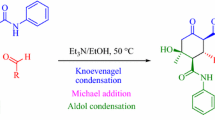
Abstract
A series of 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides were synthesized by condensing acetophenone and aromatic aldehydes with acetoacetanilide in ethanol in the presence of 2-hydroxyethylammonium acetate (2-HEAA) as a basic ionic liquid at ambient conditions. This process is simple, efficient and environmentally benign and proceeds in high yield, short reaction times and there is no need for column chromatography purification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Commonly, \(\alpha ,\!\beta \)-Enone is known as chalcone. Chalcones own an \(\alpha ,\!\beta \)-unsaturated grouping with two aromatic rings in the molecules. A highly electrophilic three-carbon \(\alpha ,\!\beta \)-unsaturated carbonyl system has interconnected the rings (Fig. 1) [1–4]. Chalcones are very reactive compounds and increase their reactivity due to a keto-ethylinic type (–CO–CH=CH–) conjugated double bond system present in the structure [5–7]. Chalcones have occupied a unique place in medicinal and biological chemistry. Chalcones and derivatives comprise a significant class of compounds possessing diverse biological and pharmacological properties including anti-bacterial [4, 8, 9], anticonvulsant [10], anti-cancer [11], anti-fungal [12, 13], antiprotozoal [14], antimalarial [1, 2, 15], larvicidal [16], antifilarial [17], anti-inflammatory [18], antioxidant [19, 20], and antimicrobial [21]. Also, they have been identified as inhibitors of enzymes, specifically mammalian alpha-amylase [3, 22], monoamine oxidase (MAO) [23], and cyclo-oxygenase (COX)[24]. The conjugate addition of a stabilized carbanion to \(\alpha ,\!\beta \)-unsaturated carbonyl compounds is one of the fundamental C–C bond-forming reactions in organic synthesis [25–27]. Therefore, an important feature of chalcones is their ability to act as activated unsaturated systems in conjugated additions of carbanions in the presence of suitable basic catalysts [28, 29]. For example, the Michael addition of ethylacetoacetate to chalcone yields 4,6-diaryl-2-oxo-cyclohex-3-ene-1-carboxylate derivatives which are efficient synthons for building spiranic compounds [30], and are also important intermediates in the synthesis of joined heterocycles, such as benzopyrazoles and benzisoxazoles [31], benzoselenadiazoles and benzothiadiazoles [32], 2H-indazoles [33], and carbazole derivatives [34]. Cyclohexenone derivatives are well-known lead molecules for the treatment of inflammation and autoimmune diseases [35, 36]. Also, cyclohexenone and inazole derivatives exhibit a variety of pharmacological properties, such as antitumor [37], tyrosine kinases inhibitor [38], antipyretic [39], antiasthametic [40], antiviral [41], anti-bacterial, anti-fungal, anti-cancer, and anti-tubercular activity [42]. Because of the importance of cyclohexenone derivatives from a pharmaceutical and biological point of view, there is still a need to develop efficient, mild, and environmentally benign protocols for the synthesis of these compounds. Herein we present an eco-friendly procedure for the synthesis simple and effi cient and characterization of a novel series cyclohexenone derivatives called 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides via a one-pot three-component reaction using acetophenone 1, aromatic aldehydes 2, and 3-oxo-N-phenylbutanamide (acetoacetanilide) 3 in the presence of 2-hydroxyethylammonium acetate as a ionic liquid in ethanol at room temperature (Scheme 1).
Results and discussion
We first examined the reaction of 4-methoxy benzaldehyde and acetophenone with acetoacetanilide using 15 mol% of different catalysts. In the initial search for an efficient catalyst, four different ionic liquids were screened in our model reaction. The reaction did not progress even after 48 h in the absence of catalyst. As shown in Table 1, the reaction was performed in the presence of diethylammonium hydrogen sulfate (DEAS), triethylammonium hydrogen sulfate (TEAS), and triethylammonium dihydrogen phosphate (TEAP), the desired product was obtained in low yields along with undesired by-products. However, after optimizing the catalyst amount, we found that 20 mol% 2-hydroxyethylammonium acetate (2-HEAA) gave desired product in high yields. Increasing the catalyst loading did not improve product yields (entries, 8 and 9). Also, we found that when the reactions were run under solvent-free and under optimized conditions, the reaction did not lead to the desired product (Table 1, entry 10) which could be due to the lack of effective interaction between reactants and the catalyst in the absence of solvent.
During the course of screening a variety of reaction conditions such as solvent, reaction temperature, and the amount of the catalyst, we found that the use of ethanol as a solvent was essential for the efficient conversion of raw materials to (1R,6S)/(1S,6R)-6-(4-methoxyphenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide 4d. It is remarkable that, ethanol is a green solvent. The structure of (1R,6S)/(1S,6R)-6-(4-methoxyphenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide 4d was confirmed by spectral techniques, such as like \(^{1}\hbox {H-NMR}, ^{13}\!\hbox {C-NMR}\), elemental, and mass spectral analysis. The products obtained in this work are chiral compounds. They have two chiral centers on C-1 and C-6, with protons in anti-configuration. Also, the protons on C-5 are diastereotopic. This reaction leads to the creation of two stereogenic centers (Fig. 2).
To explore the scope of our methodology, we extended this reaction to various aromatic aldehydes in the presence of electron-withdrawing (\(\hbox {NO}_{2}\), Cl, CN, and Br) and electron-releasing (OMe) substituents, both of which gave the desired product. The results are shown in Table 2. These results show that the functional groups did not play any significant role in the reactivity of the substrate.
The cyclo-condensation of acetoacetanilide with chalcones leads to the generation of two chiral centers at C-1 and C-6 in the structure of cyclohexenones. The products were fully characterized by melting points, elemental analyses, IR, \(^{1}\hbox {H}\) and \(^{13}\hbox {C}\) NMR, and mass spectra. For example, the IR spectra of 4g revealed a sharp absorption band at \(3308~\hbox {cm}^{-1}\) associated to NH, while a strong stretching band at \(2230~\hbox {cm}^{-1}\) was attributed to \(\hbox {C}\equiv \hbox {N}\). Furthermore, two sharp strong absorption bands were noticed at approximately 1672 and 1656 \(\hbox {cm}^{-1}\) and were assigned to the carbonyl groups. The \(^{1}\hbox {H}\) and \(^{13}\hbox {C}\) NMR, and mass spectra substantiated the results of the IR analysis. The mass spectrum of 4g recorded a molecular ion signal \((\hbox {M}^{+})\) at \(m/z = 392\), which is consistent with the proposed structure. The \(^{1}\hbox {H}\) NMR spectrum of compound 4g, exhibited two doublets of doublets at 3.07 ppm \((J = 18.0, 4.0 \ \hbox {Hz})\) and 3.18 ppm \((J = 17.6, 10.4 \ \hbox {Hz})\) for methylene protons of cyclohexenone ring (H-5, \(\hbox {H}^{{\prime }}\)-5) respectively. One of the methine protons of cyclohexenone ring (H-6) was observed as a triplet of doublet (td) at \(\delta \) 3.95 ppm \((J = 13.2, 4.8\ \hbox {Hz})\) and another methine proton (H-1) appeared as a doublet at \(\delta \) 4.02 ppm \((J = 13.2 \ \hbox {Hz})\). The vinyl proton (H-3) was observed as a doublet at 6.60 ppm (\(J\) = 1.6 Hz). The aromatic protons were recorded as doublets and triplets at \(\delta \) 7.01–7.80 ppm. The NH proton was observed at \(\delta \) 10.10 ppm, indicating an intramolecular hydrogen bond interaction with the vicinal carbonyl group on cyclohexenone ring. The \(^{13}\)C-NMR spectrum of compound 4g showed 20 distinct signals consistent with the cyclohexenone structure. According to the structure of 4g, 25 carbon signals should be present in the \(^{13}\hbox {C}\) NMR spectrum. However, due to the same carbons of (C-3, C-5) and (C-2, C- 6) in the aromatic rings in the structure of 4g, on the \(^{13}\hbox {C}\) NMR spectrum one signal is observed for each of the pairs. In the \(^{13}\hbox {C}\) NMR spectrum of this compound, the C-6 carbon was observed at \(\delta \) 35.1 ppm and C-5 at \(\delta \) 43.4 ppm. The C-1, C-3, and C-4 carbon signals were observed at \(\delta \) 59.3, 126.9, and 159.2 ppm, respectively. The \(\hbox {C}\equiv \hbox {N}\) and aromatic carbons were found at \(\delta \) 110.2-148.3 ppm. In addition, the carbon of carbonyl of amide group was shown at \(\delta \) 167.4 ppm. And, the carbon of carbonyl of conjugated double bond C=C system (C-2) was observed at 195.3 ppm.
A part of the \(^{1}\hbox {H}\) NMR spectrum of 4b is shown in Fig. 3. In this figure, four protons of cyclohexenone, H-5, H’-5, H-1, and H-6 were characterized and assigned.
A reasonable pathway for the synthesis of highly substituted cyclohexenone derivatives is given in Scheme 2. The Claisen-Schmidt condensation method for the synthesis of chalcones is very attractive, since it specifically generates the trans (E)-isomer [43–45]. Hence, the reaction of acetophenone 1 with different aryl aldehydes 2 in the presence of catalytic amount of 20 mol% 2-hydroxyethylammonium acetate afforded the desired chalcone 5. Subsequently, Michel addition of chalcone with acetoacetanilide 3 in the presence of IL followed by internal Claisen condensation gives 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides 4.
In conclusion, we have disclosed an extremely facile and environmentally benign synthesis of a series of 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides via reaction of acetophenone and different aromatic aldehydes with acetoacetanilide under room temperature with ethanol as green solvent by the use of a catalytic amount of 2-hydroxyethylammonium acetate (2-HEAA). This work not only offers substantial improvements in reaction rates and yields, but also avoids the use of hazardous catalysts or solvents. Moreover, the mild reaction conditions, high yields, easy work-up, and clean reaction profiles are some of the advantages of this procedure. Therefore, this protocol is eco-friendly.
It is worth noting that literature procedures generally point to the synthesis of cyclohexenone derivatives in two steps. Such as in the first step chalcone is synthesized from the reaction of aldehyde and acetophenone. After isolation, purification and characterization, in the second step chalcone would react in new conditions for preparing cyclohexenone derivatives. But in the present work, synthesis of novel cyclohexenones (2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides) is discussed in a one-pot three-component reaction.
Experimental
General
Melting point and IR spectra of all compounds were obtained on an Electrothermal 9100 apparatus and a JASCO FT/IR-460 plus spectrometer, respectively. \(^{1}\hbox {H}\) and \(^{13}\hbox {C}\) NMR spectra of compounds were recorded on a Bruker DRX-400 Avance instrument using DMSO or \(\hbox {CDCl}_{3}\) as the solvent and TMS as an internal standard at 400 and 100 MHz, respectively. Elemental analyses for C, H, and N for the new compounds were performed using a Heraeus CHN-O-Rapid analyzer. The mass spectra for the new compounds were recorded on an Agilent Technology (HP) mass spectrometer, operating at an ionization potential of 70 eV. All reagents were purchased from Merck (Darmastadt, Germany), Acros (Geel, Belgium) and Fluka (Buchs, Switzerland), and use without further purification.
Typical procedure for the preparation of 2-hydroxyethylammonium acetate (2-HEAA)
As reported in the literature [46, 47], for the synthesis of ionic liquid (2-HEAA) a solution of acetic acid (50 mmol, 3.00 g) in EtOH (1.5 mL) was added dropwise to a stirring solution of 2-aminoethanol (50 mmol, 3.05 g) in EtOH (1.5 mL) at room temperature within 1 h. The resulting solution was stirred at room temperature for another 20 h. Ethanol was removed in vacuo and the residual was dried in vacuo at \(50\,^{\circ }\hbox {C}\) for 48 h to give 2-hydroxyethylammonium acetate (2-HEAA) as a light yellow, viscous liquid.
General procedure for the preparation 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamide derivatives
To a solution of acetophenone (1 mmol), aromatic aldehyde (1 mmol), and acetoacetanilide (1 mmol) was added 2-HEAA 20 mol%. The resulting mixture was stirred for the appropriate time (Table 2) at room temperature. After completion of the reaction, as indicated by TLC (ethyl acetate/n-hexane, 1:3), a thick precipitate was obtained. The solid was filtered, washed with ethanol, and crystallized from ethanol to give pure product. The pure products were characterized by conventional spectroscopic methods. Physical and spectral data for the synthesized compounds are represented below:
6-(2-bromophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4a)
White solid, yield: (99 %); mp 220–222 \(^{\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3411, 1676, 1629, 1601, 1557, 1445, 1370, 1248, 1181, 757, 693. \(^{1}\hbox {H}\) NMR (400 MHz, DMSO): 3.01 (s, 2H, H-5), 4.20 (d, \(J = 13.2 \ \hbox {Hz}\), 1H, H-1), 4.26 (dd, \(J \)= 9.6, 5.2 Hz, 1H, H-6), 6.63 (s, 1H, H-3), 7.01 (t, \(J = 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.17 (t, \(J = 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.24 (t, \(J = 7.6 \ \hbox {Hz}\), 2H, Ar-H), 7.40 (t, \(J = 7.6 \ \hbox {Hz}\), 1H, Ar-H), 7.46 (d, \(J = 6.0 \ \hbox {Hz}\), 5H, Ar-H), 7.62 (dd, \(J = 11.6, 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.72 (d, \(J = 7.6 \ \hbox {Hz}\), 2H, Ar-H), 10.21 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, DMSO): \(\delta \) 34.8, 42.2, 58.7, 119.5, 123.8, 123.9, 124.5, 126.8, 128.2, 128.5, 129.1, 129.3, 129.4, 131.0, 133.4, 137.6, 139.1, 141.1, 158.7, 167.3, 195.7. MS (EI, 70 eV) \(m/z\) (%): 447 (\(\hbox {M}^{+}+1\), 19), 366 (98), 327 (20), 298 (1), 273 (19), 247 (31), 215 (19), 183 (8), 157 (17), 115 (44), 93 (100), 65 (18). Anal. Calcd. for \(\hbox {C}_{25}\hbox {H}_{20}\hbox {BrNO}_{2}\): C, 67.27; H, 4.52; N, 3.14. Found: C, 67.40; H, 4.64; N, 3.22.
6-(3-nitrophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4b)
Pale yellow solid, yield: (98 %); mp 200–202 \(^{\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3413, 1632, 1601, 1558, 1531, 1445, 1353, 758, 689. \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)): 3.08 (dd, \(J = 18.0, 8.4\) Hz, 1H, H-5), 3.38 (dd, \(J = 18.2\), 4.8 Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.76 (d, \(J= 8.8 \ \hbox {Hz}\), 1H, H-1), 4.32 (dd, \(J = 13.1\), 8.8 Hz, 1H, H-6), 6.63 (s, 1H, H-3), 7.10 (t, \(J = 7.6 \ \hbox {Hz}\), 1H, Ar-H), 7.28 (dd, \(J = 9.2\), 5.2 Hz, 2H, Ar-H), 7.45 (d, \(J = 2.4 \ \hbox {Hz}\), 2H, Ar-H), 7.48 (d, \(J = 7.2 \ \hbox {Hz}\), 3H, Ar-H), 7.52 (d, \(J = 8.0 \ \hbox {Hz}\), 1H, Ar-H), 7.57 (t, \(J = 6.4 \ \hbox {Hz}\), 2H, Ar-H), 7.69 (d, \(J = 7.6 \ \hbox {Hz}\), 1H, Ar-H), 8.13 (d, \(J = 8.4 \ \hbox {Hz}\), 1H, Ar-H), 8.19 (s, 1H, Ar-H), 8.25 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, \(\hbox {CDCl}_{3}\)): \(\delta \) 33.8, 41.0, 58.5, 120.0, 122.0, 122.3, 124.4, 124.6, 126.3, 128.9, 129.0, 129.8, 131.0, 134.2, 137.3, 137.3 148.5, 159.6, 164.4, 194.5. MS (EI, 70 eV) \(m/z\) (%): 412 (\(\hbox {M}^{+}\), 29), 369 (1), 344 (1), 320 (9), 293 (87), 270 (1), 246 (16), 224 (1), 202 (30), 176 (39), 144 (27), 115 (69), 93 (100), 65 (24). Anal. Calcd. for \(\hbox {C}_{25}\hbox {H}_{20}\hbox {N}_{2}\hbox {O}_{4}\): C, 72.80; H, 4.89; N, 6.79. Found: C, 72.86; H, 4.96; N, 6.85.
6-(2-chlorophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4c)
White solid, yield: (97 %); mp 224–226 \(^{\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3383, 3084, 1674, 1627, 1601, 1559, 1445, 1249, 1180, 757, 693. \(^{1}\hbox {H}\) NMR (400 MHz, DMSO): 3.03 (d, \(J = 7.2 \ \hbox {Hz}\), 2H, H-5), 4.24 (d, \(J = 13.2 \ \hbox {Hz}\), 1H, H-1), 4.30 (dd, \(J= 14.0\), 7.2 Hz, 1H, H-6), 6.63 (s, 1H, H-3), 7.00 (t, \(J = 7.6 \ \hbox {Hz}\), 1H, Ar-H), 7.24 (t, \(J= 7.6 \ \hbox {Hz}\), 3H, Ar-H), 7.35 (t, \(J= 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.46 (dd, \(J= 15.2\), 9.2 Hz, 6H, Ar-H), 7.67 (d, \(J= 7.6 \ \hbox {Hz}\), 1H, Ar-H), 7.72 (d, \(J= 7.6 \ \hbox {Hz}\), 2H, Ar-H), 10.26 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, DMSO): \(\delta \) 34.6, 39.6, 58.6, 119.4, 123.7, 124.0, 126.8, 127.0, 127.9, 128.4, 128.9, 129.1, 129.3, 130.1, 131.0, 133.4, 137.6, 139.2, 139.6, 158.7, 167.4, 195.7. MS (EI, 70 eV) \(m/z\) (%): 401 (\(\hbox {M}^{+}\), 3), 347 (2), 283 (3), 265 (4), 236 (3), 219 (5), 197 (3), 179 (5), 160 (4), 132 (5), 108 (48), 81 (28), 52 (100). Anal. Calcd. for \(\hbox {C}_{25}\hbox {H}_{20}\hbox {ClNO}_{2}\): C, 74.71; H, 5.02; N, 3.49. Found: C, 74.75; H, 5.06; N, 3.55.
6-(4-methoxyphenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4d)
White solid, yield: (96 %); mp 217–219 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3306, 3203, 1680, 1666, 1651, 1603, 1547, 1444, 1362, 1175, 759, 692. \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)): 2.32 (s, 3H, \(\hbox {OCH}_{3}\)), 3.06 (dd, \(J = 18.4\), 7.6 Hz, 1H, H-5), 3.36 (dd, \(J = 18.4\), 4.8 Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.71 (d, \(J = 8.0 \ \hbox {Hz}\), 1H, H-1), 4.17 (dd, \(J= 12.8\), 7.6 Hz, 1H, H-6), 6.58 (s, 1H, H-3), 7.08 (t, \(J= 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.12 (d, \(J= 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.21 (d, \(J= 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.28 (t, \(J = 8.4 \ \hbox {Hz}\), 3H, Ar-H), 7.40–7.47 (m, 5H, Ar-H), 7.56 (t, \(J= 6.4 \ \hbox {Hz}\), 2H, Ar-H), 8.01 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, \(\hbox {CDCl}_{3}\)): \(\delta \) 21.0, 34.1, 40.9, 59.5, 124.2, 124.3, 126.3, 126.4, 127.2, 128.8, 128.9, 129.4, 129.4, 130.6, 136.7, 137.6, 137.8 139.2, 160.0, 165.4, 195.8; MS (EI, 70 eV) \(m/z\) (%): 397 (\(\hbox {M}^{+}\), 1), 392 (1), 381 (35), 344 (1), 289 (4), 261 (100), 228 (6), 202 (3), 171 (4), 145 (25), 115 (23), 93 (37), 65 (6). Anal. Calcd. for \(\hbox {C}_{25}\hbox {H}_{20}\hbox {N}_{2}\hbox {O}_{4}\): C, 78.57; H, 5.83; N, 3.52. Found: C, 78.70; H, 5.94; N, 3.60.
6-(4-nitrophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4e)
Pale yellow solid, yield: (94 %); mp 202–204 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3415, 3304, 1663, 1606, 1555, 1517, 1445, 1349, 1176, 854, 759, 752, 692. \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)): 3.05 (dd, \(J= 18.0\), 8.4 Hz, 1H, H-5), 3.33 (dd, \(J = 18.0\), 4.8 Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.76 (d, \(J= 9.2 \ \hbox {Hz}\), 1H, H-1), 4.27 (m, 1H, H-6), 6.58 (s, 1H, H-3), 7.09 (t, \(J \)= 7.2 Hz, 1H, Ar-H), 7.27 (t, \(J= 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.39–7.48 (m, 5H, Ar-H), 7.49–7.55 (m, 4H, Ar-H), 8.16 (d, \(J= 8.4 \ \hbox {Hz}\), 2H, Ar-H), 8.32 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, \(\hbox {CDCl}_{3}\)): \(\delta \) 33.8, 41.3, 58.4, 120.0, 124.0, 124.2, 124.7, 126.3, 126.3, 128.4, 128.7, 128.9, 129.0, 131.0, 137.2, 137.3 146.9, 149.8, 159.5, 164.7, 194.7. MS (EI, 70 eV) \(m/z\) (%): 412 (\(\hbox {M}{+}\), 44), 344 (7), 320 (7), 292 (82), 266 (14), 228 (4), 202 (12), 157 (30), 115 (27), 93 (100), 66 (19). Anal. Calcd. for \(\hbox {C}_{25}\hbox {H}_{20}\hbox {N}_{2}\hbox {O}_{4}\): C, 72.80; H, 4.89; N, 6.79. Found: C, 72.87; H, 4.94; N, 6.85.
6-(2,6-dichlorophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4f)
White solid, yield: (95 %); mp 188–190 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3418, 3387, 1698, 1659, 1600, 1517, 1436, 1360, 1172, 768, 757, 691. \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)): 2.98 (dd, \(J= 18.0\), 4.8 Hz, 1H, H-5), 3.60 (ddd, \(J= 18.8\), 12.0, 2.4 Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 4.78 (d, \(J = 13.2 \ \hbox {Hz}\), 1H, H-1), 5.01 (td, \(J = 12.4\), 4.8 Hz, 1H, H-6), 6.64 (d, \(J = 2.4 \ \hbox {Hz}\), 1H, H-3), 7.05 (t, \(J = 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.15 (t, \(J = 8.0 \ \hbox {Hz}\), 1H, Ar-H), 7.25 (t, \(J = 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.30 (d, \(J = 8.0 \ \hbox {Hz}\), 1H, Ar-H), 7.41 (dd, \(J = 8.0\), 1.2 Hz, 1H, Ar-H), 7.44 (d, \(J = 3.2 \ \hbox {Hz}\), 2H, Ar-H), 7.46–7.49 (m, 3H, Ar-H), 7.59–7.61 (m, 2H, Ar-H), 8.21 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, \(\hbox {CDCl}_{3}\)): \(\delta \) 30.4, 38.7, 55.6, 120.1, 124.2, 126.2, 128.8, 128.9, 129.3, 130.2, 130.7, 133.9, 135.9, 137.1, 137.5 137.6, 159.6, 165.5, 195.1. MS (EI, 70 eV) \(m/z\) (%): 436 (M\(^{+}\), 6), 435 (14), 400 (100), 372 (1), 343 (6), 316 (36), 281 (36), 256 (9), 226 (6), 199 (32), 157 (35), 115 (45), 93 (71), 65 (20). Anal. Calcd. for \(\hbox {C}_{25}\hbox {H}_{19}\hbox {Cl}_{2}\hbox {NO}_{2}\): C, 68.82; H, 4.39; N, 3.21. Found: C, 68.88; H, 3.30; N, 6.85.
6-(4-cyanophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4g)
White solid, yield: (99 %); mp 224–226 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3308, 2230, 1672, 1656, 1603, 1543, 1443, 757, 692. \(^{1}\hbox {H}\) NMR (400 MHz, DMSO): 3.07 (dd, \(J = 18.0\), 4.0 Hz, 1H, H-5), 3.18 (dd, \(J= 17.6\), 10.4 Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.95 (td, \(J= 13.2\), 4.8 Hz, 1H, H-6), 4.02 (d, \(J = 13.2 \ \hbox {Hz}\), 1H, H-1), 6.60 (d, \(J= 1.6 \ \hbox {Hz}\), 1H, H-3), 7.01 (t, \(J= 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.24 (d, \(J= 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.44 (d, \(J= 8.8 \ \hbox {Hz}\), 3H, Ar-H), 7.47 (d, \(J= 0.8 \ \hbox {Hz}\), 2H, Ar-H), 7.66 (d, \(J= 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.73 (t, \(J = 5.2 \ \hbox {Hz}\), 2H, Ar-H), 7.80 (d, \(J= 8.0 \ \hbox {Hz}\), 2H, Ar-H), 10.10 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, DMSO): \(\delta \) 35.1, 43.4, 59.3, 110.2, 119.2, 119.5, 123.8, 123.9, 126.9, 129.1, 129.2, 129.3, 131.0, 132.8, 137.7 139.0, 148.3, 159.2, 167.4, 195.3. MS (EI, 70 eV) \(m/z (\%)\): 392 (\(\hbox {M}^{+}\), 41), 344 (5), 300 (7), 272 (100), 243 (6), 196 (4), 157 (38), 115 (35), 93 (70), 65 (14). Anal. Calcd. for \(\hbox {C}_{26}\hbox {H}_{20}\hbox {N}_{2}\hbox {O}_{2}\): C, 79.57; H, 5.14; N, 7.14. Found: C, 79.70; H, 5.22; N, 7.21.
6-(3-chlorophenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4h)
White solid, yield: (95 %); mp 187–189 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3368, 3302, 3086, 1665, 1628, 1600, 1560, 1498, 1445, 1368, 1247, 758, 692. \(^{1}\hbox {H}\) NMR (400 MHz, DMSO): 3.06 (dd, \(J= 18.0\), 4.4 Hz, 1H, H-5), 3.17 (m, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.86 (td, \(J= 12.8\), 4.4 Hz, 1H, H-6), 3.97 (d, \(J= 13.2 \ \hbox {Hz}\), 1H, H-1), 6.58 (d, \(J= 2.0 \ \hbox {Hz}\), 1H, H-3), 7.01 (t, \(J \)= 7.2 Hz, 1H, Ar-H), 7.24 (d, \(J= 8.4 \ \hbox {Hz}\), 2H, Ar-H), 7.27 (t, \(J= 2.0 \ \hbox {Hz}\), 1H, Ar-H), 7.34 (t, \(J \)= 8.0 Hz, 1H, Ar-H), 7.40 (d, \(J = 8.0 \ \hbox {Hz}\), 1H, Ar-H), 7.44–7.47 (m, 5H, Ar-H), 7.55 (s, 1H, Ar-H), 7.74 (dd, \(J = 7.2\), 2.4 Hz, 2H, Ar-H), 10.08 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, DMSO): \(\delta \) 35.5, 43.1, 59.7, 119.6, 123.8, 126.8, 126.9, 127.3, 128.0, 129.1, 129.3, 130.6, 130.9, 133.4, 137.8, 139.1, 145.1, 159.4, 167.6, 195.5. MS (EI, 70 eV) \(m/z\) (%): 401 (\(\hbox {M}^{+}\), 39), 342 (3), 309 (7), 281 (90), 252 (5), 228 (13), 202 (13), 179 (1), 157 (34), 115 (45), 93 (100), 65 (20). Anal. Calcd. for \(\hbox {C}_{25}\hbox {H}_{20}\hbox {ClNO}_{2}\): C, 74.71; H, 5.02; N, 3.49. Found: C, 74.78; H, 5.15; N, 3.62.
2-oxo-N,4-diphenyl-6-(pyridin-3-yl)cyclohex-3-enecarboxamide (4i)
White solid, yield: (92 %); mp 195–197 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3399, 3086, 1696, 1633, 1607, 1557, 1444, 1369, 1235, 1176, 756, 715, 693. \(^{1}\hbox {H}\) NMR (400 MHz, DMSO): 3.09 (dd, \(J = 18.2\), 4.4 Hz, 1H, H-5), 3.21 (ddd, \(J= 18.0\), 11.4, 2.0 Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.88 (td, \(J= 13.0\), 4.8 Hz, 1H, H-6), 3.51 (d, \(J= 12.8 \ \hbox {Hz}\), 1H, H-1), 6.60 (d, \(J= 2.0 \ \hbox {Hz}\), 1H, H-3), 7.01 (t, \(J= 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.24 (t, \(J= 12.8 \ \hbox {Hz}\), 2H, Ar-H), 7.35 (dd, \(J= 4.8\), 7.6 Hz, 1H, Ar-H), 7.43 (d, \(J= 7.6 \ \hbox {Hz}\), 2H, Ar-H), 7.47 (dd, \(J= 2.4\), 5.2 Hz, 2H, Ar-H), 7.75 (dd, \(J= 7.4\), 2.4 Hz, 2H, Ar-H), 7.89 (d, \(J= 8.0 \ \hbox {Hz}\), 1H, Ar-H), 8.42 (dd, \(J= 4.8\), 1.2 Hz, 1H, Ar-H), 8.65 (d, \(J \)= 1.6 Hz, 1H, Ar-H), 10.10 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, DMSO): \(\delta \) 35.2, 41.1, 59.6, 119.5, 123.9, 126.9, 129.1, 129.3, 130.9, 135.5, 137.8, 137.9, 139.0, 148.6, 149.7, 159.4, 167.5, 195.4. MS (EI, 70 eV) \(m/z\) (%): 368 (\(\hbox {M}^{+}\), 43), 344 (1), 309 (2), 281 (39), 248 (100), 220 (14), 197 (3), 157 (21), 132 (28), 93 (96), 65 (13). Anal. Calcd. for \(\hbox {C}_{24}\hbox {H}_{20}\hbox {N}_{2}\hbox {O}_{2}\): C, 78.24; H, 5.47; N, 7.60. Found: C, 78.31; H, 5.58; N, 7.69.
2-oxo-N,4-diphenyl-6-(pyridin-4-yl)cyclohex-3-enecarboxamide (4j)
White solid, yield: (97 %); mp 202–204 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3467, 3267, 3073, 1695, 1647, 1603, 1555, 1497, 1444, 1366, 1177, 756, 692. \(^{1}\hbox {H}\) NMR (400 MHz, DMSO): 3.08 (dd, \(J= 18.0\), 4.8 Hz, 1H, H-5), 3.17 (dd, \(J= 17.6\), 11.4, Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.87 (td, \(J = 13.2\), 4.8 Hz, 1H, H-6), 4.01 (d, \(J= 13.2 \ \hbox {Hz}\), 1H, H-1), 6.60 (d, \(J= 1.6 \ \hbox {Hz}\), 1H, H-3), 7.01 (t, \(J = 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.25 (t, \(J= 8.0 \ \hbox {Hz}\), 2H, Ar-H), 7.45 (t, \(J = 7.6 \ \hbox {Hz}\), 7H, Ar-H), 7.74 (t, \(J= 5.6 \ \hbox {Hz}\), 2H, Ar-H), 8.51 (d, \(J= 6.0 \ \hbox {Hz}\), 2H, Ar-H), 10.13 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, DMSO): \(\delta \) 34.8, 42.7, 59.0, 119.5, 123.4, 123.8, 123.9, 126.9, 129.1, 129.3, 131.0, 137.7, 139.0, 150.1, 151.3, 159.2, 167.4, 195.2. MS (EI, 70 eV) \(m/z\) (%): 368 (\(\hbox {M}^{+}\), 30), 344 (1), 309 (2), 281 (29), 248 (19), 220 (10), 197 (3), 171 (15), 144 (34), 115 (55), 93 (100), 65 (25). Anal. Calcd. for \(\hbox {C}_{24}\hbox {H}_{20}\hbox {N}_{2}\hbox {O}_{2}\): C, 78.24; H, 5.47; N, 7.60. Found: C, 78.31; H, 5.58; N, 7.69.
6-(2,3-dimethoxyphenyl)-2-oxo-N,4-diphenylcyclohex-3-enecarboxamide (4k)
White solid, yield: (90 %); mp 196–198 \(^ {\circ }\hbox {C}\). IR (KBr) \((\nu _{\mathrm{max}}/\hbox {cm}^{-1})\): 3413, 1666, 1600, 1556, 1477, 1445, 1365, 1274, 1073, 1002, 756, 693. \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)): 3.01 (d, \(J= 17.6 \ \hbox {Hz}\), 1H, H-5), 3.44 (ddd, \(J= 17.5\), 10.0, 3.2 Hz, 1H, \(\hbox {H}^{{\prime }}-5\)), 3.88 (s, 3H, \(\hbox {OCH}_{3}\)), 4.06 (s, 3H, \(\hbox {OCH}_{3}\)), 4.49 (d, \(J= 8.8 \ \hbox {Hz}\), 2H, H-1 and H-6), 6.53 (d, \(J= 2.8 \ \hbox {Hz}\), 1H, H-3), 6.79 (dd, \(J= 8.0\), 1.2 Hz, 1H, Ar-H), 6.96 (t, \(J= 8.0 \ \hbox {Hz}\), 1H, Ar-H), 7.08 (t, \(J= 7.2 \ \hbox {Hz}\), 1H, Ar-H), 7.22 (dd, \(J= 8.0\), 1.2 Hz, 1H, Ar-H), 7.32 (t, \(J= 8.4 \ \hbox {Hz}\), 3H, Ar-H), 7.39 (d, \(J= 7.2 \ \hbox {Hz}\), 2H, Ar-H), 7.51-7.55 (m, 4H, Ar-H), 8.24 (s, 1H, NH). \(^{13}\hbox {C}\) NMR (100 MHz, \(\hbox {CDCl}_{3}\)): \(\delta \) 28.6, 36.0, 55.6, 58.2, 60.8, 111.3, 119.8, 120.3, 121.6, 123.9, 124.8, 125.1, 126.4, 127.7, 128.9, 130.5, 135.7, 137.7, 146.7, 152.9, 160.1, 165.5, 196.0. MS (EI, 70 eV) \(m/z\) (%): 354 (\(\hbox {M}^{+}-1\), 2), 368 (3), 344 (1), 299 (7), 265 (1), 241 (100), 198 (26), 172 (1), 150 (74), 115 (15), 91 (7), 57 (9). Anal. Calcd. for \(\hbox {C}_{27}\hbox {H}_{25}\hbox {NO}_{4}\): C, 75.86; H, 5.89; N, 3.28. Found: C, 75.95; H, 5.94; N, 3.40.
References
Awasthi SK, Mishra N, Kumar B, Sharma M, Bhattacharya A, Mishra LC, Bhasin VK (2009) Potent antimalarial activity of newly synthesized substituted chalcone analogs in vitro. Med Chem Res 18:407–420. doi:10.3390/molecules170910331
Lim SS, Kim HS, Lee DU (2007) In vitro antimalarial activity of flavonoids and chalcones. Bull Korean Chem Soc 28:2495–2497
Rahman MA (2011) Chalcone: a valuable insight into the recent advances and potential pharmacological activities. Chem Sci J 2011:1–16. doi:10.4172/2150-3494.1000021
Saini RK, Choudhary AS, Joshi YC, Joshi P (2005) Solvent free synthesis of chalcones and their antibacterial activities. E-J Chem 2:224–227. doi:10.1155/2005/294094
Hussain MA (2001) Synthesis of aurentiacin. Indian J Chem 40B:324–326
Baradia R, Rao JT (2004) Synthesis and antimicrobial activity of some \(\alpha,\!\beta \)-unsaturated aromatic ketones. Asian J Chem 16:1194–1196
Ilango K, Valentina P, Saluja G (2010) Synthesis and in vitro anticancer activity of some substituted chalcones derivatives. Res J Pharm Biol Chem Sci 1:354–359
Zhang XW, Zhao DH, Quan YC, Sun LP, Yin XM, Guan LP (2010) Synthesis and evaluation of anti-inflammatory activity of substituted chalcone derivatives. Med Chem Res 19:403–412. doi:10.1007/s00044-009-9202-z
Bhatia NM, Mahadik KR, Bhatia MS (2009) QSAR analysis of 1,3-diaryl-2-propen-1-ones and their indole analogs for designing potent antibacterial agents. Chem Pap 63:456–463
Kaushik S, Kumar N, Drabu S (2010) Synthesis and anticonvulsant activities of phenoxychalcones. T Ph Res 3:257–262
Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Preti D (2008) Design, synthesis, and biological evaluation of thiophene analogues of chalcones. Bioorg Med Chem 16:5367–5376. doi:10.1016/j.bmc.2008.04.026
Lahtchev KL, Batovska DI, Parushev SP, Ubiyvovk VM, Sibirny AA (2008) Antifungal activity of chalcones: a mechanistic study using various yeast strains. Eur J Med Chem 43:2220–2228. doi:10.1016/j.ejmech.2007.12.027
Bag S, Ramar S, Degani MS (2009) Synthesis and biological evaluation of \(\alpha, \!\beta \)-unsaturated ketone as potential antifungal agents. Med Chem Res 18:309–316. doi: 10.1007/s00044-008-9128-x
Lunardi F, Guzela M, Rodrigues AT, Corre R, Eger-Mangrich I, Steindel M, Grisard EC, Assreuy J, Calixto JB, Santos ARS (2003) Trypanocidal and leishmanicidal properties of substitution-containing chalcones. Antimicrob Agents Chemother 47:1449–1451. doi:10.1128/AAC.47.4.1449-1451.2003
Cheng MS, Shi R, Kenyon G (2000) A solid phase synthesis of chalcones by Claisen-Schmidt condensations. Chin Chem Lett 11:851–854
Begum NA, Roy N, Laskar RA, Roy K (2010) Mosquito larvicidal studies of some chalcone analogues and their derived products: structure-activity relationship analysis. Med Chem Res 19: 1–14
Awasthi SK, Mishra N, Dixit SK, Singh A, Yadav M, Yadav SS, Rathaur S (2009) Antifilarial activity of 1,3-diarylpropen-1-one: effect on glutathione-S-transferase, a phase II detoxification enzyme. Am J Trop Med Hyg 80:764–768
Hamdi N, Fischmeister C, Puerta MC, Valerga P (2010) A rapid access to new coumarinyl chalcone and substituted chromeno[4,3-c]pyrazol-4(1H)-ones and their antibacterial and DPPH radical scavenging activities. Med Chem Res 19:1–16. doi:10.2478/s11696-009-0026-6
Vogel S, Ohmayer S, Brunner G, Heilmann J (2008) Natural and non-natural prenylated chalcones: synthesis, cytotoxicity and anti-oxidative activity. Bioorg Med Chem 16:4286–4293. doi:10.1016/j.bmc.2008.02.079
Sivakumar PM, Prabhakar PK, Doble M (2010) Synthesis, antioxidant evaluation and quantitative structure-activity relationship studies of chalcones. Med Chem Res 19:1–17. doi:10.1007/s00044-010-9342-1
Yayli N, Ucuncu O, Yasar A, Kucuk M, Akyuz E, Karaoglu SA (2006) Synthesis and biological activities of N-alkyl derivatives of o-, m-, and p-nitro (E)-4-azachalcones and stereoselective photochemistry in solution with theoretical calculations. Turk J Chem 30:505–514
Najafian M, Ebrahim-Habibi A, Hezareh N, Yaghmaei P, Parivar K, Larijani B (2010) Trans-chalcone: a novel small molecule inhibitor of mammalian alpha-amylase. Mol Biol Rep 10:271–274. doi:10.1007/s11033-010-0271-3
Chimenti F, Fioravanti R, Bolasco A, Chimenti P, Secci D, Rossi F, Yanez M, Francisco OF, Ortuso F, Alcaro S (2009) Chalcones: a valid scaffold for monoamine oxidases inhibitors. J Med Chem 10:1–8. doi:10.1021/jm801590u
Zarghi A, Zebardast T, Hakimion F, Shirazi FH, Rao PNP, Knaus EE (2006) Synthesis and biological evaluation of 1,3-diphenylprop-2-en-1-ones possessing a methanesulfonamido or an azido pharmacophore as cyclooxygenase-1/-2 inhibitors. Bioorg Med Chem 14:7044–7050. doi:10.1016/j.bmc.2006.06.022
Sibi MP, Manyem S (2000) Enantioselective conjugate additions. Tetrahedron 56:8033–8061. doi:10.1016/S0040-4020(00)00618-9
Berner OE, Tedeschi L, Enders D (2002) Asymmetric Michael additions to nitroalkenes. Eur J Org Chem 2002:1877–1894. doi:10.1002/1099-0690(200206)2002:12<1877::aid-ejoc1877>3.0.co;2-u
Dalko PI, Moisan L (2004) In the golden age of organocatalysis. Angew Chem Int Ed 43:5138–5175. doi:10.1002/anie.200400650
House HO (1972) Modern synthetic reactions, 2nd ed. Benjamin WA, Menlo Park, p 595
Jung ME (1991) In: Trostand BM, Fleming I (eds) Comprehensive organic synthesis. Pergamon Press, Oxford, p 1
Padmavathi V, Sharmila K, Reddy AS, Reddy DB (2001) Reactivity of 3,5-diaryl cyclohexanones—synthesis of spiro cyclohexanes. Indian J Chem 40B:11–14
Padmavathi V, Sharmila K, Balaiah A, Reddy AS, Reddy DB (2001) Cyclohexenone carboxylates. A versatile source for fused isoxazoles and pyrazoles. Synth Commun 31:2119–2126. doi:10.1081/SCC-100104462
Reddy DB, Reddy AS, Padmavathi V (1998) Synthesis of annelated 1,2,3-selena- or -thia-diazoles. J Chem Res 784–785. doi: 10.1039/A804381G
Gopalakrishnan M, Thanusu J, Kanagarajan V (2008) Synthesis and characterization of 4,6-diaryl-4,5-dihydro-2H-indazol-3-ols and 4,6-diaryl-2-phenyl-4,5-dihydro-2H-indazol-3-ols - a new series of fused indazole derivatives. Chem Hetero Comp 44:950–955. doi:10.1007/s10593-008-0137-y
Padmavathi V, Sharmila K, Padmaja A, Reddy DB (1999) An efficient synthesis of 6,8-diarylcarbazoles via Fischer indole cyclizations. Heterocycl Commun 5:451–456. doi:10.1515/HC.1999.5.5.451
Hoye TR, Tennakoon MA (2000) Synthesis (and alternative proof of configuration) of the scyphostatin C(1’)-C(20’) trienoyl fragment. Org Lett 2:1481–1483. doi:10.1021/ol0058386
Hiromichi F, Naoyuki K, Yoshinari S, Yasushi N, Yasuyuki K (2002) Concise asymmetric synthesis of a model compound, \((4S,\!5S,\!6S)\)-6-(2,2-dimethoxy)ethyl-4,5-epoxy-6-hydroxy-2-cyclohexenone, for the cyclohexenone core of scyphostatin. Tetrahedron Lett 43:4825–4828. doi:10.1016/s0040-4039(02)00916-4
Safaei-Ghomi J, Alishahi Z (2005) The preparation of some novel indazole derivatives by using chalcones. J Fudan Univ Nat Sci 44(5):789–790
Mc Bride C, Renhowe P, Heise C, Jansen J, Lapointe G, Ma S, Pineda R, Vora J, Wiesmann M, Shafer C (2006) Design and structure-activity relationship of \(3\!-\!\text{ benzimidazol }\!-\!2\!-\!\text{ yl }\!-\!1{\rm H}\)-indazoles as inhibitors of receptor tyrosine kinases. Bioorg Med Chem Lett 16:3595–3599. doi: 10.1016/j.bmcl.2006.03.069
Kim BC, Kim JL, Jhang YU (1994) Bull Korean Chem Soc 15(2): 97. Chem. Abstr 121:50097z
Yamaguchi M, Maruyama N, Koga T, Kamei K, Akima M, Kuroki T, Hamana M, Ohi N (1995) Novel antiasthmatic agents with dual activities of thromboxane A2 synthetase inhibition and bronchodilation, VI: Indazole derivatives. Chem Pharm Bull 43(2):332–334
Gorge V, De L, Kim UT, Jing L (1998) Nonsymmetric P2/P2‘ cyclic urea HIV protease inhibitors. structure-activity relationship, bioavailability, and resistance profile of monoindazole-substituted P2 analogues. J Med Chem 41(13):2411–2423. doi:10.1021/jm980103g
Vyas DH, Tala SD, Akbari JD, Dhaduk MF, Joshi HS (2009) Synthesis, antimicrobial and antitubercular activity of some cyclohexenone and indazole derivatives. Indian J Chem 48B:1405–1410
Abdel-Rahman AAH, Abdel-Megied AES, Hawata MAM, Kasem ER, Shabaan MT (2007) Synthesis and antimicrobial evaluation of some chalcones and their derived pyrazoles, pyrazolines, isoxazolines, and 5,6-dihydropyrimidine-2-(1H)-thiones. Monatshefte Chem 138:889–897. doi:10.1007/s00706-007-0700-8
Ducki S, Forrest R, Hadfield JA, Kendall A, Lawrence NJ, McGown AT, Rennisonl D (1998) Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorg Med Chem Lett 8:1051–1056. doi:10.1016/S0960-894X(98)00162-0
Bhat BA, Dhar KL, Puri SC, Saxena AK, Shanmugavel M, Qazi GN (2005) Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents. Bioorg Med Chem Lett 15:3177–3180. doi:10.1016/j.bmcl.2005.03.121
Sobhani S, Nasseri R, Honarmand M (2012) 2-Hydroxyethylammonium acetate as a reusable and cost-effective ionic liquid for the efficient synthesis of bis(pyrazolyl)methanes and 2-pyrazolyl-1-nitroalkanes. Can J Chem 90:798–804. doi:10.1139/v2012-059
Yue C, Mao A, Wei Y, Lü M (2008) Knoevenagel condensation reaction catalyzed by task-specific ionic liquid under solvent-free conditions. Catal Commun 9:1571–1574. doi:10.1016/j.catcom.2008.01.002
Acknowledgments
Financial support from the Research Council of the University of Sistan and Baluchestan is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mousavi, M.R., Maghsoodlou, M.T. & Habibi-Khorassani, S.M. One-pot diastreoselective synthesis of highly functionalized cyclohexenones: 2-oxo-N,4,6-triarylcyclohex-3-enecarboxamides. Mol Divers 18, 821–828 (2014). https://doi.org/10.1007/s11030-014-9541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9541-7