Abstract
In this investigation, a practical green chemistry procedure for synthesis of highly functionalized cyclohexenone derivatives according to the Claisen–Schmidt condensation and Michael reaction using acetoacetanilide, acetophenone, and appropriate aromatic aldehydes in the presence of 15 mol% of Na2CO3 as a catalyst in H2O/EtOH at ambient conditions is described. This methodology is of interest due to the use of water/ethanol as a solvent without the use of any harmful and toxic organic solvents and toxic metals as catalysts. Neutral conditions, excellent yields, a short reaction time, a simple work-up procedure of products and use of an inexpensive catalyst are some of the main advantages of this environmentally benign process. Also, there is no need for column chromatography to obtain pure products.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern synthetic design demands high efficiency in terms of minimizing synthetic steps together with maximization of complexity [1]. One of the ways to fulfill these goals is the development and use of multi-component reactions (MCRs) which consist of several simultaneous bond-forming reactions. Therefore, MCRs have emerged as a valuable tool in the context of modern combinatorial synthesis. Moreover, one-pot MCRs due to their productivity, facile execution and simple reaction profile are one of the important strategies in MCRs, which have expanded rapidly in organic chemistry [2–8].
The Michael addition reaction is widely recognized as one of the key reactions for carbon–carbon bond formation [9]. The nucleophilic addition of enolates or their analogues to the carbon–carbon double bond of α,β-unsaturated ketones, aldehydes, nitriles, or carboxylic acid derivatives, usually referred to as the Michael reaction, is a fundamental but useful carbon–carbon bond forming reaction (Scheme 1) [10].
One of the most important α,β-unsaturated compounds are chalcones. Usually, α,β-enone is known as chalcone [11]. α,β-enones are widely used as versatile precursors for synthesis of cyclohexenone derivatives [9, 12–17], and several types of heterocyclic compounds, such as isoxazoles [18], thiazoles [19], thiadiazoles [20], oxazolopyridines, pyrans, pyridines and pyrimidines [21, 22], and triazines [23]. Also, chalcones are structurally simple compounds of the flavonoid family and are present in a variety of plant species [24, 25]. Chemically, these are 1,3-diphenyl-2-propen-1-ones and have wide range of reported biological activities, including anti-leishmanial, anti-fungal, anti-inflammatory, anticancer, antimitotic, modulation of P-glycoprotein-mediated multi-drug resistance, anticonvulsant, and antimalarial activities, etc. [26–42].
When enolates or enamines react with α,β-unsaturated systems several new chiral centers can be generated and it is very useful to be able to carry out such reactions with good, predictable stereochemical control. Michael addition reaction of 1,3-dicarbonyl compounds such as acetoacetic esters to chalcones leads to the synthesis of cyclohexenone derivatives, which become interesting intermediates for the synthesis of a variety of heterocyclic systems with diverse biological activities. Cyclohexenone derivatives are well known lead molecules for the treatment of autoimmune and inflammation diseases [43]. Also, cyclohexenones and indazole derivatives exhibit a variety of pharmacological properties involving antitumor, antipyretic, anti-fungal, tyrosine kinases inhibitor, antiviral, antiasthmatic, anticancer, anti-bacterial, and anti-tubercular activity [12, 44–49]. Therefore, the development of a milder and more efficient route for one-pot synthesis of these important molecules is still in demand.
Sodium carbonate is either found naturally or is manufactured from sodium chloride. Sodium carbonate is a fairly strong, non-volatile base which is used in the manufacture of glass, paper, soap, and many other chemicals. Sodium carbonate is also called soda ash and washing soda. Sodium carbonate is used in laundry detergents as a softening agent. Sodium carbonate is a food additive used as an acidity regulator, anti-caking agent, raising agent, and stabilizer. Also, it is used in toothpastes. The main uses and applications of sodium carbonate are summarized in Fig. 1. Moreover, it can be used as a base catalyst in the synthesis of organic compounds [50–52].
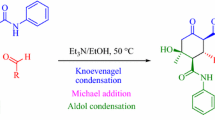
Encouraged by these findings, our continuing interests on MCRs [53–62] and as continuation of our previous work in cycloaddition reaction for synthesizing cyclohexenone derivatives [63], an efficient, entirely green and convenient synthesis of 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides has been accomplished using sodium carbonate as a natural and green catalyst with excellent yields. Herein, we have synthesized cyclohexenone derivatives via a one-pot, three-component Claisen–Schmidt condensation and Michael reaction of aromatic aldehydes, acetophenone, and acetoacetanilide at room temperature in the presence of 15 mol% of Na2CO3 as an efficient catalyst in H2O/EtOH (Scheme 2). To the best of our knowledge, there are no earlier reports for the Na2CO3-catalyzed synthesis of cyclohexenones in the open literature.
Results and discussion
Sodium carbonate is a readily available, low cost reagent, which can be conveniently handled and removed from the reaction mixture. Thus, its remarkable catalytic activities together with its operational simplicity make it the most suitable catalyst for the synthesis of 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides. Initially, the standard reaction conditions were established based on a series of trial reactions of acetoacetanilide (1 mmol) with acetophenone (1 mmol) and 3-chloro benzaldehyde (1 mmol) in the presence or in the absence of catalyst in a 5-mL mixture of ethanol and water as solvent at a ratio of 1:1 at room temperature with various catalysts, while changing the amounts of catalyst-loading of the best catalyst and solvents (Table 1). In order to optimize the reaction conditions, the model reaction was carried out in the presence of 15 mol% of various catalysts (entries 1–8, Table 1). The results are summarized in Table 1. As can be seen in Table 1, maximum yield was obtained with 15 mol% of Na2CO3 under reaction conditions (entry 8, Table 1). Poor yield (trace-38 %) was obtained when the reaction was carried out in the presence of KCN, LiCl, Na2SeO3 and NaF at room temperature (entries 1–4, Table 1). Also, the model reaction did not lead to the desired product 4c in the presence of acidic catalysts (Lewis and Broneshted acids) such as TiO2, AcOH and H3BO3. Therefore, this reaction is catalyzed by bases, and acidic catalysts are not an appropriate media.
After choosing the best catalyst, in the next inspection, a series of comparative experiments were performed to compare the effectiveness of different amounts of Na2CO3 for the formation of (1S,6R)-6-(3-chlorophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide 4c (entries 7–13). As indicated in Table 1, the best result was obtained in 15 mol% of Na2CO3 (entry 8, Table 1). As can be seen, a further increase in the amount of Na2CO3 in the aforementioned reaction did not have any significant effect on the product yield (entries 11-13). In addition, the effects of various solvents including H2O/EtOH (1:1), H2O, EtOH, MeCN, EtOAc and CH2Cl2 were studied on the model reaction. Among the screened solvent systems, a 50:50 ratio of water:ethanol was the solvent of choice that was an appropriate and entirely green system, since the reaction proceeded smoothly and afforded the desired adducts in high yields.
Remarkably, the reaction did not progress even after 48 h in the absence of catalyst and under solvent-free conditions (entry 19 and 20). That might have been due to the lack of effective interaction between reactants in the absence of the catalyst and the solvent.
To explore the scope and generality of this three-component reaction under optimized conditions, a wide range of aromatic aldehydes were subjected to reaction with acetophenone and acetoacetanilide in the presence of a catalytic amount of Na2CO3, and the results are summarized in Table 2. A variety of aromatic aldehydes possessing electron-withdrawing and electron-donating groups, such as –OMe, CN, –Cl, –Br, and –NO2, were allowed to react with acetophenone and acetoacetanilide under standard conditions, affording corresponding poly-substituted cyclohexenoes in good to high yields, with the exception of 4k of which, unfortunately, we did not get the desired cyclohexenone derivative after 48 h of stirring (Table 2, entry 11).
Therefore, diversely substituted aromatic aldehydes underwent this three-component cyclo-condensation reaction with acetophenone and acetoacetanilide to produce 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides in excellent yields (Table 2, entries 4a–l). The reactions were remarkably clean, and no chromatographic separation was required.
The cyclo-condensation of acetoacetanilide with chalcones leads to the generation of two chiral centers at C-1 and C-6 in the structure of cyclohexenones. The structures of all known products were characterized by comparison of the melting points and the analytical data [infrared (IR), proton nuclear magnetic resonance ( 1H NMR)] with those reported in our recently published work [63]. The constitution of the synthesized product was characterized by using elemental analysis, infrared spectroscopy and 1H and 13C-NMR spectroscopy and further supported by mass spectroscopy. For example, the IR spectra of 4l revealed a sharp absorption band at 3387 cm−1 was due to NH. Furthermore, two sharp strong absorption bands were noticed at approximately 1698 and 1659 cm−1 and were assigned to the carbonyl groups. The 1H and 13C NMR, and mass spectra substantiated the results of the IR analysis. The mass spectrum of 4l displayed a molecular ion peak (M+) at m/z = 401, which is consistent with the proposed structure. The 1H NMR spectrum of compound 4l exhibited two doublets of doublets at 3.04 ppm (J = 20.0, 4.0 Hz) and 3.44 ppm (J = 10.5, 2.0 Hz) for methylene protons of a cyclohexenone ring (H-5, H′-5). One of the methine protons of the cyclohexenone ring (H-6) was observed as a triplet of doublet at δ 3.85 ppm (J = 13.2, 4.8 Hz) and another methine proton (H-1) appeared as a doublet at δ 3.96 ppm (J = 13.2 Hz). The vinyl proton (H-3) was observed as a doublet at 6.59 ppm (J = 2.0 Hz). The aromatic proton's resonance was observed as doublets and triplets at δ 7.01–7.73 ppm. The NH proton was observed at δ 10.08 ppm, indicating intra-molecular hydrogen bonding with the vicinal carbonyl group on the cyclohexenone ring. The 13C NMR spectrum of compound 4l showed 20 distinct resonances consistent with the cyclohexenone structure. According to the structure of 4l, it should contain 25 carbons in the 13C NMR spectrum. However, due to the same carbons of (C-3, C-5) and (C-2, C-6) in the aromatic rings in the structure of 4l, on the 13C NMR spectrum, one peak is observed for each of the pairs. In the13C NMR spectrum of this compound, the C-6 carbon was observed at δ 35.6 ppm and C-5 was exhibited at δ 42.8 ppm. The C-1, C-3 and C-4 carbons were observed at δ 59.9, 126.9 and 159.3 ppm, respectively. The aromatic carbons were exhibited at δ 119.5–141.6 ppm. In addition, the carbon of the carbonyl of an amide group was observed at δ 167.6 ppm. Also, the carbon of the carbonyl of a conjugated double bond C=C system (C-2) was observed at 195.6 ppm.
The synthetic pathway via Claisen–Schmidt condensation and Michael reaction for the preparation of the targeted 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides is shown in Scheme 3. This reaction may proceed via Claisen–Schmidt condensation for the formation of chalcones (6) upon the loss of a water molecule. Therefore, in this research, the reaction of acetophenone (2) with different arylaldehydes (3) in the presence of a catalytic amount of Na2CO3 afforded the desired chalcone (6) upon the loss of a water molecule. Afterwards, Michael addition of chalcone with acetoacetanilide (1) in the presence of Na2CO3 pursued by internal Claisen condensation gave 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides (4) by the loss of a water molecule.
In the 1H NMR spectra, the deshielded CH (H-3) group on the cyclohexene ring, which, in all of these derivatives, resonates as a singlet and in some cases as a doublet at δ > 6.5 ppm, can be reliable evidence for the formation of the cyclohexenone framework.
Experimental
General
Melting point and IR spectra of all compounds were obtained on an Electrothermal 9100 apparatus and a JASCO FT/IR-460 plus spectrometer, respectively. 1H and 13C NMR spectra of compounds were recorded on a Bruker DRX-400 Avance instrument in dimethyl sulfoxide (DMSO) or deuterated chloroform (CDCl3 ) as the solvent with trimethylsilane (TMS) as the internal standard at 400 and 100 MHz, respectively. Elemental analyses for C, H, and N for the new compound were performed using a Heraeus CHN–O–Rapid analyzer. The mass spectrum for the new compound was recorded on an Agilent Technology (HP) mass spectrometer, operating at an ionization potential of 70 eV. All reagents were purchased from Merck (Darmastadt, Germany), Acros (Geel, Belgium) and Fluka (Buchs, Switzerland) and used without further purification.
General procedure for the synthesis of 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides
To a solution of 15 mol% Na2CO3 in H2O/EtOH (1:1, 5 mL), arylaldehyde (1 mmol), acetophenone (1 mmol), and acetoacetanilide (1 mmol) were added and the resulting mixture was stirred at room temperature for appropriate time as indicated in Table 2. After completion of the reaction, as indicated by TLC (ethylacetate:n-hexane, 2:3), the products were precipitated from the reaction mixtures, filtered off and washed with H2O/EtOH (2 × 5 mL); the solid residue was crystallized from hot ethanol (98 %) to give pure product 4 in high yield. Physical and spectral data for the selected compounds are represented below.
(1S,6R)-6-(2-Bromophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4a)
White solid, yield: (96 %); mp 223–224 °C. 1H NMR (400 MHz, DMSO): 3.01(s, 2H, H-5), 4.20 (d, J = 13.2 Hz, 1H, H-1), 4.26 (dd, J = 5.2, 9.6 Hz, 1H, H-6), 6.63 (s, 1H, H-3), 7.01 (t, J = 7.2 Hz, 1H, Ar–H), 7.17 (t, J = 7.2 Hz, 1H, Ar–H), 7.24 (t, J = 7.6 Hz, 2H, Ar–H), 7.40 (t, J = 7.6 Hz, 1H, Ar–H), 7.46 (d, J = 6.0 Hz, 5H, Ar–H), 7.62 (dd, J = 11.6, 8.0 Hz, 2H, Ar–H), 7.72 (d, J = 7.6 Hz, 2H, Ar–H), 10.21 (s, 1H, NH).
(1S,6R)-6-(3-Chlorophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4c)
White solid, yield: (94 %); mp 186–188 °C. 1H NMR (400 MHz, DMSO): 3.06 (dd, J = 18.0, 4.4 Hz, 1H, H-5), 3.17 (m, 1H, H′-5), 3.86 (td, J = 12.8, 4.4 Hz, 1H, H-6), 3.97 (d, J = 13.2 Hz, 1H, H-1), 6.58 (d, J = 2.0 Hz, 1H, H-3), 7.01 (t, J = 7.2 Hz, 1H, Ar–H), 7.24 (d, J = 8.4 Hz, 2H, Ar–H), 7.27 (t, J = 2.0 Hz, 1H, Ar–H), 7.34 (t, J = 8.0 Hz, 1H, Ar–H), 7.40 (d, J = 8.0 Hz, 1H, Ar–H), 7.44–7.47 (m, 5H, Ar–H), 7.55 (s, 1H, Ar–H), 7.74 (dd, J = 7.2, 2.4 Hz, 2H, Ar–H), 10.08 (s, 1H, NH).
(1S,6R)-6-(2,6-Dichlorophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4d)
White solid, yield: (96 %); mp 186–188 °C. 1H NMR (400 MHz, CDCl3): 2.98 (dd, J = 18.0, 4.8 Hz, 1H, H-5), 3.60 (ddd, J = 18.8, 12.0, 2.4 Hz, 1H, H′-5), 4.78 (d, J = 13.2 Hz, 1H, H-1), 5.01 (td, J = 12.4, 4.8 Hz, 1H, H-6), 6.64 (d, J = 2.4 Hz, 1H, H-3), 7.05 (t, J = 7.2 Hz, 1H, Ar–H), 7.15 (t, J = 8.0 Hz, 1H, Ar–H), 7.25 (t, J = 8.0 Hz, 2H, Ar–H), 7.30 (d, J = 8.0 Hz, 1H, Ar–H), 7.41 (dd, J = 8.0, 1.2 Hz, 1H, Ar–H), 7.44 (d, J = 3.2 Hz, 2H, Ar–H), 7.46–7.49 (m, 3H, Ar–H), 7.59–7.61 (m, 2H, Ar–H), 8.21 (s, 1H, NH).
(1S,6R)-6-(4-Methoxyphenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4f)
White solid, yield: (90 %); mp 220 °C. 1H NMR (400 MHz, CDCl3): 2.32 (s, 3H, OCH3), 3.06 (dd, J = 18.4, 7.6 Hz, 1H, H-5), 3.36 (dd, J = 18.4, 4.8 Hz, 1H, H′-5), 3.71 (d, J = 8.0 Hz, 1H, H-1), 4.17 (dd, J = 12.8, 7.6 Hz, 1H, H-6), 6.58 (s, 1H, H-3), 7.08 (t, J = 7.2 Hz, 1H, Ar–H), 7.12 (d, J = 8.0 Hz, 2H, Ar–H), 7.21 (d, J = 8.0 Hz, 2H, Ar–H), 7.28 (t, J = 8.4 Hz, 3H, Ar–H), 7.40–7.47 (m, 5H, Ar–H), 7.56 (t, J = 6.4 Hz, 2H, Ar–H), 8.01 (s, 1H, NH).
(1S,6R)-6-(4-Cyanophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4g)
White solid, yield: (98 %); mp 225 °C. 1H NMR (400 MHz, DMSO): 3.07 (dd, J = 18.0, 4.0 Hz, 1H, H-5), 3.18 (dd, J = 17.6, 10.4 Hz, 1H, H′-5), 3.95 (td, J = 13.2, 4.8 Hz, 1H, H-6), 4.02 (d, J = 13.2 Hz, 1H, H-1), 6.60 (d, J = 1.6 Hz, 1H, H-3), 7.01 (t, J = 7.2 Hz, 1H, Ar–H), 7.24 (d, J = 8.0 Hz, 2H, Ar–H), 7.44 (d, J = 8.8 Hz, 3H, Ar–H), 7.47 (d, J = 0.8 Hz, 2H, Ar–H), 7.66 (d, J = 8.0 Hz, 2H, Ar–H), 7.73 (t, J = 5.2 Hz, 2H, Ar–H), 7.80 (d, J = 8.0 Hz, 2H, Ar–H), 10.10 (s, 1H, NH).
(1S,6R)-2-Oxo-N,4-diphenyl-6-(pyridin-3-yl)cyclohex-3-enecarboxamide (4i)
White solid, yield: (88 %); mp 196–198 °C. 1H NMR (400 MHz, DMSO): 3.09 (dd, J = 18.2, 4.4 Hz, 1H, H-5), 3.21 (ddd, J = 18.0, 11.4, 2.0 Hz, 1H, H′-5), 3.88 (td, J = 13.0, 4.8 Hz, 1H, H-6), 3.51 (d, J = 12.8 Hz, 1H, H-1), 6.60 (d, J = 2.0 Hz, 1H, H-3), 7.01 (t, J = 7.2 Hz, 1H, Ar–H), 7.24 (t, J = 12.8 Hz, 2H, Ar–H), 7.35 (dd, J = 4.8, 7.6 Hz, 1H, Ar–H), 7.43 (d, J = 7.6 Hz, 2H, Ar–H), 7.47 (dd, J = 2.4, 5.2 Hz, 2H, Ar–H), 7.75 (dd, J = 7.4, 2.4 Hz, 2H, Ar–H), 7.89 (d, J = 8.0 Hz, 1H, Ar–H), 8.42 (dd, J = 4.8, 1.2 Hz, 1H, Ar–H), 8.65 (d, J = 1.6 Hz, 1H, Ar–H), 10.10 (s, 1H, NH).
(1S,6R)-6-(4-Chlorophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4l)
Pale yellow, yield: (98 %); mp: 225–227 °C. IR (KBr) (νmax/cm−1): 3418, 3387, 1698, 1659, 1600, 1517, 1436, 1360, 1172, 888, 768, 757, 691. 1H NMR (400 MHz, CDCl3): 3.04 (dd, J = 20.0, 4.0 Hz, 1H, H-5), 3.44 (dd, J = 10.5, 2.0 Hz, 1H, H′-5), 3.85 (td, J = 13.2, 4.8 Hz, 1H, H-6), 3.96 (d, J = 13.2 Hz, 1H, H-1), 6.59 (d, J = 2.0 Hz, 1H, H-3), 7.01 (t, J = 8.0 Hz, 1H, Ar–H), 7.25 (t, J = 8.0 Hz, 2H, Ar–H), 7.38 (d, J = 8.0 Hz, 2H, Ar–H), 7.43–7.48 (m, 7H, Ar–H), 7.73 (dd, J = 1.2, 8.0 Hz, 1H, Ar–H), 10.08 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ 35.6, 42.8, 59.9, 119.5, 123.8, 123.9, 126.9, 128.7, 129.1, 129.3, 129.9, 130.9, 131.8, 137.8, 139.1, 141.6, 159.3, 167.6, 195.6. MS (EI, 70 eV) m/z (%): 401 (M+, 20), 309 (4), 281 (100), 252 (4), 228 (12), 202 (12), 179 (1), 157 (39), 115 (38), 93 (85), 65 (13), 43 (2). Anal. Calcd for C25H20ClNO2: C, 74.71; H, 5.02; N, 3.49. Found: C, 74.84; H, 5.12; N, 3.57.
Conclusion
In conclusion, a practical, efficient, ecofriendly and convenient method for the synthesis of a series of biologically relevant 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides (poly substituted cyclohexenones) via a one-pot, three-component reaction of acetoacetanilide, acetophenone and aromatic aldehydes is described using Na2CO3–H2O/EtOH as the catalytic system. A short reaction time, high yields, a clean process, a simple methodology, easy workup, and green sustainable conditions are the key features involved in the present protocol. Also, the products have two stereocenters and are synthesized in a diastereoselective manner. These features will enable this protocol to find widespread applications in the field of organic synthesis.
References
B.M. Trost, Science 254, 1471 (1991)
A.J. Von Wangelin, H. Neumann, D. Gördes, S. Klaus, D. Strübing, M. Beller, Chem. Eur. J. 9, 4286 (2003)
S.J. Park, G. Keum, S.B. Kang, H.Y. Koh, Y. Kim, D.H. Lee, Tetrahedron Lett. 39, 7109 (1998)
R.V.A. Orru, M. de Greef, Synthesis 1471 (2003)
F. Tamaddon, M. Farahi, B. Karami, J. Mol. Catal. A: Chem. 356, 85 (2011)
M.B. Deshmukh, S.M. Salunkhe, D.R. Patil, P.V. Anbhule, Eur. J. Med. Chem. 44, 2651 (2009)
H. Bienayme, C. Hulme, G. Oddon, P. Schmidt, Chem. Eur. J. 6, 3321 (2000)
A. Hasaninejad, M. Shekouhy, N. Golzar, A. Zare, M.M. Doroodmand, Appl. Catal. A: Gen. 402, 11 (2011)
S. Samshuddin, B. Narayana, B.K. Sarojini, L.N. Madhu, Med. Chem. Res. 22, 3002 (2013)
N. Krause, A. Hoffmann-Röder, Synthesis 171 (2001)
S.K. Awasthi, N. Mishra, B. Kumar, M. Sharma, A. Bhattacharya, L.C. Mishra, V.K. Bhasin, Med. Chem. Res. 18, 407 (2009)
D.H. Vyas, S.D. Tala, J.D. Akbari, M.F. Dhaduk, H.S. Joshi, Indian J. Chem. 48B, 1405 (2009)
N.A. Shakil, M.K. Singh, J. Kumar, M. Sathiyendiran, G. Kumar, M.K. Singh, R.P. Pandey, A. Pandey, V.S. Parmar, J. Environ. Sci. Health, Part B 45, 524 (2010)
N.B. Ayrim, Al- Mustansiriyah J. Sci. 23, 99 (2012)
K.L. Ameta, Res. Chem. Intermed. 41, 3433 (2015)
E. Findik, Y. Budak, M. Ceylan, Synth. Commun. 39, 3647 (2009)
S. Samshuddin, B. Narayana, B.K. Sarojini, H.S. Yathirajanc, R. Raghavendr, Der Pharm. Chem. 4, 1445 (2012)
F.A. Lakhvich, E.V. Koroleva, A.A. Akhrem, Chem. Heterocycl. Comp. 25, 359 (1989)
S. Mukhtar, M. Ramadan, W. Ansari, G. Lemiere, A. De Groot, R. Dommisse, Molecules 4, 232 (1999)
S.H. Bae, K.S. Kim, Y. Park, Heterocycles 53, 159 (2000)
R.H. Swellem, Y.A. Allam, G.A. Nawwar, Z. Naturforsch. 54b, 1197 (1999)
E.A. Bakhite, J. Chem. Res., Synop. 11, 500 (2000)
M.A. Al-Shikh, A.M.S. El-Din, E.A. Hafez, M.H. Elnagdi, J. Chem. Res. 174 (2004)
S.S. Lim, S.H. Jung, J. Ji, K.H. Shin, S.R. Keum, J. Pharm. Pharmacol. 53, 653 (2001)
N.A. Begum, N. Roy, R.A. Laskar, K. Roy, Med. Chem. Res. 20, 184 (2011)
M.L. Go, X. Wu, X.L. Liu, Curr. Med. Chem. 12, 481 (2005)
F. Chimenti, R. Fioravanti, A. Bolasco, P. Chimenti, D. Secci, R. Rossi, M. Yáñez, F. Orallo, F. Ortuso, S. Alcaro, J. Med. Chem. 52, 2818 (2009)
M. Liu, P. Wilairat, M.-L. Go, J. Med. Chem. 44, 4443 (2001)
S. Ducki, R. Forrest, J.A. Hadfield, A. Kendall, N.J. Lawrence, A.T. McGown, D. Rennison, Bioorg. Med. Chem. Lett. 8, 1051 (1998)
F. Bois, A. Boumendjel, A. Mariotte, G. Conseil, A. Di Petro, Bioorg. Med. Chem. 7, 2691 (1999)
J.R. Dimmock, D.W. Elias, M.A. Beazely, N.M. Kandepu, Curr. Med. Chem. 6, 1125 (1999)
S. Kaushik, N. Kumar, S. Drabu, T. Ph. Res. 3, 257 (2010)
R. Romagnoli, P.G. Baraldi, M.D. Carrion, C.L. Cara, O. Cruz-Lopez, D. Preti, Bioorg. Med. Chem. 16, 5367 (2008)
K.L. Lahtchev, D.I. Batovska, S.P. Parushev, V.M. Ubiyvovk, A.A. Sibirny, Eur. J. Med. Chem. 43, 2220 (2008)
S. Bag, S. Ramar, M.S. Degani, Med. Chem. Res. 18, 309 (2009)
F. Lunardi, M. Guzela, A.T. Rodrigues, R. Corre, I. Eger-Mangrich, M. Steindel, E.C. Grisard, J. Assreuy, J.B. Calixto, A.R.S. Santos, Antimicrob. Agents Chemother. 47, 1449 (2003)
M.S. Cheng, R. Shi, G. Kenyon, Chin. Chem. Lett. 11, 851 (2000)
N.A. Begum, N. Roy, R.A. Laskar, K. Roy, Med. Chem. Res. 19, 1 (2010)
S.K. Awasthi, N. Mishra, S.K. Dixit, A. Singh, M. Yadav, S.S. Yadav, S. Rathaur, Am. J. Trop. Med. Hyg. 80, 764 (2009)
N. Hamdi, C. Fischmeister, M.C. Puerta, P. Valerga, Med. Chem. Res. 19, 1 (2010)
S. Vogel, S. Ohmayer, G. Brunner, J. Heilmann, Bioorg. Med. Chem. 16, 4286 (2008)
P.M. Sivakumar, P.K. Prabhakar, M. Doble, Med. Chem. Res. 19, 1 (2010)
F. Hiromichi, K. Naoyuki, S. Yoshinari, N. Yasushi, K. Yasuyuki, Tetrahedron Lett. 43, 4825 (2002)
J. Safaei-Ghomi, Z. Alishahi, J. Fudan. Univ. Nat. Sci. 44, 789 (2005)
C. Mc Bride, P. Renhowe, C. Heise, J. Jansen, G. Lapointe, S. Ma, R. Pineda, J. Vora, M. Wiesmann, C. Shafer, Bioorg. Med. Chem. Lett. 16, 3595 (2006)
B.C. Kim, J.L. Kim, Y.U. Jhang, Bull. Korean. Chem. Soc. 15(2), 97 (1994)
B.C. Kim, J.L. Kim, Y.U. Jhang, Chem. Abstr. 121, 50097z (1994)
M. Yamaguchi, N. Maruyama, T. Koga, K. Kamei, M. Akima, T. Kuroki, M. Hamana, N. Ohi, Chem. Pharm. Bull. 43, 332 (1995)
V. Gorge, L. De, U.T. Kim, L. Jing, J. Med. Chem. 41, 2411 (1998)
S. Budavari (ed.), The Merck Index (Merck & Co Inc., Rahway, 1989)
W.L. Faith, D. Keyes, R. Clark, Industrial Chemicals (Wiley, New York, 1966)
D.R. Lide (ed.), CRC Handbook of Chemistry and Physics (CRC Press, Boca Raton, 2001)
M.R. Mousavi, N. Hazeri, M.T. Maghsoodlou, S. Salahi, S.M. Habibi-Khorassani, Chin. Chem. Lett. 24, 411 (2013)
N. Hazeri, M.T. Maghsoodlou, M.R. Mousavi, J. Aboonajmi, M. Safarzaei, Res. Chem. Intermed. doi:10.1007/s11164-013-1179-z
M.T. Maghsoodlou, N. Khorshidi, M.R. Mousavi, N. Hazeri, S.M. Habibi-Khorassani, Res. Chem. Intermed. doi:10.1007/s11164-014-1839-7
M.R. Mousavi, M.T. Maghsoodlou, J. Iran. Chem. Soc. 12, 743 (2015)
M.R. Mousavi, J. Aboonajmi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, M. Safarzaei, Lett. Org. Chem. 10, 171 (2013)
J. Aboonajmi, M.R. Mousavi, M.T. Maghsoodlou, N. Hazeri, A. Masoumnia, Res. Chem. Intermed. 41, 1925 (2015)
M.R. Mousavi, M.T. Maghsoodlou, N. Hazeri, J. Aboonajmi, S.M. Habibi-Khorassani, Iranian J. Org. Chem. 6, 1323 (2014)
M.R. Mousavi, J. Aboonajmi, M.T. Maghsoodlou, N. Hazeri, J. Chem. Res. 76 (2014)
M.R. Mousavi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, J. Iran Chem. Soc. 12, 1419 (2015)
M.R. Mousavi, M.T. Maghsoodlou, Monatsh. Chem. 145, 1967 (2014)
M.R. Mousavi, M.T. Maghsoodlou, S.M. Habibi-Khorassani, Mol. Divers. 18, 821 (2014)
Acknowledgments
The University of Sistan and Baluchestan is thanked for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousavi, M.R., Maghsoodlou, M.T. & Gharari, H. Sodium carbonate-catalyzed Claisen–Schmidt condensation: one-pot synthesis of highly functionalized cyclohexenones under environmental conditions. Res Chem Intermed 42, 2233–2246 (2016). https://doi.org/10.1007/s11164-015-2146-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2146-7