer and Hochholdinger 2011; Porco et al. 2016). AtLBD37/38/39 are strongly induced by nitrate and negatively regulate anthocyanin synthesis (Rubin et al. 2009), indicating that LBD genes play an important role in plant secondary metabolism.
In this study, MdLBD13, a nitrate-induced LBD gene was identified. MdLBD13 mediated a repressive effect on the nitrate response in transgenic apple calli and Arabidopsis. It was also observed that MdLBD13 repressed anthocyanin biosynthesis by down-regulating the expression of anthocyanin biosynthesis-related genes, resulting in reduced anthocyanin accumulation.
Materials and methods
Plant materials and growth conditions
The ‘Royal Gala’ (Malus × domestica ‘Gala’) cultivars were grown on Murashige and Skoog (MS) medium with 0.1 mg L−1 of gibberellins, 0.5 mg L−1 of 6-benzylaminopurine (6-BA) and 0.2 mg L−1of 1-naphthaleneacetic acid (NAA) at 25 °C.The tissue cultures were subcultured at monthly intervals under a long-day photoperiod (16-h-light/8-h-dark). The ‘Orin’ calli were grown on MS solid medium containing 1.5 mg L−1of 2, 4-dichlorophenoxy (2, 4-D) and 0.5 mg L−1 of 6-BA in the dark and were subcultured at 15–20 days intervals at 25 °C. The Arabidopsis was germinated and grown on MS medium at 22 °C with a 16-h-light/8-h-dark photoperiod.
The root, stem, flower, leaf and fruit of self-rooting ‘Gala’ apple seedlings were used to analyze tissue expression. One-month-old ‘Gala’ apple seedlings were treated with 5 mM KNO3 and 5 mM KCl for expression analysis. The ‘Orin’ apple calli and Arabidopsis were cultured in media with 5 mM KNO3 to examine anthocyanin accumulation and nitrate content.
Sequence alignment and phylogenetic tree analysis
The LBD protein sequences were input into the software DNAMAN, and a graphics file was obtained as output. The phylogenetic tree of LBD proteins was obtained by the neighbor-joining method using the MEGA5 program (http://www.megasoftware.net/). A graphical representation of the phylogenetic tree for LBD proteins was obtained using MEGA5 (Tamura et al. 2011).
RNA extraction, reverse transcription and RT-qPCR assays
The total RNAs of ‘Gala’ cultivars were extracted using the RNA plant reagent (Tiangen, China). The total RNAs of apple calli and Arabidopsis were extracted using Trizol reagent (Invitrogen, USA). The RNA (1–7 μg) was reverse transcribed using the PrimeScript first-strand cDNA synthesis kit (Takara, China).
The real-time quantitative polymerase chain reaction (RT-qPCR) assays were performed with 2 × UltraSYBR mixture (10 μL), forward primers (1.0 μL), reverse primers (1.0 μL), cDNA (1.0 μL) and ddH2O (7.0 μL). The RT-qPCR was performed under the following conditions: 95 °C for 15 s, 56 °C for 15 s, and 65 °C for 10 s consisted of 40 cycles. Each reaction was performed thrice. The MdACTIN and AtACTIN genes were used as controls. The 2−∆∆CT method was used for data analysis. The primers used in this study are listed in supplementary Table 1.
Construction of overexpression vector of MdLBD13 and genetic transformation
The open reading frame (ORF) of MdLBD13 is 753 bp long. The PCR products were digested with BamHI/SalI and then introduced into the pCAMBIA 1300 plant expression vector downstream of the 35S cauliflower mosaic virus (CaMV) promoter. Next, the plasmid construct was transformed into Agrobacterium tumefaciens LBA4404 using electroporation.
The transgenic apple calli were obtained using the Agrobacterium-mediated method described by An et al. (2015). The wild-type (WT) Arabidopsis (Columbia) was transformed by Agrobacterium using the floral-dipping method (Clough and Bent 1998). Transgenic seedlings were selected on MS medium containing 500 mg L−1 hygromycin. The T3 homozygous seeds were used for all subsequent experiments.
Yeast two-hybrid assays
Yeast (Saccharomyces cerevisiae) two-hybrid assays were performed according to the manufacturer’s instructions (Clontech, USA). The full-length cDNAs of MdLBD13, MdMYB1, MdMYB9, MdMYB11, MdbHLH3 and MdbHLH33 were inserted into pGAD424 and pGBT9 vectors (Clontech). All of the plasmids were co-transformed into the yeast strain Y2H Gold using the lithium acetate method. The cells were cultured on medium lacking Trp and Leu (SD/-Trp-Leu) at 28 °C for 2days. Subsequently, putative transformants were transferred to a medium lacking Trp, Leu, His and Ade (SD/-Leu-Trp-His/-Ade) with or without 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside to detect their interactions.
Measurement of the total anthocyanin content
The total anthocyaninin apple calli and Arabidopsis was extracted by the methanol-HCl method (Lee and Wicker 1991). Approximately 0.5 g samples were soaked and incubated in 5 mL of 1%(v/v) methanol-HCl in the dark for 24 h at room temperature. Subsequently, the absorbance of the extracts was measured at 530, 620 and 650 nm using a spectrophotometer (UV-1600, Shimadzu). The following formula was used to quantify the anthocyanin content: OD = (A530–A620) – 0.1 (OD650–OD620) (Lee and Wicker 1991). These experiments were performed at least thrice.
Measurement of the nitrate content
The nitrate content was measured using the salicylic acid method (Cataldo et al. 2008; Vendrell and Zupancic 1990). First, approximately 1 g of the samples were frozen in liquid nitrogen and milled into powder. Afterwards, 10 mL of deionized water was added to the tubes. The samples were boiled at 100 °C for 20 min, centrifuged at 15,000 g for 10 min, and 0.1 mL of the supernatant was transferred into a new tube. Next, 0.4 mL of 5% salicylic acid-sulfuric acid solution was added to the tubes. The reactions were allowed to proceed at room temperature for 20 min. Subsequently, 9.5 mL of 8% NaOH solution was added slowly to the tubes. After cooling to room temperature, absorbance values were measured at 410 nm; deionized water was used as the control to measure the OD410 values. The nitrate content was calculated using the following equation: N = C·V/W (N, nitrate content; C, nitrate concentration calculated using OD410 in the regression equation; V, total volume of the extracted sample; W, weight of the sample). Known concentrations of KNO3 (10 to 120 mg L−1) were used to make a standard curve. The regression equation was determined based on the standard curve.
Measurement of nitrate reductase activity
The nitrate reductase activity was measured as described in Freschi et al. (2010). Samples (0.5 g) were put into test tubes after washing with distilled water and weighed; 1 mL trichloroacetic acid was used as the control. Next, 9 ml of 0.1 M phosphate buffer (pH7.5) with 3% propanol and 0.1 M KNO3 was added to the tubes, and the samples were vacuum infiltrated until the samples sank to the bottom of the tubes. The reactions were allowed to proceed at 30 °C in the dark for 30 min, and 1 mL trichloroacetic acid was added to stop the reactions. After 2 min of incubation, 4 mL sulphanilamide with 3 M HCl and 4 mL of 0.2% N-(1-naphthyl) ethylenediamine was added to the supernatants (2 mL), which had been transferred to new tubes. Finally, the absorbance was determined at 540 nm after 30 min. NaNO2 (0–2 g per reaction) was used to make a standard curve. The regression equation was calculated based on the standard curve. The nitrate reductase activity was expressed as the amount of nitrite produced per hour per gram of fresh weight (nmol nitriteh−1 g−1FW).
Results
Phylogenetic tree analysis and protein structure alignment of the MdLBD13 protein from different species
In a previous study, members of the AtLBD gene family were identified as playing an important role in nitrate utilization and anthocyanin biosynthesis (Rubin et al. 2009). To identify the apple homolog of AtLBD37/38 that is involved in the nitrate signaling, genome-wide analysis was performed, and MdLBD13 (MDP0000317227), which exhibited high similarity to AtLBD37/38, was identified. Next, phylogenetic analysis was carried out to determine the relationship between MdLBD13 and LBD proteins from other plant species. The results showed that MdLBD13 and AtLBD37/38 were highly homologous (Fig. 1a). The protein structure analysis of LBDs showed that MdLBD13 and LBD proteins from other species contained a highly conserved CX2CX6CX3C zinc-finger domain at their N-termini (Fig. 1b). These results indicated that MdLBD13 belonged to the class II LBD family of TFs and was homologous to Arabidopsis LBD37/38.
a Phylogenetic tree of MdLBD13 and other LBDs from different species. The tree was drawn with MEGA5.0 using the neighbor-joining method. b Comparison of the putative MdLBD13 protein sequence with other LBDs. There is a highly conserved CX2CX6CX3C zinc finger-like motif in the LOB domain at the N-terminus of the MdLBD13 protein. MdLBD13: Malus × domestica MDP0000317227; AtLBD37/38/39: Arabidopsis thaliana AT5G67420, AT3G49940, AT4G37540; RcLBD37: Ricinus communis XP_002525255.1; TcLBD38: Theobroma cacao XP_007047485.1; PtLBD39-1/2: Populus trichocarpa XP_006380633.1, XP_002306497.1; VaLBD37: Vigna angularis XP_017421901.1; CcLBD38: Cajanus cajan KYP52401.1; GmLBD37/38: Glycine max XP_003517339.1, XP_003539286.1; CsLBD38: Citrus sinensis XP_006466482.1; ZjLBD38: Ziziphus jujuba XP_015890312.1; CsLBD37: Cucumis sativus XP_004141875.1; PbLBD37: Pyrus × bretschneideri XP_009379314.1; VvLBD38: Vitis vinifera XP_002284296.1; NtLBD37-1/2: Nicotiana tabacum XP_016476789.1, XP_016476601.1
Expression pattern of MdLBD13
To elucidate the function of MdLBD13 in planta, the expression patterns of MdLBD13 were analyzed by RT-qPCR. The results showed that the transcripts of MdLBD13 were detected in all organs in the apple with the highest expression levels in the stems (Fig. 2a). RT-qPCR was also carried out to determine whether MdLBD13 was responsive to nitrate. The results showed that compared with the control, transcripts of MdLBD13 were remarkably increased (approximately 8.5-fold) after treatment with 5 mM KNO3 for 4 h in the root (Fig. 2b). A similar expression pattern was also found in the shoot with highest levels of the transcript found at 32 h after nitrate treatment (Fig. 2c).Statistical analysis indicates that the transcripts of MdLBD13 were significantly regulated by KNO3, while there was no significant difference in MdLBD13transcript levels with KCl treatment (Fig. 2b, c). These results demonstrated that nitrate, and not potassium, induced the expression of MdLBD13. Meanwhile, the highest expression of MdLBD13 after nitrate treatment was found at 32 h in the shoots, which was longer than that in the roots. This may be due to the time lag in the transport of nitrates from the roots to the shoot.
Expression patterns of MdLBD13. a The expression levels of MdLBD13 in different organs (roots, stems, flowers, leaves and fruits). The value in fruits was set to 1. b and c Expression levels of MdLBD13 at different periods in the root and shoot after the addition of 5 mM KNO3 and 5 mM KCl to N-limited apple seedlings. Expression levels of MdLBD13 were determined by RT-qPCR, and the data were analyzed by the 2−∆∆CT method
MdLBD13 inhibits anthocyanin accumulation by regulating gene expression in apple calli
To examine the role of MdLBD13 in anthocyanin accumulation, the expression construct 35S::MdLBD13 was obtained. Next, it was transformed into the ‘Orin’ apple calli by Agrobacterium-mediated genetic transformation. Phenotypically, the wild-type control appeared redder than the 35S::MdLBD13 transgenic calli, and the35S::MdLBD13transgenic calli accumulated lower levels of anthocyanin than the WT control (Fig. 3a–c). These results indicated that MdLBD13 inhibited anthocyanin accumulation in apple calli.
Overexpression of MdLBD13 inhibits anthocyanin accumulation in apple calli. a Anthocyanin accumulation in 35S::MdLBD13 and WT apple calli. b and c The color-based and spectrophotometric analysis of anthocyanin content. d The anthocyanin biosynthesis pathway. e RT-qPCR experiments analyzed the expression of genes related to anthocyanin biosynthesis in apple (MdMYB1, MdMYB9, MdMYB11, MdbHLH3, MdbHLH33, MdCHS, MdCHI, MdF3H, MdDFR1, MdANR1 and MdUFGT). The results are expressed as the means ± SD (standard deviation) from three independent experiments. The data were analyzed using the 2−∆∆CT method
As is well-known, MdMYB1, MdMYB9/11 and MdbHLH3/33 are crucial transcription factors that regulate anthocyanin accumulation in apple (Takos et al. 2006; Xie et al. 2012; An et al. 2015). To test whether MdLBD13 regulated anthocyanin biosynthesis by interacting with MdMYB or MdbHLH proteins, yeast two-hybrid assays were performed. The full-length cDNAs of MdMYB and MdbHLH genes were cloned into the pGAD vector (fused with the sequence of the GAL4-DNA activation domain), while theMdLBD13 gene was cloned into pGBD (fused with the sequence of the DNA binding domain). The two-hybrid assay results showed that yeast cells cotransfected with pGBD-MdLBD13 and either pGAD-MdMYBs or pGAD-MdbHLHs were unable to grow on SD/-Trp-Leu-His-Ade selection plates. However, positive β-gal activity was observed in yeast containing pGAD-MdMYB1 and pGBD-MdbHLH3, which served as the positive control (Figure. S1). These results showed that MdLBD13 did not interact with the anthocyanin-related TFs.
It is well-known that the anthocyanin biosynthesis pathway is regulated by several enzymes (Fig. 3d). Furthermore, the expression levels of MdMYBs, MdbHLHs and the flavonoid structural genes (MdCHS, MdCHI, MdF3H, MdDFR, MdANR1, and MdUFGT) were analyzed by RT-qPCR. The expression of MdMYBs, MdbHLHs and anthocyanin-related genes was down-regulated in 35S::MdLBD13 transgenic calli (Fig. 3e) indicating that MdLBD13 acted a negative regulator in the regulation of anthocyanin biosynthesis. Therefore, MdLBD13 inhibited the accumulation of anthocyanin by repressing the expression of MdMYBs, MdbHLHs and anthocyanin biosynthesis-related structural genes.
MdLBD13 represses the absorption and assimilation of nitrate in apple calli
The nitrate content and nitrate reductase activity (NRA) were then examined in 35::MdLBD13 and WT apple calli. Both nitrate content and NRA were significantly reduced in the 35::MdLBD13transgenic calli compared to the WT control (Fig. 4a, b). The expression of genes involved in nitrate uptake, transport and assimilation were then analyzed. The results showed that transcript levels of all nitrate-related genes were repressed compared with the WT control (Fig. 4c). For instance, the expression levels of MdNRT1.1, MdNRT1.7, and MdNIA2 were down-regulated approximately 0.6-fold, the expression levels of MdNRT2.1, MdNRT2.5, MdNRT2.7 and MdNIA1 were down-regulated approximately 0.4-fold and the expression level of MdNRT2.4 was down-regulated approximately 0.8-fold. These results supported the hypothesis that MdLBD13 reduced nitrate utilization by repressing the expression of genes that are responsible for nitrate uptake and assimilation.
Overexpression of MdLBD13 reduces the nitrate content and NRA in apple calli. a and b The nitrate content and NRA of the 35S::MdLBD13 and WT apple calli cultured with 5 mM KNO3. c MdLBD13 affected expression of the key genes (MdNRT1.1, MdNRT1.7, MdNRT2.1, MdNRT2.4, MdNRT2.5, MdNRT2.7, MdNIA1 and MdNIA2) that are responsible for nitrate uptake and assimilation. The results are expressed as the means ± SD from three independent experiments. The data were analyzed using the 2−∆∆CT method
Ectopic expression of MdLBD13 represses anthocyanin accumulation as well as nitrate uptake and utilization in transgenic Arabidopsis
To characterize the function of MdLBD13 in planta, three independent MdLBD13 transgenic Arabidopsis lines (35::MdLBD13-1/3/8) were obtained. RT-qPCR analysis showed that the expression of MdLBD13 was much higher in the transgenic lines than that in the WT control (Col) (Fig. 5a). To determine whether the ectopic expression of MdLBD13 affects anthocyanin accumulation, the three transgenic lines and the WT control were treated with 5 mM KNO3. Analysis of the pigmentation and spectrophotometric analysis showed that the transgenic seedlings accumulated less anthocyanin than the WT seedlings (Fig. 5b–d). The expression levels of AtPAP1 and other anthocyanin-related genes including AtCHS, AtCHI, AtDFR, and AtUFGT were then examined with RT-qPCR. As shown in Fig. 5e, AtPAP1 was down-regulated approximately 0.2-fold, and the other genes were all significantly repressed in the transgenic lines. These results indicated that overexpression of MdLBD13 either directly down-regulated the expression of anthocyanin-related structural genes or down-regulated their expression by regulating the expression of AtPAP1, which directly binds to the promoters of the structural genes in anthocyanin biosynthesis. These findings demonstrated that MdLBD13 acted as a negative regulator of anthocyanin biosynthesis in apple calli and Arabidopsis.
Ectopic expression of MdLBD13 represses anthocyanin accumulation in Arabidopsis. a Three independent transgenic Arabidopsis lines were examined by RT-qPCR. b Phenotypes of transgenic Arabidopsis and WT (Col) grown in 5mM KNO3 with UV irradiation. c and d The colors and anthocyanin content of the transgenic Arabidopsis lines and WT shown in b were analyzed. e Ectopic expression of MdLBD13 affected the expression of the anthocyanin biosynthesis-related genes (AtPAP1, AtCHS, AtCHI, AtDFR1 and AtUFGT). The results are expressed as the means ± SD from three independent experiments. The data were analyzed using the 2−∆∆CT method
Subsequently, nitrate content and NRA were examined in the WT and transgenic Arabidopsis. The results showed that nitrate content and NRA were noticeably reduced in the transgenic lines compared to the WT control (Fig. 6a, b). RT-qPCR assays were performed to check the expression levels of the genes involved in nitrate uptake and assimilation. The results showed that the transcript levels ofAtNRT1.1, AtNRT1.7, AtNRT2.1 and AtNIA1/2 were remarkably repressed in the transgenic lines and especially in the line 35S::MdLBD13-3 (Fig. 6c), indicating that these genes were directly or indirectly regulated by MdLBD13.Therefore, it was concluded that MdLBD13 repressed nitrate uptake and assimilation when it was ectopically expressed in Arabidopsis. This result is again consistent with the notion that the nitrate responsive genes are repressed by MdLBD13 in the nitrate signaling pathway.
Ectopic expression of MdLBD13 decreased nitrate content and NRA in transgenic Arabidopsis. a and b Nitrate and NRA were significantly reduced under normal conditions. c MdLBD13 regulated the expression levels of nitrate-responsive genes. The results are expressed as the means ± SD from three independent experiments. The data were analyzed using the 2−∆∆CT method
Ectopic expression of MdLBD13 promotes lateral root development
As is well-known, nitrate plays an important role in root development (Zhang and Forde 2000). To examine whether MdLBD13 influenced the development of roots, the seeds of transgenic and WT Arabidopsis were sown in MS medium and left to germinate for 2 days. The young seedlings were then transferred to new MS medium and left to grow for another 10 days; subsequently, primary root length and lateral root number were determined (Fig. 7a). The results showed that the 35S::MdLBD13 transgenic seedlings generated more lateral roots than the WT control, although they had similar primary root length as the WT control (Fig. 7b, c). Therefore, MdLBD13 promotes the development of lateral, but not primary roots in Arabidopsis.
Ectopic expression of MdLBD13 promotes lateral root development in transgenic Arabidopsis. a Root growth of three transgenic Arabidopsis lines and WT under normal conditions. b and c Primary root length and lateral root numbers in transgenic Arabidopsis and WT seedlings. The results are expressed as the means ± SD from three different seedlings
Discussion
As the major nitrogen source and a key signaling molecule, nitrate plays an essential role in plant growth, development and metabolism (Crawford and Forde 2002). In recent years, several studies have shown that LBD TFs are involved in plant growth and development in many species (Husbands et al. 2007; Majer and Hochholdinger 2011). Members of the LBD gene family from different plants have been characterized as key regulators of reproductive growth (Bortiri et al. 2006), leaf development (Shuai et al. 2002; Sun et al. 2010), husk development (Li et al. 2008) and lateral root formation (Zhu et al. 2016; Porco et al. 2016). However, more work needs to be done on the functional characterization of LBD genes, especially in apple and other woody plants.
In this study, MdLBD13, a nitrate responsive LBD gene was isolated. The pigmentation phenotypes observed in transgenic apple calli and Arabidopsis prompted us to perform further analysis of anthocyanin accumulation at the metabolite and transcript levels. MYB TFs have been reported to act as key regulators in anthocyanin biosynthesis in different species (Borevitz et al. 2000; Cone et al. 1993; Schwinn et al. 2006). In apple, MdMYB and MdbHLH proteins have been characterized as essential regulators of anthocyanin accumulation (Takos et al. 2006; Ban et al. 2007). The MYB proteins can interact with bHLH proteins to activate the expression of structural genes in anthocyanin biosynthesis (Liu et al. 2013). In a previous study, the expression of AtPAP1 and AtPAP2 is strongly repressed in response to nitrate (Scheible et al. 2004). MdLBD13 did not interact with anthocyanin-related TFs in yeast two hybrid assays. However, the expression of MYBs and bHLHs was repressed in MdLBD13 transgenic apple calli and Arabidopsis suggesting that MdLBD13 is a transcription repressor that acts upstream of MdMYBs and MdbHLHs. Whether MdLBD13 can directly bind to the promoter regions of MdMYBs and MdbHLHs needs to be explored in future work.
Alteration of many secondary metabolism pathways is part of the main response to the presence of nitrate. That MdLBD13 was rapidly induced by nitrate, inhibited anthocyanin biosynthesis and strongly repressed the expression of MYB and bHLH genes lead to the conclusion that MdLBD13 acts as an important regulator component in anthocyanin biosynthesis and the nitrate signaling pathway. In Arabidopsis, transcriptome profiling indicates that AtLBD37/38/39 play a profound role in anthocyanin accumulation, as well as nitrate uptake and assimilation (Rubin et al. 2009). In this study, it was also found that besides anthocyanin biosynthesis and nitrate utilization, MdLBD13 also regulated lateral root development in transgenic Arabidopsis suggesting an important signaling role for MdLBD13 in nitrate-mediated root growth and development. It is well-known that lateral root development is regulated by multiple hormones, such as the auxins, cytokinins (Peret et al. 2009) and strigolactones (Kapulnik et al. 2011). A previous study has shown that cytokinins accumulate in Arabidopsis roots after nitrate replenishment (Takei et al. 2002). It is interesting to speculate whether MdLBD13 affects root development by regulating the biosynthesis or the signaling pathway of these hormones.
In summary, MdLBD13 from apple was identified as a member of the LBD family. This gene functions as a regulator in anthocyanin synthesis and nitrate uptake/assimilation and thereby acts as a key molecular component in plant nitrate signaling.
Abbreviations
- LBD:
-
Lateral organ boundaries domain
- MBW:
-
R2R3 MYB TFS, basic helix–loop–helix TFS and WD40 proteins
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- MYB1:
-
MYB domain protein 1
- PAP1/2:
-
Production of anthocyanins pigment 1/2
- CHS:
-
Chalcone synthase (CHS; EC 2.3.1.74)
- CHI:
-
Chalcone flavanone isomerase (CHI; EC 5.5.1.6)
- F3H:
-
Flavanone 3-hydroxylase (F3H, EC 1.14.11.9)
- DFR:
-
Dihydroflavonol 4-reductase (DFR, EC 1.1.1.219)
- ANR:
-
Anthocyanidin reductase (ANR, EC 1.3.1.77)
- UFGT:
-
UDP-glucose:flavonoid 3-O-glucosyltransferase (UFGT; EC 2.4.1.9 1)
- NRT:
-
Nitrate response transporter
- NIA1/2 :
-
Nitrate reductase1/2
- NR:
-
Nitrate reductase (NR, EC 1.7.1.1)
- NRA:
-
Nitrate reductase activity
- WT:
-
Wild type
References
Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM (2011) Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J 65:771–784
Alboresi A, Gestin C, Leydecker MT, Bedu M, Meyer C, Truong HN (2005) Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ 28:500–512
Allan AC, Hellens RP, Laing WA (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13:99–102
Alvarez JM, Riveras E, Vidal EA, Gras DE, Contreras-López O, Tamayo KP, Aceituno F, Gómez I, Ruffel S, Lejay L, Jordana X, Gutiérrez RA (2014) Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J 80:1–13
An XH, Tian Y, Chen KQ, Liu XJ, Liu DD, Xie XB, Cheng CG, Cong PH, Hao YJ (2015) MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol 56(4):650–662
Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48:958–970
Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P (1993) Physiological signals that induce flowering. Plant Cell 5:1147–1155
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12:2383–2394
Bortiri E, Chuck G, Vollbrecht E, Rocheford T, Martienssen R, Hake S (2006) ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18:574–585
Cataldo DA, Maroon M, Schrader LE, Youngs VL (2008) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6(1):71–80
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Cone KC, Cocciolone SM, Burr FA, Burr B (1993) Maize anthocyanins regulatory geneplis a duplicate of c1 that functions in the plant. Plant Cell 5:1795–1805
Crawford NM, Forde BG (2002) Molecular and developmental biology of inorganic nitrogen nutrition. In: Somerville CR, Meyerowitz EM (ed) The Arabidopsis book, American Society of Plant Biologists, Rockville, http://www.aspb.org/publications/Arabidopsis/
Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC (2007) Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49(3):414–427
Freschi L, Rodrigues MA, Tiné MAS, Mercier H (2010) Correlation between citric acid and nitrate metabolisms during CAM cycle in the atmospheric bromeliad Tillandsia pohliana. J Plant Physiol 167:1577–1583
Gan Y, Filleur S, Rahman A, Gotensparre S, Forde BG (2005) Nutritional regulation of ANR1 and other root-expressed MADS-box genes in Arabidopsis thaliana. Planta 222:730–742
Guo FQ, Young J, Crawford NM (2003) The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell 15:107–117
Huang NC, Liu KH, Lo HJ, Tsay YF (1999) Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. The Plant Cell 11(8):1381–1392
Husbands A, Bell EM, Shuai B, Smith HM, Springer PS (2007) LATERAL OGRAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res, 35:6663–6671
Ji XH, Wang YT, Zhang R, Wu SJ, An MM, Li M, Wang CZ, Chen XL, Zhang YM, Chen XS (2015) Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult 120(1):325–337
Kapulnik Y, Delaux PM, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Beeckman T (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233(1):209–216
Kiba T, Feria-Bourrellier AB, Lafouge F, Lezhneva L, Boutet-Mercey S, Orsel M, Brehaut V, Miller A, Daniel-Vedele F, Sakakibara H, Krapp A (2012) The Arabidopsis nitrate transporter NRT2. 4 plays a double role in roots and shoots of nitrogen-starved plants. The Plant Cell 24(1):245–258
Konishi M, Yanagisawa S (2013) Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat Commun 4:1617
Krouk G, Mirowski P, LeCun Y, Shasha DE, Coruzzi GM (2010) Predictive network modeling of the high-resolution dynamic plant transcriptome in response to nitrate. Genome Biol 11:R123
Lee HS, Wicker L (1991) Anthocyanins pigments in the skin of lychee fruit. J Food Sci 56:466–468
Lezhneva L, Kiba T, Feria-Bourrellier AB, Lafouge F, Boutet-Mercey S, Zoufan P, Sakakibara H, Daniel-Vedele F, Krapp A (2014) The Arabidopsis nitrate transporter NRT2. 5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J 80(2):230–241
Li S (2014) Transcriptional control of flavonoid biosynthesis: Fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant signal behav 9(1), e27522.
Li W, Wang Y, Okamoto M, Crawford NM, Siddiqi MY, Glass AD (2007) Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol 143:425–433
Li A, Zhang Y, Wu X, Tang W, Wu R, Dai Z, Liu G, Zhang H, Wu C, Chen G (2008) DH1, a LOB domain-like protein required for glume formation in rice. Plant Mol Biol, 66:491–502
Li YY, Mao K, Zhao C, Zhao XY, Zhang HL, Shu HR, Hao YJ (2012) MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light induced anthocyanins biosynthesis and red fruit coloration in apple. Plant Physiol 160:1011–1022
Lin SH, Kuo HF, Canivenc G, Lin CS, Lepetit M, Hsu PK, Tillard P, Lin HL, Wang YY, Tsai CB, Gojon A, Tasy YF (2008) Mutation of the Arabidopsis NRT1. 5 nitrate transporter causes defective root-to-shoot nitrate transport. The Plant Cell 20(9):2514–2528
Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA 102:13693–13698
Liu XF, Yin XR, Allan AC, Lin-Wang K, Shi YN, Huang YJ, Ferguson IB, Xu CJ, Chen KS (2013) The role of MrbHLH1 and MrMYB1 in regulating anthocyanin biosynthetic genes in tobacco and Chinese bayberry (Myrica rubra) during anthocyanin biosynthesis. Plant Cell Tissue Organ Cult 115(3):285–298
Majer C, Hochholdinger F (2011) Defining the boundaries: structure and function of LOB domain proteins. Trends Plant Sci 16:47–52
Matsumura Y, Iwakawa H, Machida Y, Machida C (2009) Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J 58:525–537
Nagata T, Todoriki S, Masumizu T, Suda I, Furuta S, Du Z, Kikuchi S (2003) Levels of active oxygen species are controlled by ascorbic acid and anthocyanins in Arabidopsis. J Agric Food Chem 51:2992–2999
Nakabayashi R, Saito K (2015) Integrated metabolomics for abiotic stress responses in plants. Curr opin plant biol 24:10–16
Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Bennett MJ (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14(7):399–408
Porco S, Larrieu A, Du Y, Gaudinier A, Goh T, Swarup K, Swarup R, Kuempers B, Bishopp A, Lavenus J, Casimiro I, Hill K, Benkova E, Fukaki H, Brady SM, Scheres B, Péret B, Bennett MJ (2016) Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulation of auxin influx carrier LAX3. Development 143(18):3340–3349
Ramsay NA, Glover BJ (2005) MYB–bHLH–WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci 10(2):63–70
Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR (2009) Members of the LBD family of transcription factors repress anthocyanins synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 21:3567–3584
Scheible WR, Lauerer M, Schulze ED, Caboche M, Stitt M (1997) Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J 11:671–691
Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499
Schwinn K, Venail J, Shang Y, Mackay S, Alm V, Butelli E, Oyama R, Bailey P, Davies K, Martin C (2006) A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. Plant Cell 18(4):831–851
Shuai B, Reynaga-Peña CG, Springer PS (2002) The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol 129(2):747–761
Sun XD, Meng LS, Feng ZH, Zhu J (2010) ASYMMETRIC LEAVES2-LIKE11 gene, a member of the AS2/LOB family of Arabidopsis, causes pleiotropic alteration in transgenic cockscomb (Celosia cristata). Plant Cell Tissue Organ Cult (PCTOC), 101(2):193–200
Takei K, Takahashi T, Sugiyama T, Yamaya T, Sakakibara H (2002) Multiple routes communicating nitrogen availability from roots to shoots: A signal transduction pathway mediated by cytokinin. J Exp Bot 53:971–977
Takos AM, Jaffe FW, Jacob SR, Bogs J, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142(3):1216–1232
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tsay Y (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72(5):705–713
Vendrell PF, Zupancic J (1990) Determination of soil nitrate by transnitration of salicylic acid. Commun. Soil. Sci. Plant Anal 21:1705–1713.
Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, Gutiérrez RA (2010) Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci USA 107:4477–4482
Vidal EA, Moyano TC, Riveras E, Contreras-López O, Gutiérrez RA (2013) Systems approaches map regulatory networks downstream of the auxin receptor AFB3 in the nitrate response of Arabidopsis thaliana roots. Proc Natl Acad Sci USA 110:12840–12845
Walch-Liu P, Neumann G, Bangerth F, Engels C (2000) Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J Exp Bot 51:227–237
Wang XF, Zhang SZ, Su L, Liu X, Hao YJ (2013) A genome-wide analysis of the LBD (LATERAL ORGAN BOUNDARIES domain) gene family in Malus domestica with a functional characterization of MdLBD11. PloS ONE 8(2):e57044
Xie XB, Li S, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, You CX, Zhang XS, Hao YJ (2012) The bHLH transcription factor MdbHLH3 promotes anthocyanins accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ 35:1884–1897
Xu N, Wang R, Zhao L, Zhang C, Li Z, Lei Z, Wang Y (2016) The Arabidopsis NRG2 protein mediates nitrate signaling and interacts with and regulates key nitrate regulators. Plant Cell 28(2):485–504
Zhang H, Forde BG (2000) Regulation of Arabidopsis root development by nitrate availability. J Exp Bot 51(342):51–59
Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci 96(11):6529–6534
Zhu L, Zheng C, Liu R, Song A, Zhang Z, Xin J, Jiang J, Chen S, Zhang F, Fang W, Chen F (2016) Chrysanthemum transcription factor CmLBD1 direct lateral root formation in Arabidopsis thaliana. Sci Rep. doi:10.1038/srep20009
Acknowledgements
This work was supported by grants from NSFC (31325024, 31601742 and 31272142), Ministry of Education of China (IRT15R42) and Shandong Province (SDAIT-06-03).
Author contributions
Chun-Xiang You and Xiao-Fei Wang designed the experiments. Hao-Hao Li and Xin-Liu carried out the experiments and analyzed the data. Jian-Ping An carried out analysis of the content of nitrate and NRA. Hao-Hao Li, Xiao-Fei Wang and Yu-Jin Hao wrote the manuscript. Hao-Hao Li and Xin-Liu contributed to revised paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hao-Hao Li and Xin Liu have contributed equally to the article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, HH., Liu, X., An, JP. et al. Cloning and elucidation of the functional role of apple MdLBD13 in anthocyanin biosynthesis and nitrate assimilation. Plant Cell Tiss Organ Cult 130, 47–59 (2017). https://doi.org/10.1007/s11240-017-1203-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1203-x