Abstract
In the current study, magnetite-silica core–shell nanoparticles modified with Cu-salen complex (Fe3O4@SiO2-imine/phenoxy-Cu(II)) was utilized as a heterogeneous catalyst for the one-pot multicomponent synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione and dihydropyrano[3,2-c]chromene derivatives under solvent-free conditions, without using any harmful organic reagents/solvent. The current synthetic protocol demonstrates that the reactions proceeds to completing step, leading to the successful synthesis of high purity compounds. Advantages of this method include easy purification, reusability of the catalyst, green and mild procedure and synthesis of new derivatives in high yields within short reaction time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis of new heterocyclic compounds is of paramount importance, because of their abundance, pharmaceutical and industrial applications [1]. So far, many methods have been proposed to synthesize these compounds. In the last two decades, multi-component reactions (MCRs) have become a powerful, rapid and efficient approach for the synthesis of heterocyclic compounds [2,3,4,5,6,7]. The utilization of MCRs offers several advantages such as increased reaction rate, reduction of time, high atom economy and avoidance of complicated purification processes. These benefits make MCRs a considerable method for the synthesis of complex heterocyclic compounds [8]. One the key driving forces of the MCRs is the utilization of heterogeneous recyclable catalysts, that very recently, the efforts have been directed to surface and structural modification of the heterogeneous catalysts by using the recent trends in the nanoarchitecture [9,10,11,12,13,14,15,16].
1H-pyrazolo[1,2-b]phthalazine-5,10-dione and dihydropyrano[3,2-c]chromene derivatives are two crucial classes of heterocycles for which several synthetic methods have been developed for the synthesis of their derivatives. Their biological and pharmacological properties and thus, their clinical applications are the notable reasons for the interest in these compounds [17,18,19,20,21,22,23]. Anti-convulsant, vasorelaxant, cardiotonic, antipyretic, anti-inflammatory, anticancer, antifungal and antibacterial activities are some examples of biological properties of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives [24,25,26,27]. Also, dihydropyrano[3,2-c]chromenes are well known for anti-anaphylactic, antitumor, antimicrobial, anti-tuberculosis agents, analgesic, anti-cancer and anticoagulant activities [28,29,30,31]. Owing to all the above-mentioned importance and applications of these compounds, it is of primary importance to find the superior synthesis approaches for them by scientists. So far, innumerable approaches have been proposed by researchers for the manufacture of these compounds, among which there is a commonly employed strategy which consists of one-pot three-component reaction. This reaction has been studied with different catalysts and conditions. As an illustration, in 2019, 1H-pyrazolo[1,2-b]phthalazine-5,10-dione was synthesized by Pati et al. using CuO nanoparticles as a reusable catalyst under solvent-free conditions [32]. Piltan et al. in 2017, synthesized 1H-pyrazolo[1,2-b]phthalazine-5,10-dione using the ZrO2 nanoparticles as a catalyst, at 100 °C and under solvent-free conditions [2,3,4,5,6,7]. In 2019, dihydropyrano[3,2-c]chromenes derivatives were synthesized by Hallaoui et al. using Co3(PO4)2 as a catalyst at 80 °C. This reaction was performed in a mixture of water/ethanol (4:1) [33]. In another research work, Sharma et al. in 2018, reported the new derivatives of dihydropyrano[3,2-c]chromenes at 80 °C in the presence of [Bmim] Sac as a non-toxic and green ionic liquid in aqueous media [34]. In the last few decades, magnetic nanoparticles (MNPs) have attracted a lot of attention as sustainable and efficient catalysts for multi-component reactions (MCRs) due to their features such as large surface to volume ratio, high thermal stability, chemical stability, easy magnetic separation and high potential of recyclability.
Given the fact that we have recently introduced Copper‐based Schiff Base Complex Immobilized on Core‐shell Fe3O4@SiO2 as a magnetically recyclable and highly efficient nanocatalyst for green synthesis of 2‐amino‐4H‐chromene derivatives [35], in this article, we have presented another important application of Fe3O4@SiO2-Imine/Phenoxy-Cu(II) catalyst as an efficient, low price, safe, catalytic performance and magnetic powerful solid acid catalyst for one-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione and dihydropyrano[3,2-c]chromenes. The schematic illustration of the synthesis route is exhibited in Scheme 1.
Experimental
Chemicals and instruments
All chemicals were purchased from Merck and Fluka Companies. 1H NMR and 13C NMR spectra were measured on a spectrometer at 250, 400, 300, 90 MHz and 62.5, 100, 75 MHz, respectively, in DMSO-d6. Fourier transform infrared (FT-IR) spectra were obtained using the Shimadzu 435-U-04 FT spectrophotometer from KBr pellets. Melting points were recorded on a BUCHI 510 apparatus in open capillary tubes.
Preparation of Fe3O4–SiO2–(CH2)3–1,3-Phenylenediamine-Schiff base Complex of Cu(II)
Magnetic Fe3O4 nanoparticles were synthesized via co-precipitation of solutions containing Fe3+ and Fe2+ ions [36]. FeCl3·6H2O (11.3 g, 0.0418 mol) and FeCl2·4H2O (5.648 g, 0.0209 mol) were dissolved in 100 ml of deionized water. The mixture was stirred at 85 °C for 30 min, and then 25 ml of ammonia (28% aqueous solution) was added rapidly to the reaction mixture. The reaction was stirred for another 30 min at 85 °C. Then, the black Fe3O4 nanoparticles were separated with an external magnet. They were washed with distilled water followed by EtOH and dried at 80 °C in an oven. Then, 1.0 g of the MNPs was dispersed in a mixture of 80 ml of deionized water, 20 ml of EtOH and 2.0 ml of ammonia (28 wt%), and then 2.0 mL of tetraethyl orthosilicate (TEOS) was added to the mixture and stirred at 50 °C for 2 h. The core–shell MNPs (Fe3O4@SiO2 MNPs) were separated using a magnet and washed with deionized water, EtOH, and then dried in an oven [37]. In the following, Fe3O4@SiO2–functionalized Cl was synthesized via surface reaction of Fe3O4@SiO2 using (3‐chloropropyl) triethoxysilane as the silylation agent. 1.0 g of Fe3O4@SiO2 and 50.0 ml of toluene were mixed into a 250 ml round flask. Then, (3 Chloropropyl)triethoxysilane (2.0 ml) was added to the mixture and refluxed at 110 °C with stirring for 12 h. In the end, Fe3O4@SiO2@Cl MNPs were separated by a magnet and washed with toluene and EtOH several times. Finally, the product was dried in an oven. Then, for Synthesis of Fe3O4@SiO2–(CH2)3–NH–(3-NH2C6H4) MNPs,1, 3-phenylenediamine (1.3 g, 0.012 mol) and a few drops of triethylamine as a base were dissolved in ethanol (70 ml). Then Fe3O4@SiO2–(CH2)3–Cl (1 g) was added to the mixture and dispersed (15 min). The resulting mixture was refluxed in N2 for 24 h. After refluxing, the final precipitate was separated with a magnet, washed several times with ethanol and dried at room temperature. For the synthesis of Fe3O4@SiO2- iminomethyl/phenol,at First, Fe3O4@SiO2–(CH2)3–NH–(3-NH2C6H4) nanoparticles (1 g) was dispersed in 50 ml of methanol (15 min) and refluxed. Then, salicylaldehyde (2.93 g, 24 mmol) was dissolved in ethanol (30 ml) and added dropwise to the nanoparticles using a decanter funnel. The reaction mixture was refluxed for 24 h and the product was separated using a magnet, washed several times with ethanol, and dried in an oven. Finally, 1 g from the previously synthesized nanoparticles was dispersed in 50 ml ethanol (15 min). Then 5% (w/v) solution of Cu(OAc)2 in ethanol (5 ml) was added dropwise to the previous mixture and the resulting mixture was refluxed for 48 h. After the reaction completed, the product was separated using the magnet, washed with 50 ml ethanol and water to remove the remaining unreacted materials and dried in an oven. The resulted Fe3O4@SiO2‐imine/phenoxy‐Cu(II) magnetic nanoparticles (MNPs) were characterized by various techniques including SEM, TEM, XRD, XPS, EDX, VSM, FT‐IR and ICP [35].
General procedure for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives
Under the optimum reaction conditions, phthalhydrazide (1 mmol), malononitrile (1 mmol), aldehyde (1 mmol) and the catalyst (0.02 g) were added to a test tube. The mixture was stirred magnetically and heated at 80 °C under solvent-free conditions. After completion of the reaction (judged by the TLC (n-hexane/ethyl acetate 10:3)), 10 ml of hot ethanol was added to the reaction mixture and the catalyst was separated using an external magnet. Then, 20 ml of distilled water was added to the reaction mixture and precipitated product isolated by filtration. The product was washed with NaOH (0.5 mol) to produce a pure product. The product was characterized using physical and spectroscopic data (FTIR, NMR and MS).
General procedure for the synthesis of dihydropyrano[3,2-c]chromene derivatives
Under the optimum reaction conditions, 4-hydroxycoumarin (1 mmol), malononitrile (1 mmol), aldehyde (1 mmol) and 0.03 g catalyst were added to a test tube. The mixture was stirred magnetically and heated at 90 °C under solvent-free conditions. After completion of the reaction (by the TLC (n-hexane/ethyl acetate 7:4)), 10 ml of hot ethanol was added to the reaction mixture and the catalyst separated using an external magnet. Then, 20 ml distilled water was added to the reaction mixture and precipitated product isolated by filtration. The product was washed with NaOH (0.5 mol) to produce a pure product. The product was characterized using physical and spectroscopic data (FT-IR, NMR and MS).
Analytical data of selected products
3-amino-5,10-dioxo-1-phenyl-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4a) Yellow solid; mp 249–251 °C. FT-IR (KBr, cm−1): 3361, 3260, 2197, 1682, 1660. 1H NMR (90 MHz, DMSO-d6, δ ppm): 6.12 (s, 1H, CH), 7.31–7.87 (m, 9H, Ar–H), 8.29 (t, 2H, NH2). 13C NMR (75 MHz, DMSO-d6, δ ppm): 61.4, 62.9, 116.2, 126.5, 126.6, 127.3, 128.1, 128.5, 128.6, 128.7, 133.6, 134.7, 138.4, 150.6, 153.7, 156.5 ppm.
3-amino-1-(naphthalen-1-yl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4b) Yellow solid; mp 275–276 °C. FT-IR (KBr, cm−1): 3365, 3260, 2192, 1682, 1655. 1H NMR (300 MHz, DMSO-d6, δ ppm): 6.27 (s, 1H, CH), 7.50–8.28 (m, 11H, Ar–H), 8.29 (t, 2H, NH2). 13C NMR (75 MHz, DMSO-d6, δ ppm): 61.3, 63.2, 116.0, 124.2, 125.0, 125.1, 126.3, 126.6, 126.9, 127.5, 127.7, 127.9, 128.4, 128.7, 128.9, 132.8, 132.9, 133.6, 134.5, 135.8, 150.7, 153.8, 156.7 ppm.
3-amino-1-(naphthalen-2-yl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4c) Yellow solid; mp 265–269 °C. FT-IR (KBr, cm−1): 3367, 3258, 2192, 1682, 1655. 1H NMR (250 MHz, DMSO-d6, δ ppm): 6.27 (s, 1H, CH), 7.51–8.13 (m, 11H, Ar–H), 8.25 (t, 2H, NH2).
3-amino-1-(2,4-dichlorophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4d) Yellow solid; mp 230–234 °C. FT-IR (KBr, cm−1): 3370, 3260, 2204, 1676, 1632, 698. 1H NMR (90 MHz, DMSO-d6, δ ppm): 6.35 (s, 1H, CH), 7.43–7.90 (m, 7H, Ar–H), 8.45 (t, 2H, NH2). 13C NMR (75 MHz, DMSO-d6, δ ppm): 59.7, 60.6, 116.1, 125.5, 127.2, 127.9, 128.7, 128.7, 128.8, 129.3, 129.7, 132.7, 133.0, 134.0, 134.3, 135.0, 151.3, 154.1, 157.2 ppm.
3-amino-1-(4-chloro-3-nitrophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4e) Yellow solid; mp 262–264 °C. FT-IR (KBr, cm−1): 3364, 3299, 2190, 1682, 1662, 1567, 1383, 693. 1H NMR (250 MHz, DMSO-d6, δ ppm): 6.24 (s, 1H, CH), 7.78–8.18 (m, 7H, Ar–H), 8.29 (t, 2H, NH2). 13C NMR (62 MHz, DMSO-d6, δ ppm): 60.5, 62.0, 116.2, 124.1, 124.8, 127.1, 127.6, 128.8, 129.5, 132.0, 132.7, 134.2, 135.0, 140.3, 148.4, 151.5, 154.2, 157.2. MS: m/z: 395, 239, 217, 187, 162, 130, 104, 76, 50 [M + H]+.
3-amino-5,10-dioxo-1-(3,4,5-trimethoxyphenyl)-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4f) Yellow solid; mp 253–256 °C. FT-IR (KBr, cm−1): 3372, 3295, 2841, 2185, 1681, 1658. 1H NMR (300 MHz, DMSO-d6, δ ppm): 3.32–3.75 ( s, 9H, OCH3), 6.11 (s, 1H, CH), 6.73–8.26 (m, 6H, Ar–H), 8.27 (t, 2H, NH2). 13C NMR (75 MHz, DMSO-d6, δ ppm): 56.0, 59.8, 61.2, 63.3, 104.1, 116.2, 126.7, 127.2, 128.7, 128.8, 133.7, 134.1, 134.5, 137.3, 150.6, 152.8, 153.9, 156.6 ppm.
3-amino-1-(4-methoxyphenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4 g) Yellow solid; mp 239–241 °C. FT-IR (KBr, cm−1): 3376, 3266, 2197, 1677, 1661. 1H NMR (300 MHz, DMSO-d6, δ ppm): 3.73 ( s, 3H, OCH3), 6.07 (s, 1H, CH), 6.89–8.24 (m, 8H, Ar–H), 8.36 (t, 2H, NH2). 13C NMR (75 MHz, DMSO-d6, δ ppm): 55.1, 61.3, 62.6, 113.9, 116.2, 126.5, 127.1, 128.5, 128.6, 130.2, 133.1, 134.6, 150.6, 153.4, 156.6, 159.1 ppm.
3-amino-5,10-dioxo-1-(p-tolyl)-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (4 h) Yellow solid; mp 253–255 °C. FT-IR (KBr, cm−1): 3366, 3263, 3036, 2197, 1682, 1658. 1H NMR (90 MHz, DMSO-d6, δ ppm): 2.31 ( s, 3H, CH3), 6.11 (s, 1H, CH), 7.09–7.83 (m, 8H, Ar–H), 8.32 (t, 2H, NH2). 13C NMR (75 MHz, DMSO-d6, δ ppm): 61.5, 62.8, 116.0, 126.6, 126.7, 127.3, 128.5, 129.2, 133.6, 134.6, 135.3, 137.5, 150.4, 153.6, 156.6 ppm.
2-amino-4-(4-chloro-3-nitrophenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5b) Cream solid; mp 252–258 °C. FT-IR (KBr, cm−1): 3392, 3319, 2198, 1694, 1530, 1380, 1255. 1H NMR (250 MHz, DMSO-d6, δ ppm): 4.65 (s, 1H, CH), 7.45–7.85 (m, 7H, Ar–H), 8.02 (t, 2H, NH2). 13C NMR (62 MHz, DMSO-d6, δ ppm): 36.4, 57.0, 102.7, 113.4, 116.9, 119.3, 123.0, 123.7, 125.0, 131.8, 133.5, 133.7, 144.8, 148.2, 152.7, 154.5, 158.5, 160.0. MS: m/z: 395, 378, 348, 295, 282, 239, 120, 92, 66 [M + H]+.
2-amino-4-(naphthalen-1-yl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5c) Cream solid; mp 259–264 °C. FT-IR (KBr, cm−1): 3311, 2188, 1696, 1255. 1H NMR (250 MHz, DMSO-d6, δ ppm): 5.45 (s, 1H, CH), 7.36–7.95 (m, 11H, Ar–H), 8.42 (t, 2H, NH2). 13C NMR (62 MHz, DMSO-d6, δ ppm): 31.9, 59.0, 105.0, 113.3, 117.0, 119.6, 122.9, 123.8, 125.1, 126.3, 126.5, 127.9, 128.9, 131.4, 133.3, 133.7, 141.1, 152.5, 154.2, 158.3, 160.0.
2-amino-4-(4-hydroxyphenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5e) Cream solid; mp 264–266 °C. FT-IR (KBr, cm−1): 3407, 3286, 3218, 2196, 1696, 1215. 1H NMR (300 MHz, DMSO-d6, δ ppm): 4.31 (s, 1H, CH), 6.66–7.86 (m, 8H, Ar–H), 7.88 (t, 2H, NH2), 9.34 (s, 1H, OH).
2-amino-4-(2,4-dichlorophenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5f) Cream solid; mp 254–256 °C. FT-IR (KBr, cm−1): 3296, 3160, 2200, 1716, 1258. 1H NMR (400 MHz, DMSO-d6, δ ppm): 4.98 (s, 1H, CH), 7.35–7.90 (m, 7H, Ar–H), 7.92 (t, 2H, NH2). 13C NMR (100 MHz, DMSO-d6, δ ppm): 39.9, 56.4, 102.9, 113.3, 117.1, 119.1, 123.0, 125.2, 128.3, 129.3, 132.5, 132.8, 133.6, 139.9, 152.7, 154.6, 156.5, 158.6, 159.9.
2-amino-4-(2-chlorophenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5 g) Cream solid; mp 265–268 °C. FT-IR (KBr, cm−1): 3284, 3179, 2200, 1709, 1259. 1H NMR (400 MHz, DMSO-d6, δ ppm): 4.99 (s, 1H, CH), 6.81–7.90 (m, 8H, Ar–H), 7.92 (t, 2H, NH2). 13C NMR (100 MHz, DMSO-d6, δ ppm): 36.4, 56.6, 103.1, 112.8, 116.5, 118.6, 122.5, 122.8, 124.6, 128.2, 129.0, 130.6, 132.7, 133.0, 152.1, 153.9, 158.0, 159.3.
2-amino-4-(3-bromophenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5 h) Cream solid; mp 272–276 °C. FT-IR (KBr, cm−1): 3380, 3292, 2195, 1716, 1258. 1H NMR (400 MHz, DMSO-d6, δ ppm): 5.00 (s, 1H, CH), 7.17–7.91 (m, 8H, Ar–H),7.93 (t, 2H, NH2). 13C NMR (100 MHz, DMSO-d6, δ ppm): 36.9, 57.1, 103.6, 113.3, 117.0, 119.2, 123.0,123.4, 125.1, 128.8, 129.3, 131.1, 133.2, 133.8, 142.4, 152.6, 154.4, 158.4, 159.5, 159.8.
Result and discussion
In this work, Fe3O4@SiO2-imine/phenoxy-Cu(II) was synthesized as an efficient and recyclable catalyst for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione and dihydropyrano[3,2-c]chromenes derivatives. For synthesis of the catalyst, the Fe3O4 nanoparticles were first prepared via the co-precipitation method, in which the silica was utilized as a protecting shell to coat the Fe3O4 nanoparticles and form a core–shell structure (Fe3O4@SiO2), the nanoparticles of which were coated by (3-chloropropyl)triethoxysilane, yielding the Fe3O4@SiO2–(CH2)3-Cl MNPs. In the next step, the Fe3O4@SiO2–(CH2)3-Cl nanoparticles were reacted with 1,3-phenylenediamine to yield the Fe3O4@SiO2–(CH2)3–NH–(3-NH2C6H4) nanoparticles. Conversion of the Fe3O4@SiO2–(CH2)3–NH–(3-NH2C6H4) nanoparticles into Fe3O4@SiO2-Iminomethyl/phenol was accomplished via the reaction of Fe3O4@SiO2–(CH2)3–NH–(3-NH2C6H4) nanoparticles with salicylaldehyde. Ultimately, Fe3O4@SiO2-imine/phenoxy-Cu (II) was synthesized by dispersing the material in Cu(OAc)2 solution [35].
To check the catalytic activity of Fe3O4@SiO2-imine/phenoxy-Cu(II) in the one-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione the reaction of phthalhydrazide, malononitrile and aldehyde was selected as a model reaction. The reaction conditions, including solvent, temperature and different amount of catalyst, were optimized and listed in Table 1. Based on these data, no reaction progress was observed in the absence of a catalyst and 25 mg of the catalyst was a suitable amount for this reaction. Then, a variety of temperatures were monitored in the model reaction, by which the highest yield of product was achieved at 80 °C. Additionally, various solvents were also employed to study the reaction. Therefore, The best result for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives can be obtained by using 20 mg of catalyst, solvent-free conditions and a temperature of 80 °C.
This reaction was studied under the optimum condition for different aromatic aldehydes with different electron‐withdrawing and electron‐donating groups and the results were presented in Table 2. In this work, it was concluded that the reaction could proceed much better when utilizing an aldehyde of the electron‐withdrawing group.
A schematic illustration of a feasible mechanism for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives by using Fe3O4@SiO2-imine/phenoxy-Cu(II) catalyst, is presented in Scheme 2. Initially, the activation of C–H bond in malononitrile by the catalyst (as a Lewis acid catalyst) leads to the attack of malononitrile on the activated carbonyl group of aldehyde. Knoevenagel condensation reaction between malononitrile and aldehyde was followed by dehydration to provide the intermediate (b). Then, the michael addition between Intermediate b and phthalhydrazide, forms intermediate (c). Then intramolecular cyclization and tautomerization in presence of the catalyst give 1H-pyrazolo[1,2-b]phthalazine-5,10-dione.
After the successful synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones, we evaluated the catalytic activity of Fe3O4@SiO2-imine/phenoxy-Cu(II) for the synthesis of dihydropyrano[3,2-c]chromene derivatives. For this purpose, the reaction of aldehyde (1 mmol), malononitrile (1 mmol) and 4-hydroxycoumarin (1 mmol) were applied as a model, and diverse solvents, temperature and catalyst content were used to optimize the reaction (Table 3). The best result for this reaction can be obtained by using 30 mg of catalyst, solvent-free conditions and a temperature of 90 °C.
After optimizing the reaction parameters, the catalyst was investigated on different aromatic aldehydes with different electron‐withdrawing and electron‐donating groups and the results are presented in Table 4. In this work, it was concluded that the reaction could proceed more efficiently when utilizing an aldehyde possessing an electron‐withdrawing group.
Scheme 3 shows the probable mechanism for one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives by using Fe3O4@SiO2-imine/phenoxy-Cu(II) as a Lewis acid catalyst. Based on this mechanism, the activation of C–H bond in malononitrile by the catalyst leads to the attack of malononitrile on an activated carbonyl group of aldehyde. Knoevenagel condensation reaction between malononitrile and aldehyde followed by dehydration to provide the intermediate (b). Michael addition between Intermediate b and 4-hydroxycoumarin further forms the intermediate (d). Then, the intramolecular cyclization and tautomerization in presence of the catalyst results in dihydropyrano[3,2-c]chromene.
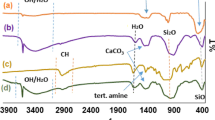
The model reaction was also used to assess the reusability of the catalyst. For this purpose, after which the reaction was completed, the separated catalyst was washed several times with water and hot ethanol, dried and reused in the next cycle. Figure 1 exhibits the results (FT-IR,TEM,XRD of reused catalyst in the fifth run), confirming the recyclability of the catalyst. Hot-filtration test also indicated that the catalyst has no leaching in each cycle.
Our method is advantageous to other methods from viewpoint of the shorter reaction time and higher yield of production with an insignificant fraction of the side products. This method compared with other methods shown in Table 5.
Conclusion
The application of Fe3O4@SiO2-imine/phenoxy-Cu(II) as a catalyst shows a highly efficient catalytic activity for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione and dihydropyrano[3,2-c]chromene derivatives due to the presence of Cu species in the catalyst which act as Lewis acid. The newly synthesized derivatives were analyzed and their structure was corroborated. The main advantages of this work are facile reaction workup, recyclability and reusability of the catalyst, low catalyst loading, short reaction times and considerable yield of the product. Briefly, our method is advantageous to other methods from viewpoint of the shorter reaction time and higher yield of production with an insignificant fraction of the side products.
References
P. Panahi, N. Nouruzi, E. Doustkhah, H. Mohtasham, A. Ahadi, A. Ghiasi-Moaser, S. Rostamnia, G. Mahmoudi, A. Khataee, Ultrason. Sonochem. 58, 104653 (2019)
M. Piltan, Heterocycl. Comm. 23, 401 (2017)
M. Saraei, N. Valizadeh, H. Ebrahimi-asl, Monatsh. Chem. 146, 345 (2015)
D. Azarifar, H. Ebrahimiasl, R. Karamian, M. Ahmadi-Khoei, J. Iran. Chem. Soc. 16, 341 (2019)
H. Ebrahimiasl, D. Azarifar, M. Mohammadi, H. Keypour, Res. Chem. Intermed. 47, 683 (2020)
S. Rostamnia, E. Doustkhah, A. Baghban, B. Zeynizadeh, J. Appl. Polym. Sci. 133, 1 (2016)
E. Doustkhah, S. Rostamnia, A. Hassankhani, J. Porous Mater. 23, 549 (2016)
I.A. Ibarra, A. Islas-Jácome, E. González-Zamora, Org. Biomol. Chem. 16, 1402 (2018)
R. Tahawy, E. Doustkhah, E.S.A. Abdel-Aal, M. Esmat, F.E. Farghaly, H. El-Hosainy, N. Tsunoji, F.I. El-Hosiny, Y. Yamauchi, M.H.N. Assadi, Y. Ide, Appl. Catal. B: Environ. 286, 119854 (2021)
S.S. Mofarah, L. Schreck, C. Cazorla, X. Zheng, E. Adabifiroozjaei, C. Tsounis, J. Scott, R. Shahmiri, Y. Yao, R. Abbasi, Y. Wang, Nanoscale 13, 6764 (2021)
E. Doustkhah, R. Hassandoost, A. Khataee, R. Luque, M.H.N. Assadi, Chem. Soc. Rev. 50, 2927 (2021)
T.S. Rad, Z. Ansarian, A. Khataee, B. Vahid, E. Doustkhah, Sep. Purif. Technol. 256, 117811 (2021)
A. Zebardasti, M.G. Dekamin, E. Doustkhah, M.H.N. Assadi, Inorg. Chem. 59, 11223 (2020)
E. Doustkhah, H. Mohtasham, M. Farajzadeh, S. Rostamnia, Y. Wang, H. Arandiyan, M.H.N. Assadi, Micropor. Mesopor. Mat. 293, 109832 (2020)
M. Aalinejad, N.N. Pesyan, E. Doustkhah Mol. Catal. 494, 111117 (2020)
E. Doustkhah, H. Mohtasham, M. Hasani, Y. Ide, S. Rostamnia, N. Tsunoji, M.H.N. Assadi, Mol. Catal. 482, 110676 (2020)
J. Li, Y.F. Zhao, X.Y. Yuan, J.X. Xu, P. Gong, Molecules 11, 574 (2006)
S.S. Karbasaki, G. Bagherzade, B. Maleki, M. Ghani, J. Taiwan Inst. Chem. Eng. 118, 342 (2021)
B. Maleki, R. Nejat, H. Alinezhad, S.M. Mousavi, B. Mahdavi, M. Delavari, Org. Prep. Proced. Int. 52, 328 (2020)
B. Maleki, Org. Prep. Proced. Int 48, 303 (2016)
R. Tayebee, M. Jomei, B. Maleki, M.K. Razi, H. Veisi, M. Bakherad, J. Mol. Liq. 206, 119 (2015)
R.W. Carling, K.W. Moore, L.J. Street, D. Wild, C. Isted, P.D. Leeson, S. Thomas, D. O’Connor, R.M. McKernan, K. Quirk, S.M. Cook, J.R. Atack, K.A. Wafford, S.A. Thompson, G.R. Dawson, P. Ferris, J.L. Castro, J. Med. Chem. 47, 1807 (2004)
M.A. Shaikh, M. Farooqui, S. Abed, Res. Chem. Intermed. 44, 5483 (2018)
S. Grasso, G. De Sarro, A. De Sarro, N. Micale, M. Zappala, G. Puja, M. Baraldi, C. De Micheli, J. Med. Chem. 43, 2851 (2000)
E. Doustkhah, A. Baghban, M.H.N. Assadi, R. Luque, S. Rostamnia, Catal. Lett. 149, 591 (2019)
Y.A. Tayade, D.S. Dalal, Catal. Lett. 147, 1411 (2017)
A.V. Chatea, P.K. Bhadkea, M.A. Khandea, J.N. Sangshettib, C.H. Gill, Chin. Chem. Lett. 28, 1577 (2017)
D.C. Mungra, M.P. Patel, D.P. Rajani, R.G. Patel, Eur. J. Med. Chem. 46, 4192 (2011)
M. Kidwai, S. Saxena, M.K.R. Khan, S.S. Thukral, Bioorg. Med. Chem. Lett. 15, 4295 (2005)
J.Y.C. Wu, W.F. Fong, J.X. Zhang, C.H. Leung, H.L. Kwong, M.S. Yang, D. Li, H.Y. Cheung, Eur. J. Pharmacol. 473, 9 (2003)
L. Bonsignorel, G. Loyl, D. Seccil, A. Calignanoz, Eur. J. Med. Chem. 28, 517 (1993)
S. Patil, A. Mane, S. Dhongade-Desai, J. Iran. Chem. Soc. 16, 1665 (2019)
A. El Hallaoui, S. Chehab, B. Malek, O. Zimou, T. Ghailane, S. Boukhris, A. Souizi, R. Ghailane, ChemistrySelect 4, 3062 (2019)
H. Sharma, S. Srivastava, RSC Adv. 8, 38974 (2018)
H. Ebrahimiasl, D. Azarifar, Appl. Organomet. Chem. 34, 5359 (2019)
U.C.R. Divya, D.S. Rawat, RSC Adv. 4, 41323 (2014)
M. Tajbakhsh, M. Farhang, R. Hosseinzadeh, Y. Sarrafi, RSC Adv. 4, 23116 (2014)
J. Safaei-Ghomi, H. Shahbazi-Alavi, A. Ziarati, R. Teymuri, M.R. Saberi, Chin. Chem. Lett. 25, 401 (2014)
R. Ghahremanzadeh, G.I. Shakibaei, A. Bazgir, Synlett. 8, 1129 (2008)
M.R. Nabid, S.J.T. Rezaei, R. Ghahremanzadeh, A. Bazgir, Ultrason. Sonochem. 17, 159 (2010)
M. Piltan, Heterocycl. Commun. 23, 401 (2017)
G. Karthikeyan, A. Pandurangan, J. Mol. Catal. A: Chem. 361, 58 (2012)
A. Montaghami, N. Montazeri, Orient. J. Chem. 30, 1361 (2014)
H.J. Wang, J. Lu, Z.H. Zhang, Mon. für Chem.-Chem. Mon. 141, 1107 (2010)
A. Alizadeh, M.M. Khodaei, M. Beygzadeh, D. Kordestani, M. Feyzi, Bull. Korean Chem. Soc. 33, 2546 (2012)
M.M. Heravi, B.A. Jani, F. Derikvand, F.F. Bamoharram, H.A. Oskooie, Catal. Commun. 10, 272 (2008)
M. Seifi, H. Sheibani, Catal. Lett. 126, 275 (2008)
S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett. 48, 3299 (2007)
H. Azizi, A. Khorshidi, K. Tabatabaeian, J. Iran. Chem. Soc. 15, 1023 (2018)
Acknowledgements
We gratefully acknowledge the Research Council of Bu‐Ali Sina University for providing facilities and financial support of this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nesarvand, M., Azarifar, D. & Ebrahimiasl, H. One‐pot and green synthesis 1H-pyrazolo[1,2-b]phthalazine-5,10-dione and dihydropyrano[3,2-c]chromene derivatives by Fe3O4@SiO2-imine/phenoxy-Cu(II) as an efficient and reusable catalyst. Res Chem Intermed 47, 3629–3644 (2021). https://doi.org/10.1007/s11164-021-04498-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04498-4