Abstract
Differentiated thyroid cancer (DTC) is a rare disease in the paediatric population (≤ 18 years old. at diagnosis). Increasing incidence is reflected by increases in incidence for papillary thyroid carcinoma (PTC) subtypes. Compared to those of adults, despite aggressive presentation, paediatric DTC has an excellent prognosis. As for adult DTC, European and American guidelines recommend individualised management, based on the differences in clinical presentation and genetic findings. Therefore, we conducted a systematic review to identify the epidemiological landscape of all genetic alterations so far investigated in paediatric populations at diagnosis affected by thyroid tumours and/or DTC that have improved and/or informed preventive and/or curative diagnostic and prognostic clinical conduct globally. Fusions involving the gene RET followed by NTRK, ALK and BRAF, were the most prevalent rearrangements found in paediatric PTC. BRAF V600E was found at lower prevalence in paediatric (especially ≤ 10 years old) than in adults PTC. We identified TERT and RAS mutations at very low prevalence in most countries. DICER1 SNVs, while found at higher prevalence in few countries, they were found in both benign and DTC. Although the precise role of DICER1 is not fully understood, it has been hypothesised that additional genetic alterations, similar to that observed for RAS gene, might be required for the malignant transformation of these nodules. Regarding aggressiveness, fusion oncogenes may have a higher growth impact compared with BRAF V600E. We reported the shortcomings of the systematized research and outlined three key recommendations for global authors to improve and inform precision health approaches, glocally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Differentiated thyroid cancer (DTC) is a rare disease in the paediatric population (≤ 18 years old. at diagnosis). Its incidence rate increased 1.1 per year from 1973 to 2006 and markedly increased from 2006 to 2013, especially in females and patients aged > 10 years. The increase in incidence rates was reflected by increases in incidence for papillary thyroid carcinoma (PTC) subtypes, being the classic PTC the most prevalent (58,6%), followed by the follicular subtype of PTC (18.9%) [1].
Compared to those of adults, paediatric DTC usually presents with more aggressive local disease and higher frequency of lymph nodes (50–75%) and distant metastasis (5–16%), being the lungs the most common site [2, 3]. It has been suggested that extra-thyroidal extension, younger age, and larger tumours (> 2 cm) are prognostic factors associated with worse prognosis [4, 5].
Despite this aggressive presentation, paediatric DTC has an excellent prognosis. Therefore, early identification of children who are at high risk of persistent or metastatic disease, and those children that may not need radioactive iodine (RAI) therapy, is a fundamental step in the therapeutic strategy. Remarkably, younger age at diagnosis (< 10 years of age) was found as an important risk factor for disease recurrence (i.e., increased rate and faster time) [6, 7]. Age (< 10 years at diagnosis), American Thyroid Association (ATA) high-risk level and poor response to therapy were found as significant negative prognostic factors for event-free survival [8].
As for adult DTC, integration of variables (i.e., prognostic markers) may offer additional help to estimate the risk of recurrence or persistent disease and, therefore, individualised management.
Recent European Thyroid Association (ETA) recommendations [4] and the first ATA guideline [9] endorsed that special recommendations, based on the differences in clinical presentation and genetic findings, are necessary for DTC management in this age group. However, the current evidence is insufficient to conclude that molecular testing of thyroid nodule or paediatric thyroid carcinoma tissue will help diagnosis or alter clinical management. Therefore, prospective studies are needed.
Although evidence suggests that BRAF V600E variant in a fine-needle biopsy may help PTC diagnosis and could be considered, BRAF V600E prevalence is considerably lower in children (mainly < 10 years of age) and was not found in some populations. In fact, the younger the age, the more important the role of fusions in the development of thyroid cancer in the paediatric age-tiers [10,11,12,13,14,15,16,17,18,19].
Due to the uncommonness of paediatric thyroid cancer and the observed difference in tumour biology and clinicopathological presentation, we believe that global evidence identification and synthesis would certainly help to outline the genetic differences among individuals from different populations, as well as validate the use of the variable (i.e., genetic findings) for prognostication and treatment decisions in paediatric age-tiers.
Therefore, we conducted a systematic review of available evidence published in peer-reviewed scientific journals to identify the epidemiological landscape of all genetic alterations so far investigated in paediatric populations (i.e. individuals ≤ 18 years of age) at diagnosis affected by thyroid tumours and/or DTC that have improved and/or informed preventive and/or curative diagnostic and prognostic clinical conduct (i.e. to guide the use of druggable targets, surgical therapeutic interventions and/or biochemical, imaging and further genetic alterations follow-up), globally. We have also investigated the epidemiological landscape of actionable targets from such genetic alterations identified, as well as key evidence gaps whose research and development must continue to produce new actionable information (as technology) and further enable adequate context-specific precision health approaches at the glocal level [20, 21] – i.e. developing strategies that are contextually relevant for local implementation but whose implementation could also be adapted at other global locations, given adequate health technology assessment [22].
2 Methodology
This systematic review was designed following a predefined protocol, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [23], which is registered in the PROSPERO database under the identification number: PROSPERO 2023 CRD42023446483 (PRISMA Checklist is available at the Supplementary File).
2.1 Eligibility criteria
This systematic review included systematic, non-systematic and rapid reviews, as well as peer-reviewed original articles, which discuss, comment and critically analyse how epidemiological data on molecular genetic alterations profiling of paediatric populations has been enabling precision health approaches, per country, to improve and/or inform diagnosis or prognosis of individuals affected by thyroid tumours and/or DTC. Therefore, we included (series of) case studies and cohort studies exemplifying mere molecular genetic alterations profiling in paediatric populations affected by thyroid tumours and/or DTC that did (or not) outline actionable targets and/or evidence gaps for improving and/or informing clinical for the purposes of precision health approaches. As such, specific familial predisposing genetic alterations, such as in the RET oncogene for Multiple Endocrine Neoplasia Type 2 (MEN2) was regarded as irrelevant and was discarded. Meanwhile, we incorporated qualitative evidence alongside a review of quantitative data when we found relevant evidence describing genotype–phenotype correlations that portrayed the diagnostic and/or prognostic roles of such molecular genetic alterations profiles per country, region or sub-region (or state).
Exclusion criteria comprised publications regarding only adult populations (older than 18 years old) and familial differentiated and medullary thyroid cancers or anaplastic and poorly differentiated tumour types. We will also did not include publications describing epigenetic events (such as microRNA, LncRNA and methylation) or conference proceedings (as they comprise only abstract with no full-text available for analysis). Full-text eligibility exclusion criteria comprised publications that described only adult populations (older than 18 years old) and/or those describing both paediatric and young adult populations (older than 18 or 21 years of age), unless individual patient data for the paediatric population could be separately extracted. Finally, we also did not include publications that did not describe the mutational profile and/or location (country and/or (sub)region/state) and/or method for genetic alteration profiling and/or tumour type and status data.
2.2 Study outcomes
The main outcome of this study was the investigation of the epidemiological genetic alterations profile landscape of known and new diagnostic and prognostic targets amongst children and adolescents affected by thyroid tumours and/or DTC per country (and sub-region or state, where applicable – namely, Brazil, China and USA) and region (i.e., Asia, Europe, Middle-East, North America, South America and those induced by radiation due to nuclear environmental exposure – namely, Hiroshima/Nagasaki in 1945, Chernobyl in 1986 and Fukushima in 2011). More specifically, the primary outcomes combined differences in the prevalence of the following tumour variables: age at diagnosis (i.e., 0 ≤ 10 years of age or children, and > 10 ≤ 18 years of age or adolescents), histological types (i.e., follicular thyroid adenoma [FTA] or carcinoma [FTC], papillary thyroid carcinoma [PTC], oncocytic thyroid adenoma [OTA] and carcinoma [OTC]), molecular biology and genomics methodological approaches for genetic alterations identification, number of patients, study design and aetiology (i.e., whether tumourigenesis and/or metastatic progression were sporadic, radiation-induced by the abovementioned environmental nuclear events, or therapy).
The secondary outcomes were related to each of the genetic alterations’ diagnostic and prognostic role in terms of adequately informing differential clinical conduct amongst paediatric age-tiers and histological types, as well as their clinicopathological characteristics, therapeutics and relationship with survival outcome per country, region or sub-region (or state), namely: previous and/or other coexisting diseases, therapeutic (i.e., different types of surgery, radiotherapy, targeted-therapies and/or other) approaches, tumour size, lymph node metastasis (TNM-staging), follow-up period, distant metastasis, Initial Dynamic Risk Stratification (IDRS, to evaluate treatment response), final disease outcome (i.e., no evidence of disease (NED), progressing or death), actionable targets, evidence gaps, and findings, including barriers and facilitators, on how (actionable) diagnostic and/or prognostic molecular biomarkers are being implemented for specific populations within given locations (i.e., one or more institution(s), city(ies), sub-region(s)/state(s), country(ies), region(s)). Data on study limitations and funding support were also collected for study bias and conflict of interest evaluations.
2.3 Information sources
Relevant publications over the past thirty years (since 1995), describing and evaluating the diagnostic and/or prognostic role(s) of known and new (actionable) genetic alterations prevalence, and evidence gaps, in paediatric populations affected by thyroid tumours and/or DTC per country, were identified by searching the following electronic bibliographic databases: PubMed, EMBASE, The Cochrane Library, BVS, Google Scholar, and Web of Science. Searches were performed in English on 17 July 2023, using a combination of relevant terms such as ‘paediatric thyroid tumours’, ‘paediatric differentiated thyroid cancer’, ‘molecular landscape’ or ‘genetic landscape’ or ‘mutational analysis’ or ‘genetic alterations identification’, and they were adapted according to the bibliographic databases. There were no time or language restrictions. We did not include grey literature publications due to time constraints, as we were only able to search on Brazilian grey literature databases and it was not representative of all countries/regions whose peer-reviewed publications were included. However, we thoroughly examined bibliographies to manually include relevant studies meeting the eligibility criteria that were identified as primary studies on (non-) systematic literature reviews and original (primary) studies. We did not re-run searches on all databases just before the final analyses (only PubMed), because we finalised these on 28 August 2023. Therefore, there we no further studies to be retrieved for inclusion after such a short period (approximately 40 days).
2.4 Screening, data collection and analysis
Four reviewers independently screened titles and abstracts of publications as duos (JMC and YPC; INN and LS). Disagreements regarding eligibility of (full-text) studies were resolved by discussion and consensus (INN and LS duo) and/or by a fifth reviewer (MSAS for: JMC and YPC duo’s title/abstract eligibility; both duos’ full-text eligibility). Rayyan was used for study selection and conflicts’ resolution.
Data was extracted by all five reviewers with all revising data extracted from documents, including information about location, study design, participants demographics and baseline characteristics, as well as numbers of events or measures of effect (primary and secondary outcomes), as previously detailed. Missing data was not included as study investigators were not contacted for unreported data or additional details. All extracted data was recorded on excel spreadsheets by all five reviewers that also synthesised findings. Full-text eligibility and data extraction occurred simultaneously due to time constraints for submission of systematic review manuscript for publication. Any disagreements were resolved by all five reviewers’ consensus and/or JMC and/or MSAS.
2.5 Quality assessment of included studies
Quality assessment of the included original (primary) studies was conducted by four reviewers (MSAS, INN, YPC and JMC) using specific Critical Appraisal Checklist JBI Tools, developed by the Joanna Briggs Institute, Faculty of Health and Medical Sciences at the University of Adelaide, according to each study design. Each tool consists of six (Text or Opinion [24]), eight (Case Reports [25]), ten (Case Series [26]), eleven (Cohort Studies [25]) or thirteen (Randomised Controlled Trials [27]) different questions that assess the methodological quality of each study, determining the extent to which the possibility of bias has been addressed in the study design, conduction and analysis. Each question was assessed by the four reviewers as ‘Yes’, ‘No’, or ‘Unclear’ and overall score comprised the percentual of ‘Yes’ answers.
The methodological quality of secondary studies (systematic reviews and meta-analysis) was assessed by MSAS using AMSTAR 2 [28] to assign ‘Yes’, ‘No’ or ‘Partial Yes’ on ten questions, and further six questions specific to meta-analysis. Similarly, the overall score comprised the percentual of ‘Yes’ answers. Each study was rated by the four reviewers as: ‘red’ or ‘low-level of confidence (overall score 0–25% ‘Yes’ answers); ‘yellow’ or ‘moderate-level of confidence’ (overall score 26–50% ‘Yes’ answers); ‘green’ or ‘high-level of confidence’ (overall score 51–75% ‘Yes’ answers); ‘blue’ or ‘excellent-level of confidence’ (overall score 76–100% ‘Yes’ answers). A sample of each type of study was assessed by at least two reviewers (MSAS and any other reviewer). The rest were assessed by one of the four reviewers. Discrepancies were resolved by discussion amongst all four reviewers. The four reviewers consensually decided to use JBI Text and Opinion Tool [24] to conduct critical assessment of Non-systematic Literature Reviews included, and further discount 25% in the overall score for neither conducting a systematic, rapid nor scoping review, nor a qualitative evidence synthesis, case series, cohort nor a randomised controlled study. However, when non-systematic literature reviews also presented case reports or series, the four reviewers decided to use JBI Case Reports [25] and Case Series [26] Tools for critical assessment, respectively.
2.6 Data synthesis
Quantitative outcomes regarding genetic alterations landscape was collated by region, country (sub-region or state for Brazil, China and USA) and age-tier, according to histological type and specific genetic alteration, as well as method for identification and/or (clinical and/or analytical) validation (as summarised in Supplementary Table 1). We calculated prevalence of specific genetic alterations by adding sub-region/state, then country and regional percentual of investigated positive and negative findings reported by each included study’s population sample. Since this is a meta-aggregative systematic review [29], and we did not necessarily find evidence on all quantitative and qualitative secondary outcomes, as previously described, these were textually discussed, when relevant, according to regional and country (and sub-region or state for Brazil, China and USA) epidemiological genetic alterations’ profiles.
3 Findings
3.1 Study selection and characteristics
The search strategies for all aforementioned electronic bibliographic database retrieved 3,511 results. After excluding 959 duplicates, 2,552 studies were formally screened against eligibility criteria. Among those, we included 543 studies for full-text screening against eligibility criteria. From these, we included 160 studies, which includes 5 by manual selection through reference lists regarding primary studies’ data that fell under the eligibility criteria. We included 17 Case Reports [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], 67 Case Series [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113], 56 Cohort Studies [11, 13, 14, 114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166], 1 Randomised Controlled Trial [167], 17 Non-Systematic Reviews [10, 12, 15,16,17,18,19, 168,169,170,171,172,173,174,175,176,177] and 2 Systematic Reviews and Meta-analysis [178, 179]. The selection flowchart of the research, outlining reasons for exclusion, is presented in Fig. 1.
3.2 Genetic alterations landscape global findings for sporadic, radiation-induced by nuclear environmental events and/or therapy-exposed paediatric thyroid tumours and DTC
The first question of our systematic review regarded the epidemiological landscape of genetic alterations in paediatric thyroid tumours and/or DTC that are sporadic, radiation-induced by nuclear environmental events and/or therapy-exposed. Therefore, we have summarised all epidemiological genetic alterations (mutations and fusions) identified and/or (clinically and/or analytically) validated per region and country (and sub-region or state for Brazil, China and USA) in Supplementary Table 1. These studies’ findings have several implications. First, in those state/countries that have implemented comprehensive testing methods to identify genetic alterations, they were able to uncover the unique alterations of paediatric thyroid nodules and DTC, including less prevalent novel gene fusions and SNVs, such as DICER1 and other new players. Remarkably, in most studies, neoplasms (i.e., benign, and malignant neoplasms) harbouring DICER1 mutations SNVs were most of follicular subtype of PTCs, FTCs and FTA. None had extrathyroidal extension, lymph node or distant metastasis. Although the precise role of DICER1 is not fully understood, it has been hypothesised that additional genetic alterations, similar to that observed for RAS gene, might be required for the malignant transformation of these nodules. A common finding across the countries was that in paediatric DTC, fusion alterations, most commonly involving RET and NTRK, have the highest association with invasive disease, lymph node involvement and distant metastases (mainly to the lung) while BRAF V600E is uncommon in PTC patients with less than 10 years of age, which present a more aggressive phenotype than those children with > 10–18 years of age.
Those studies that assessed mainly for genetic alterations identified in adults, performed in most countries, although showed a more limited genetic landscape, support a significantly lower rate of the BRAF V600E mutation in PTC in paediatric patients than in adults and that RAS and TERT mutations do not contribute significantly to PTC tumourigenesis. Although it has generated a BRAF and RAS-score (BRS) for paediatric thyroid carcinoma [121], the authors also agree that it will be essential to generate the transcriptional signatures of the most prevalent fusions found such as RET, NTRK and BRAF (mainly in Brazil).
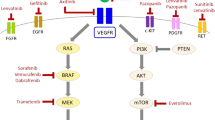
This global epidemiological landscape can enable us to better understand the populations’ geographical and age variations for detection of actionable targets such as tyrosine-kinase inhibitors (TKIs such as NTRK, RET, ALK and BRAF) that could benefit from targeted therapy for those patients presenting progressive disease. It is important to be able to indicate selective TKIs for these patients, although this is a completely infrequent situation in paediatric thyroid cancer, and we could hypothesise on the usefulness of such an approach when these patients reach adult age and have RAI therapy refractory progressive thyroid cancer.
As such, we have also visually summarised the genetic alterations profiles’ landscape in the Fig. 2 world map.
3.3 Actionable genetic alterations landscape for sporadic, radiation-induced by nuclear environmental events and/or therapy-exposed paediatric thyroid tumours and DTC
The second question of our systematic review regarded the epidemiological landscape of actionable genetic alterations in paediatric thyroid tumours and/or DTC that are sporadic, radiation-induced by nuclear environmental events and/or radiation therapy-exposed. We present findings per region and country (and sub-region or state for Brazil, China and USA). Our goal was to identify all such genetic alterations that have improved and/or informed preventive and/or curative diagnostic and prognostic clinical conduct (i.e. to guide the use of druggable targets, surgical therapeutic interventions and/or biochemical, imaging and further genetic alterations follow-up), globally (Supplementary Findings).
3.4 Evidence gaps in (actionable) genetic alterations for sporadic, radiation-induced by nuclear environmental events and/or therapy-exposed paediatric thyroid tumours and DTC
The third question of our systematic review regarded any key evidence gaps in the epidemiological landscape of (actionable) genetic alterations in paediatric thyroid tumours and/or differentiated thyroid cancer. Here, we present findings per region and country (and sub-region or state for Brazil, China and USA). Our overall goal with these three questions was to identify unresolved issues in paediatric thyroid tumours and DTC management that could be tackled by the establishment of new (actionable) information (as technology) research, development and implementation that can enable adequate context-specific precision health approaches, glocally [20, 21].
3.5 Asia
Across all Chinese sub-regions, evidence gaps we identified referred to larger amount of research, development and implementation (for both clinical and analytical validity evaluation) work to define clinical utility, elucidate biological mechanisms, and explore the potential of TERT promoter mutations as therapeutic targets in thyroid cancer [127], as well as of BRAF V600E mutation's role in risk stratification and management of children and adolescent PTC, for the purposes of updating current ETA and ATA guidelines [17, 178, 179]. There is no such evidence for fusion oncogenes in PTCs such as RET/PTC, especially regarding therapy-induced tumourigenesis. Nevertheless, RET/PTC was reported to be present in benign adenomas and also in Hashimoto thyroiditis tissues [15]. Bridging the gap between evidence generation and its implementation remains key, since preliminary reports of targeted therapy in paediatric patients with DTC with progressive disease have shown encouraging results, as well as the possible association of hyperthyroidism and PTC in resistance to thyroid hormone. Therefore, adequately-designed clinical trials must be conducted [19], also to explore the long-term effects of medication [44].
In terms of evidence gaps identified in India, we observed that whole-exome-sequencing or other more advanced molecular biology and genomics methodological approaches could be explored to unravel further genetic biomarkers for aggressiveness in paediatric PTC [119]. As for adults, BRAF V600E mutations has been associated with RAI-therapy refractoriness in childhood DTCs. However, data concerning its association with other aggressive features in paediatric cases are quite heterogeneous, and further studies are needed [179].
The evidence gaps we identified across the Republic of Korea referred to the aetiology of age-associated genetic alterations that remains unexplained, although chromosomal rearrangements have a strong association with exposure to ionizing radiation, as thyroid cells of young children may be more susceptible to its effects and/or lose key factors in the DNA repair machinery, leading to uncoupled double-stranded breaks and translocation with partner genes. Children with oncogenic fusion PTCs presented with more advanced-stage disease (especially, lung metastasis and a higher risk for recurrence or persistence) than did those with BRAF V600E PTC [135, 136]. Furthermore, DICER1 SNVs have been identified in benign and malignant thyroid neoplasms. However, the pathogenesis associated with progression of a normal thyroid to benign neoplasms and then to malignant transformation, has not yet been fully established. Therefore, further studies are needed to better understand this distinct pathogenesis [67]. As for DICER1, STK11 has also been identified as a coexisting well-known driver mutation hot-spot, whose role in tumorigenesis needs to be better investigated [117].
Regarding study limitations, in China, we observed that qualitative and quantitative analyses of genotype–phenotype association of these genetic alterations with the various clinicopathological characteristics described have not yet been done. Furthermore, a retrospective design to the included studies may signify insufficient number of cases and/or incomplete data for cases, as well as short follow-up period. This may be due to the rarity of thyroid cancer in the paediatric population. Nevertheless, large-sample, multicentre prospective studies are also needed to investigate any potential differences between children and adolescents with PTC across China and or Asia, regionally [134, 159]. Whereas in India, Chakraborty et al. [119] outlined that longer duration prospective studies must be conducted to evaluate progression-free or overall survival in BRAF V600E mutations, as well as other genetic alterations often found in paediatric DTCs, such as RET/PTC rearrangement in radiation-induced DTCs and PAX8/PPARγ fusion, as previous studies have shown high rates of such translocations in India. In the Republic of Korea, we observed that the small number of cases and the possible selection bias of some studies [34, 117, 135] make definitive conclusions difficult to draw. Most prominently, the lack of individual data available on genetic alterations and clinicopathological correlations remained as a limitation across several regional studies, preventing comparisons between different paediatric age-tiers as well as with other young adult and adult age groups. We would like to outline the gold-standard for individual patient data reporting as implemented by Lee et al. (2021).
Finally, we found little information regarding funding support to investigate potential conflicts of interests across all Chinese sub-regions. However, we observed that most studies that reported funding support were either based in Beijing [127, 128], Hiroshima, Nagasaki or Fukushima [15], mainly funded by the public rather than the private sector [159]. In India, Chakraborty et al. [119] received public sector funding. We identified that most studies received financial support by several Korean Ministries, therefore, the public sector [34, 67, 117, 136].
3.6 Europe
The evidence gaps we identified across all European countries referred to the need for further studies, particularly in regions where paediatric populations are poorly studied or without described tumour molecular profile. Few studies (i.e., first studies) did not report the RET fusion partners and, therefore, to calculate the real prevalence of the individual RET fusions was not achievable. Furthermore, follicular thyroid tumours are still very under-studied [97, 141]. Investigating novel gene fusions and assessing alterations already described in small subsets may help the better understanding of tumour’s molecular landscape, enabling robust correlations and clinical applications [11, 32, 71, 137, 141]. Lastly, studies exploring the distribution of genetic alterations across distinct foci of primary tumour and metastasis are necessary to uncover its application in target therapies for metastatic tumours [55].
Within the Czech Republic, evidence gaps revolved around the need to underscore: the mechanism of RET/PTC1ex9 formation [32] and other fusion genes by RNA in paediatric populations to assess any potential correlations between certain fusion genes and clinical-pathological features, and compared those with adult population, especially in PTC [11, 146]; the clinical impact and prognostic importance of RAS mutations in FTA, as well as the role of pathogenic variants found in PTEN or PIK3CA genes, which have been found in paediatric PTCs [146]. In France, we identified the need for new studies investigating a potential post-Chernobyl molecular signature in thyroid tumours comparison purposes with sporadic tumours samples analysed [79]. Evidence gaps in Germany related to: the role of Gsα mutation in paediatric populations [45]; and carefully designed studies to identify potential correlation between RET/PTC rearrangements and clinical behaviour or nodal metastasis [95]. In the Netherlands, studies outlined the need for more in depth investigation on kinase inhibitors and their so far limited efficacy leading to resistance and severe side effects [99]. In Poland, evidence gaps related to: further investigation on the influence of the novel mutation at codon 31 of KRAS oncogene on the development of PTC [59]; larger samples analyses as the TCGA study [84]. Evidence gaps that we identified in Portugal related to the putative role of EWSR1::FLI1 gene rearrangement in the neoplastic development of PTC, which outlined the small series of positive cases as a study limitation that should be tackled [77, 122]. In Sweden, we identified the need to identify additional gene fusions in FTC additionally to the well-described PAX8/PPARγ fusion gene and other less common fusion events such as THADA [141]. In the United Kingdom, we also identified the need for larger sample size studies to adequately identify each of the known RET translocations [102].
Regarding study limitations, in the Czech Republic, we observed the limited number of paediatric samples [146] and that no functional analysis of novel fusion genes have been performed [11]. In Germany, one study outlined several confounding variables when analysing PTC histology, irrespective of Belarusian patients' age [148]. Italian authors outlined that: resolution power of early CGH investigations was extremely limited and should be tackled [144]; case series bias lies in the limited number of paediatric cases and the controversial ‘wait and see’ approach used in Italian Classification Thyroid system [92]; the nCounter analysis displayed an important technical limitation for NTRK3 fusion detection and that the lack of clinical follow-up data is a serious limitation to also investigate the real impact of different molecular and pathological PTC characteristics in the prognostic risk stratification of patients [137]. Dutch studies mainly discussed small sample sizes of studies comparing different histological subtypes and their association with the outcome of different molecular backgrounds stratified by the histological subtype [99]. Whereas Polish studies outlined that biased and small [60] samples have prevented de facto genotype–phenotype association studies not only for BRAF and TERTp mutations [156] but also RAS, PAX8/PPARγ, RET/PTC1, and RET/PTC3 rearrangements [84]. One Swedish study also outlined the small sample size [41].
Finally, regarding information on funding support to investigate potential conflicts of interests, we observed that all studies were funded by the public sector in the Czech Republic [11, 32, 146], Germany [148], Italy [69, 92, 137], the Netherlands [99, 145], Poland [59, 60, 84, 97, 156], Portugal [77], Sweden [41, 104, 141], and the UK [102]. Whereas certain studies also received funding from the private sector in France [79], Italy [126], and Portugal [121].
3.7 Middle East
The current knowledge about sporadic paediatric PTCs at the molecular level is limited due to the scarcity of relatively small cohorts focusing on these aspects. The status of fusion oncogenes and their correlation with disease-free survival (DFS) is not addressed in paediatric PTCs. Therefore, regarding evidence gaps, we identified the need for larger and more comprehensive studies to better understand the genetic characteristics and genotype–phenotype correlations of paediatric PTCs, shedding light on their genomic landscape [78]. Additionally, the prognostic significance of the BRAF V600E mutation remains unclear in paediatric patients with thyroid cancer [149]. This highlights the need for further research to determine the impact of this mutation on the prognosis and clinical outcomes of paediatric thyroid cancer cases.
In Saudi Arabia, we identified a need for prospective larger samples studies [17] deploying additional identification methods to fixed panels that exclude other important genes, such as PAX8/PPARγ, THADA fusion genes, and EIF1AX mutations [116]. Moreover, different demographic and racial characteristics, distinct of methods of diagnosis and molecular analysis, resulting in high heterogeneity of the sample [115] and cross-sectional and retrospective previous studies [178] may have compromised the ability to demonstrate causality. Studies evaluating only point mutations in ‘target genes/exons’ that are not frequently mutated in paediatric thyroid cancer do not allow for clinical-pathological associations [48]. Although certain non-systematic literature reviews have been thoroughly developed, some are lacking a few references and did not outline clear pharmaceutical actionable genetic targets, even though they synthesised large amounts of genetic alterations prevalence per country [10].
In Turkey, retrospective studies with relatively small sample sizes have prevented the correlation between BRAF V600E mutation and recurrence or response to treatment [130].
Finally, we found no information regarding funding support to investigate potential conflicts of interests across all Middle East countries analysed.
3.8 North America
The evidence gaps that we identified in Canada revolved around the need for more research on children with CCDC6::RET-driven tumours being younger than those with BRAF V600E-driven tumours (mean age 10.1 vs. 14.5 years) to verify the hypothesis of whether tumours arising in young (pre-/peri-pubertal) children constitute a biologically and genomically unique category [96]. Regarding information on funding support to investigate potential conflicts of interests, we observed that most studies were financed by the public and third sector [40, 96] as well as the private sector [40].
Nearly 30 studies were published across 14 states in the USA, predominantly in Philadelphia. Evidence gaps that were acknowledged across all US states referred to the need for further studies using comprehensive molecular testing byNext Generation Sequencing (NGS) in all patients with unresectable or progressive and/or symptomatic distal disease that is unresponsive to standard therapy of surgery and radioactive iodine, especially including targetable oncogenic drivers including: NTRK1-3, RET and ALK gene fusions, BRAF mutations, and MET overexpression/fusion [88].
In Maryland, we identified the need for prospective studies with larger samples from more than one institution to allow meaningful statistical analysis after focused NGS targeting known genomic alterations in thyroid cancers broadly [90]. In the Tennessee, authors outlined the need to underscore the precise role of DICER1 in thyroid oncogenesis, suggesting a potential additional hits hypothesis required for nodule transformation [61]. In Wisconsin, we identified the need for further research on the genetics of paediatric PTC as a way of better stratifying patients and identifying subgroups that could safely be treated with subtotal thyroidectomy [129]. In New York, evidence gap evolved around the need for larger sample sizes to compile a variety of tumour types to identify oncogenic fusions, especially NTRK fusions [143], whereas, in Washington-DC, the target was RAS mutations involved in the extrathyroidal spread of PTC [124], while in Connecticut, authors discussed whether their small samples studies would influence the prevalence of NTRK1/NTRK3 fusions in PTCs [88].
In Pennsylvania, studies investigating BRAF positive tumours, with decreased expression of the NIS and nodal and/or distant metastases, also outlined the need for further research into RAS and PAX8/PPARγ mutations’ potential association with less aggressive disease, as patients would be able to undergo less aggressive adjunctive treatments [53]. Also, we identified the need for further investigations deploying NGS as a cost-effective approach with high throughput and parallel sequencing to analyse larger numbers of genetic variations in small volume samples in studies aiming to underscore evidence that paediatric thyroid cancer is likely biologically different than adult thyroid cancer. Still in Pennsylvania, we identified the need for larger sample studies to further determine whether DICER1 mutations are somatic or germline [153], as well as for clearly underscoring the pathological pathways through which ETV6::NTRK3, TPR::NTRK1, RET/PTC and PAX8/PPARγ gene fusions may increase the susceptibility to chromosomal fragility in paediatric radiation therapy-induced PTC [147]. Finally, tumours with no identified genetic alteration in the miRInform thyroid test should use a more comprehensive panel [125].
In Texas, we identified evidence gaps relating to the need for prospective and larger studies evaluating the benefits of molecular testing in paediatric thyroid lesions bearing TERT promoter rearrangements to verify if they play a role in paediatric thyroid carcinomas. Furthermore, it may be important to verify whether age (> 11–18 years of age) may play a role in the frequency discrepancies of RAS mutations in paediatric thyroid cancers, or if this finding is simply incidental [118].
Regarding study limitations, we identified sampling bias in retrospective studies around Tennessee – especially for patients of racial or ethnic minority groups, as well as investigations at high-volume centres with established thyroid expertise, both of which may produce results that cannot be generalisable to different settings and/or underserved communities. Furthermore, although comprehensive testing methods to detect unique genetic alterations in paediatric thyroid tumours have been implemented, certain targets may be refined to underscore key genomic and clinicopathologic findings to help with targeted therapy in the case of progression following treatment with radioactive iodine or in the neoadjuvant setting. It may also help prevent diagnostic surgeries in paediatric populations with more aggressive disease and expand the understanding of the genetic mechanisms of thyroid tumours in children [61].
In Wisconsin, we identified potential bias in COLD-PCR methodology used in DNA samples extracted from paraffin-embedded tissue from a retrospective review with a small sample size, as well as rarity of patient follow-up data, which makes association with recurrence unclear [129].
Finally, in Missouri, we outline that, beyond limitations related to retrospective analyses, BRAF mutation analysis in PTC through PCR–RFLP, which can generate both false positive and false negative results, should be avoided. Also, investigators should also test for other common mutations, such as the RET/PTC rearrangement that often occurs in paediatric PTC and can enrich diagnosis and prognosis informing tumour management [50].
Regarding information on funding support to investigate potential conflicts of interests, we observed that most US-based studies received support by both the public and private sectors [13, 18, 61, 75, 88, 90, 124, 125, 125, 129, 142, 147, 147, 151, 153, 167].
3.9 South America
Regarding evidence gaps, one of the main drawbacks of the Brazilian studies is that they are retrospective analysis, which lacks a proper follow-up for all the patients [112, 180]. This may limit the accuracy and completeness of the findings. Moreover, the rarity of this disease in the paediatric population limits the number of patients included in the studies, especially for cases under 10 years of age at diagnosis, making it challenging to gather a larger number of cases [58]. Colombian paediatric PTC patients have been tested only for point mutations (BRAF V600E, DICER1, NKX2-1, NTRK1, PTEN, RAS and TSHR), demonstrating a frequency of 6.3% BRAF V600E in the studied population and none for all the other tested mutations [56]. Evidence gaps related to the need for further studies on: how the patients evolve; if their response to RAI therapy was complete; exploring wider known genetic alterations; registering more clinical data to unveil possible familial clustering and risk factors [56].
Both in Brazil and Colombia, we identified the need for studies to investigate quite heterogeneous data concerning BRAF mutations association with aggressive features in paediatric cases. Also, both qualitative and quantitative analyses of the association of these events with various clinicopathological characteristics should be conducted [179].
Finally, regarding information on funding support to investigate potential conflicts of interests, we observed that most Brazilian studies have been financed by the public sector [38, 58, 83, 112].
3.10 Nuclear environmental events: Chernobyl and Fukushima
As for Europe and the USA, the evidence gaps we identified revolved around more and better prospective larger sample sizes studies. In Belarus, we identified the need for studies investigating structural differences between the various rearranged forms of RET, specifically in solid variant of PTC in Chernobyl-exposed paediatric populations – a rare finding in either children or in the adult population – that is poorly characterised from both a biological behaviour and patient prognosis perspectives [75, 85, 86]. Differences of RAS mutations prevalence that might be related to age, environmental or genetic factors, or techniques used should also be further investigated [94]. TK/EC expression indicating the presence of RET/PTC rearrangements should also be further characterised [164]. Further analyses of TP53 mutations need to be performed paediatric PTC without radiation history [157], especially codons 167 and 183 to investigate whether they are radiation-specific targets [109]. A matched control (age and region) would be necessary to ascertain whether the exposure to radioactive isotopes has really caused an increase in the prevalence of RET rearrangements, particularly RET/PTC3, to verify whether this is linked primarily to the nature of the carcinogenic agent or to the child’s age [158].
In Ukraine, evidence gaps related to the need for further studies addressing all driver oncogenes and other cancer genes at the genomic and transcriptomic levels to determine whether the phenotype and prognosis of the BRAF V600E-positive radiogenic PTCs will be acquiring patient age-related changes similarly to those described in sporadic PTCs [107].
Japanese evidence gaps regarded the need for further studies also outlining the clinicopathological significance of BRAF or TERT promoter mutations and their prognostic impact via advanced methods such as whole-exome-sequencing. [76]. Furthermore, given the low frequency of both child PTC and the ETV6::NTRK3 fusion, it remains unclear whether the impact of genetic alteration in PTCs varies with geographic diversity. As the increase of thyroid cancer after radiation exposure might require a relatively long period of time following the 2011 Fukushima disaster, it is important to accumulate detailed data regarding both sporadic and possible radiation-related PTCs in Japan [39]. Finally, it is important to verify any differences in genetic alteration between paediatric and adult FTCs, which have been this far poorly investigated in Fukushima radiation exposed paediatric populations [43].
Regarding study limitations, we would like to outline that sample size has been an issue for all Chernobyl and Fukushima studies and a Ukraine discussion regarding the detection of RET/PTC rearrangements via automated FISH analysis approach, which provides reliable results in higher cell numbers. Nevertheless, results indicate a genetic heterogeneity since only subpopulations of tumour cells carried the RET/PTC rearrangement [132].
Finally, we found little information on funding support to investigate potential conflicts of interests for nuclear environmental events studies. Nevertheless, we observed that all Brazilian studies received funding exclusively from the public sector [154]. German studies received financial support from the public sector [33, 152]. Japanese studies received funding from third, private and public sectors [39, 70, 76]. One Ukrainian study received funding from the third and public sectors [107]. One US-based study received funding from both public and private sectors [72, 75].
4 Final remarks
We have outlined three key recommendations for global authors as means of improving evidence generation and use in the implementation of genetic alterations identification and follow-up strategies to improve and/or inform the management of sporadic, radiation-induced by nuclear environmental events and/or therapy-exposed children and adolescents (diagnosed until 18 years of age) with thyroid tumours and/or DTC.
From the full-text studies that did not meet eligibility criteria, 133 studies from all investigated regions collected age data but did not report genotype–phenotype correlation and/or epidemiological data (Supplementary References A). Therefore, our first recommendation for authors is to stratify results by age groups (i.e. children, adolescents, young adults, adults and elderly populations) and/or report data for individual patients' age at diagnosis as supplementary/additional files.
From the full-text studies that did not meet eligibility criteria, 9 studies did not report individual country and/or sub-region or state (Supplementary References B) epidemiological data regarding genotype–phenotype correlations, following our eligibility criteria. For those studies analysing populations from more than one country and/or sub-region or state that did not report individual country and/or sub-region or state epidemiological data regarding genotype–phenotype data according to age-ranges and or individual patient data between zero and 18 years of age (inclusive) at diagnosis, we replicated the same data for all countries listed in the study. This is somewhat imprecise but close enough at regional level, as those studies analysing population samples from more than one country and/or sub-region or sate belonged to the same region and/or country, respectively. Therefore, our second recommendation for authors is to report results per location as specific as possible (i.e. individual data for each city, sub-region or state, country, especially when analysing population samples from a group of countries and/or sub-region or state).
More generally, a wide variety of studies from all investigated countries did not adequately report positive and negative findings investigated, the methodologies deployed for such investigation, specific genetic alterations identified per histological type and/or aetiology. Therefore, our final recommendation for authors is to always report each genetic alteration investigated, whether you find them or not, by which specific methodological approach for such identification and/or (clinical and/or analytical) validation, as well as from which type of histological lesion caused by specific aetiology. Further descriptions in terms of clinicopathological characteristics, therapeutics and relationship with survival outcome per genetic alteration are also key for tackling implementation barriers and/or evaluating context-specific facilitators at other global locations.
Finally, as a short-term future perspective, such an approach will enable the development of novel genetic/genomic tests from data that has been validated following each country’s specific genetic alterations landscape. This is of key importance for cost-effective implementation of precision medicine goals at the local level worldwide.
References
Qian ZJ, Jin MC, Meister KD, Megwalu UC. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973–2013. JAMA Otolaryngol-Head Neck Surg. 2019;145:617. https://doi.org/10.1001/jamaoto.2019.0898.
Hay ID, Johnson TR, Kaggal S, Reinalda MS, Iniguez-Ariza NM, Grant CS, et al. Papillary Thyroid Carcinoma (PTC) in children and adults: Comparison of initial presentation and long-term postoperative outcome in 4432 patients consecutively treated at the mayo clinic during eight decades (1936–2015). World J Surg. 2018;42:329–42. https://doi.org/10.1007/s00268-017-4279-x.
Lee YA, Jung HW, Kim HY, Choi H, Kim H-Y, Hah JH, et al. Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: A retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab. 2015;100:1619–29. https://doi.org/10.1210/jc.2014-3647.
Lebbink CA, Links TP, Czarniecka A, Dias RP, Elisei R, Izatt L, et al. European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur Thyroid J. 2022;2022:11. https://doi.org/10.1530/ETJ-22-0146.
Lazar L, Lebenthal Y, Steinmetz A, Yackobovitch-Gavan M, Phillip M. Differentiated thyroid carcinoma in pediatric patients: Comparison of presentation and course between pre-pubertal children and adolescents. J Pediatr. 2009;154:708–14. https://doi.org/10.1016/j.jpeds.2008.11.059.
Alessandri AJ, Goddard KJ, Blair GK, Fryer CJH, Schultz KR. Age is the major determinant of recurrence in pediatric differentiated thyroid carcinoma. Med Pediatr Oncol. 2000;35:41–6. https://doi.org/10.1002/1096-911X(200007)35:1%3c41::AID-MPO7%3e3.0.CO;2-7.
Liu Z, Hu D, Huang Y, Chen S, Zeng W, Zhou L, et al. Factors associated with distant metastasis in pediatric thyroid cancer: evaluation of the SEER database. Endocr Connect. 2019;8:78–85. https://doi.org/10.1530/EC-18-0441.
Redlich A, Luster M, Lorenz K, Lessel L, Rohrer TR, Schmid KW, et al. Age, American thyroid association risk group, and response to therapy are prognostic factors in children with differentiated thyroid cancer. J Clin Endocrinol Metab. 2022;107:e165–77. https://doi.org/10.1210/clinem/dgab622.
Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25:716–59. https://doi.org/10.1089/thy.2014.0460.
Cordioli MICV, Moraes L, Cury AN, Cerutti JM. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocr Relat Cancer. 2015;22:R311–24. https://doi.org/10.1530/ERC-15-0381.
Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, et al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. 2020;30:1771–80. https://doi.org/10.1089/THY.2019.0802.
Rangel-Pozzo A, Sisdelli L, Cordioli MIV, Vaisman F, Caria P, Mai S, et al. Genetic landscape of papillary thyroid carcinoma and nuclear architecture: an overview comparing pediatric and adult populations. Cancers (Basel). 2020;12:1–26. https://doi.org/10.3390/CANCERS12113146.
Hess JR, Newbern DK, Beebe KL, Walsh AM, Schafernak KT. High prevalence of gene fusions and copy number alterations in pediatric radiation therapy-induced papillary and follicular thyroid carcinomas. Thyroid. 2022;32:411–20. https://doi.org/10.1089/THY.2021.0217.
Newfield RS, Jiang W, Sugganth DX, Hantash FM, Lee E, Newbury RO. Mutational analysis using next generation sequencing in pediatric thyroid cancer reveals BRAF and fusion oncogenes are common. Int J Pediatr Otorhinolaryngol. 2022;157. https://doi.org/10.1016/J.IJPORL.2022.111121.
Mitsutake N, Saenko V. Molecular pathogenesis of pediatric thyroid carcinoma. J Radiat Res. 2021;62:I71–7. https://doi.org/10.1093/JRR/RRAA096.
Perreault S, Chami R, Deyell RJ, El DD, Ellezam B, Jabado N, et al. Canadian consensus for biomarker testing and treatment of TRK fusion cancer in pediatric patients. Curr Oncol. 2021;28:346–66. https://doi.org/10.3390/CURRONCOL28010038.
Prasad PK, Mahajan P, Hawkins DS, Mostoufi-Moab S, Venkatramani R. Management of pediatric differentiated thyroid cancer: An overview for the pediatric oncologist. Pediatr Blood Cancer. 2020;67. https://doi.org/10.1002/PBC.28141.
Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol. 2002;13:3–16. https://doi.org/10.1385/EP:13:1:03.
Zanella AB, Schefel RS, Weinert L, Dora JM, Maia AL. New insights into the management of differentiated thyroid carcinoma in children and adolescents (Review). Int J Oncol. 2021;58. https://doi.org/10.3892/IJO.2021.5193.
Kickbusch I. Global + local = glocal public health. J Epidemiol Community Health. 1978;1999(53):451–2. https://doi.org/10.1136/jech.53.8.451.
Kickbusch I, Leeuw E. Global public health: revisiting healthy public policy at the global level. Health Promot Int. 1999;14:285–8. https://doi.org/10.1093/heapro/14.4.285.
O’Rourke B, Oortwijn W, Schuller T. The new definition of health technology assessment: A milestone in international collaboration. Int J Technol Assess Health Care. 2020;36:187–90. https://doi.org/10.1017/S0266462320000215.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:n71. https://doi.org/10.1136/bmj.n71.
McArthur A, Klugárová J, Yan H, Florescu S. Innovations in the systematic review of text and opinion. Int J Evid Based Healthc. 2015;13:188–95. https://doi.org/10.1097/XEB.0000000000000060.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. JBI Manual Evid Synth. 2020. https://doi.org/10.46658/JBIMES-20-08.
Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies. JBI Database Syst Rev Implement Rep. 2019. https://doi.org/10.11124/JBISRIR-D-19-00099.
Barker TH, Stone JC, Sears K, Klugar M, Tufanaru C, Leonardi-Bee J, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth. 2023;21:494–506. https://doi.org/10.11124/JBIES-22-00430.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017:j4008. https://doi.org/10.1136/bmj.j4008.
Sousa MSA de, Wainwright M, Soares CB. Sínteses de evidências qualitativas: guia introdutório. BIS Boletim Do Instituto de Saúde. 2020;20:7–22. https://doi.org/10.52753/BIS.V20I2.34465.
Brehar AC, Terzea DC, Ioachim DL, Procopiuc C, Brehar FM, Bulgăr AC, et al. Cribriform-morular variant of papillary thyroid carcinoma at pediatric age - case report and review of the literature. Rom J Morphol Embryol. 2016;57:531–7.
Elisei R, Romei C, Soldatenko PP, Cosci B, Vorontsova T, Vivaldi A, et al. New breakpoints in both the H4 and RET genes create a variant of PTC-1 in a post-Chernobyl papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2000;53:131–6. https://doi.org/10.1046/J.1365-2265.2000.01046.X.
Halkova T, Dvorakova S, Vaclavikova E, Sykorova V, Vcelak J, Sykorova P, et al. A novel RET/PTC variant detected in a pediatric patient with papillary thyroid cancer without ionization history. Hum Pathol. 2015;46:1962–9. https://doi.org/10.1016/J.HUMPATH.2015.08.013.
Klugbauer S, Rabes HM. The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene. 1999;18:4388–93. https://doi.org/10.1038/SJ.ONC.1202824.
Kwon MJ, Rho YS, Jeong JC, Shin HS, Lee JS, Cho SJ, et al. Cribriform-morular variant of papillary thyroid carcinoma: a study of 3 cases featuring the PIK3CA mutation. Hum Pathol. 2015;46:1180–8. https://doi.org/10.1016/J.HUMPATH.2015.04.010.
Leenhardt L, Aurengo A. Post-Chernobyl thyroid carcinoma in children. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:667–77. https://doi.org/10.1053/BEEM.2000.0109.
Macerola E, Torregrossa L, Ugolini C, Bakkar S, Vitti P, Fadda G, et al. BRAFK601E mutation in a follicular thyroid adenoma: A case report. Int J Surg Pathol. 2017;25:348–51. https://doi.org/10.1177/1066896916688083.
Machado GJR, Ramos HE. Carcinoma papilífero de tireoide pediátrico associado à fusão gênica RET-PTC6: relato de caso. Revista de Ciências Médicas e Biológicas. 2021;20:472–5. https://doi.org/10.9771/cmbio.v20i3.47096.
Oler G, Nakabashi CD, Biscolla RPM, Cerutti JM. Seven-year follow-up of a juvenile female with papillary thyroid carcinoma with poor outcome, BRAF mutation and loss of expression of iodine-metabolizing genes. Arq Bras Endocrinol Metabol. 2008;52:1313–6. https://doi.org/10.1590/S0004-27302008000800017.
Otsubo R, Mussazhanova Z, Akazawa Y, Sato A, Matsuda K, Matsumoto M, et al. Sporadic pediatric papillary thyroid carcinoma harboring the ETV6/NTRK3 fusion oncogene in a 7-year-old Japanese girl: a case report and review of literature. J Pediatr Endocrinol Metab. 2018;31:461–7. https://doi.org/10.1515/JPEM-2017-0292.
Ronsley R, Rod Rassekh S, Shen Y, Lee AF, Jantzen C, Halparin J, et al. Application of genomics to identify therapeutic targets in recurrent pediatric papillary thyroid carcinoma. Cold Spring Harb Mol Case Stud. 2018;4. https://doi.org/10.1101/MCS.A002568.
Stenman A, Backman S, Johansson K, Paulsson JO, Stålberg P, Zedenius J, et al. Pan-genomic characterization of high-risk pediatric papillary thyroid carcinoma. Endocr Relat Cancer. 2021;28:337–51. https://doi.org/10.1530/ERC-20-0464.
Tfayli HM, Teot LA, Indyk JA, Witchel SF. Papillary thyroid carcinoma in an autonomous hyperfunctioning thyroid nodule: case report and review of the literature. Thyroid. 2010;20:1029–32. https://doi.org/10.1089/THY.2010.0144.
Vuong HG, Kondo T, Oishi N, Nakazawa T, Mochizuki K, Miyauchi A, et al. Paediatric follicular thyroid carcinoma - indolent cancer with low prevalence of RAS mutations and absence of PAX8-PPARG fusion in a Japanese population. Histopathology. 2017;71:760–8. https://doi.org/10.1111/HIS.13285.
Xing W, Liu X, He Q, Zhang Z, Jiang Z. BRAFV600E mutation contributes papillary thyroid carcinoma and Hashimoto thyroiditis with resistance to thyroid hormone: A case report and literature review. Oncol Lett. 2017;14:2903–11. https://doi.org/10.3892/OL.2017.6486.
Schwab KO, Pfarr N, van der Werf-Grohmann N, Pohl M, Rädecke J, Musholt T, et al. Autonomous thyroid adenoma: only an adulthood disease? J Pediatr. 2009;154. https://doi.org/10.1016/J.JPEDS.2008.12.019.
Buryk MA, Picarsic JL, Creary SE, Shaw PH, Simons JP, Deutsch M, et al. Identification of unique, heterozygous germline mutation, STK11 (p.F354L), in a child with an encapsulated follicular variant of papillary thyroid carcinoma within six months of completing treatment for neuroblastoma. Pediatr Dev Pathol. 2015;18:318–23. https://doi.org/10.2350/15-01-1597-CR.1.
Alzahrani AS, Qasem E, Murugan AK, Al-Hindi HN, Alkhafaji D, Almohanna M, et al. Uncommon TERT promoter mutations in pediatric thyroid cancer. Thyroid. 2016;26:235–41. https://doi.org/10.1089/THY.2015.0510.
Alzahrani AS, Murugan AK, Qasem E, Alswailem M, Al-Hindi H, Shi Y. Single point mutations in pediatric differentiated thyroid cancer. Thyroid. 2017;27:189–96. https://doi.org/10.1089/THY.2016.0339.
Alzahrani AS, Alswailem M, Moria Y, Almutairi R, Alotaibi M, Murugan AK, et al. Lung metastasis in pediatric thyroid cancer: radiological pattern, molecular genetics, response to therapy, and outcome. J Clin Endocrinol Metab. 2019;104:103–10. https://doi.org/10.1210/JC.2018-01690.
Henke LE, Perkins SM, Pfeifer JD, Ma C, Chen Y, DeWees T, et al. BRAF V600E mutational status in pediatric thyroid cancer. Pediatr Blood Cancer. 2014;61:1168–72. https://doi.org/10.1002/pbc.24935.
Basolo F, Giannini R, Monaco C, Melillo RM, Carlomagno F, Pancrazi M, et al. Potent mitogenicity of the RET/PTC3 oncogene correlates with its prevalence in tall-cell variant of papillary thyroid carcinoma. Am J Pathol. 2002;160:247. https://doi.org/10.1016/S0002-9440(10)64368-4.
Beimfohr C, Klugbauer S, Demidchik EP, Lengfelder E, Rabes HM. NTRK1 re-arrangement in papillary thyroid carcinomas of children after the Chernobyl reactor accident. J Cancer. 1999;80:842–7. https://doi.org/10.1002/(SICI)1097-0215(19990315)80:6.
Buryk MA, Monaco SE, Witchel SF, Mehta DK, Gurtunca N, Nikiforov YE, et al. Preoperative cytology with molecular analysis to help guide surgery for pediatric thyroid nodules. Int J Pediatr Otorhinolaryngol. 2013;77:1697–700. https://doi.org/10.1016/J.IJPORL.2013.07.029.
Buryk MA, Simons JP, Picarsic J, Monaco SE, Ozolek JA, Joyce J, et al. Can malignant thyroid nodules be distinguished from benign thyroid nodules in children and adolescents by clinical characteristics? A review of 89 pediatric patients with thyroid nodules. Thyroid. 2015;25:392–400. https://doi.org/10.1089/THY.2014.0312.
Cañadas-Garre M, Becerra-Massare P, Moreno Casares A, Calleja-Hernández MÁ, Llamas-Elvira JM. Relevance of BRAF and NRAS mutations in the primary tumor and metastases of papillary thyroid carcinomas. Head Neck. 2016;38:1772–9. https://doi.org/10.1002/HED.24517.
Castro P, Patiño E, Fierro F, Rojas C, Buitrago G, Olaya N. Clinical characteristics, surgical approach, BRAFV600E mutation and sodium iodine symporter expression in pediatric patients with thyroid carcinoma. J Pediatr Endocrinol Metab. 2020;33:1457–63. https://doi.org/10.1515/JPEM-2020-0201.
Colato C, Vicentini C, Cantara S, Pedron S, Brazzarola P, Marchetti I, et al. Break-apart interphase fluorescence in situ hybridization assay in papillary thyroid carcinoma: on the road to optimizing the cut-off level for RET/PTC rearrangements. Eur J Endocrinol. 2015;172:571–82. https://doi.org/10.1530/EJE-14-0930.
Cordioli MICV, Moraes L, Bastos AU, Besson P, Alves MTDS, Delcelo R, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid. 2017;27:182–8. https://doi.org/10.1089/THY.2016.0387.
Cyniak-Magierska A, Brzeziańska E, Januszkiewicz-Caulier J, Jarza̧b B, Lewiński A. Prevalence of RAS point mutations in papillary thyroid carcinoma; a novel mutation at codon 31 of K-RAS. Exp Clin Endocrinol Diabetes 2007;115:594–9. https://doi.org/10.1055/S-2007-981670.
Eszlinger M, Niedziela M, Typlt E, Jaeschke H, Huth S, Schaarschmidt J, et al. Somatic mutations in 33 benign and malignant hot thyroid nodules in children and adolescents. Mol Cell Endocrinol. 2014;393:39–45. https://doi.org/10.1016/J.MCE.2014.05.023.
Gallant JN, Chen SC, Ortega CA, Rohde SL, Belcher RH, Netterville JL, et al. Evaluation of the molecular landscape of pediatric thyroid nodules and use of a multigene genomic classifier in children. JAMA Oncol. 2022;8:1323. https://doi.org/10.1001/JAMAONCOL.2022.1655.
Hillebrandt S, Streffer C, Reiners C, Demidchik E. Mutations in the p53 tumour suppressor gene in thyroid tumours of children from areas contaminated by the Chernobyl accident. Int J Radiat Biol. 1996;69:39–45. https://doi.org/10.1080/095530096146165.
Iwadate M, Mitsutake N, Matsuse M, Fukushima T, Suzuki S, Matsumoto Y, et al. The clinicopathological results of thyroid cancer with BRAFV600E mutation in the young population of fukushima. J Clin Endocrinol Metab. 2020;105. https://doi.org/10.1210/CLINEM/DGAA573.
Klugbauer S, Pfeiffer P, Gassenhuber H, Beimfohr C, Rabes HM. RET rearrangements in radiation-induced papillary thyroid carcinomas: high prevalence of topoisomerase I sites at breakpoints and microhomology-mediated end joining in ELE1 and RET chimeric genes. Genomics. 2001;73:149–60. https://doi.org/10.1006/GENO.2000.6434.
Kumagai A, Namba H, Saenko VA, Ashizawa K, Ohtsuru A, Ito M, et al. Low frequency of BRAFT1796A mutations in childhood thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4280–4. https://doi.org/10.1210/JC.2004-0172.
Lam KY, Lo CY, Leung PS. High prevalence of RET proto-oncogene activation (RET/PTC) in papillary thyroid carcinomas. Eur J Endocrinol. 2002;147:741–5. https://doi.org/10.1530/EJE.0.1470741.
Lee YA, Im SW, Jung KC, Chung EJ, Shin CH, Kim J Il, et al. Predominant DICER1 pathogenic variants in pediatric follicular thyroid carcinomas. Thyroid. 2020;30:1120–31. https://doi.org/10.1089/THY.2019.0233.
Li Y, Wang Y, Li L, Qiu X. The clinical significance of BRAFV600E mutations in pediatric papillary thyroid carcinomas. Sci Rep. 2022;12. https://doi.org/10.1038/S41598-022-16207-1.
Mian C, Barollo S, Pennelli G, Pavan N, Rugge M, Pelizzo MR, et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf). 2008;68:108–16. https://doi.org/10.1111/J.1365-2265.2007.03008.X.
Motomura T, Nikiforov YE, Namba H, Ashizawa K, Nagataki S, Yamashita S, et al. ret rearrangements in Japanese pediatric and adult papillary thyroid cancers. Thyroid. 1998;8:485–9. https://doi.org/10.1089/THY.1998.8.485.
Musholt TJ, Brehm C, Hanack J, Von Wasielewski R, Musholt PB. Identification of differentially expressed genes in papillary thyroid carcinomas with and without rearrangements of the tyrosine kinase receptors RET and/or NTRK1. J Surg Res. 2006;131:15–25. https://doi.org/10.1016/J.JSS.2005.08.013.
Nikiforov YE, Koshoffer A, Nikiforova M, Stringer J, Fagin JA. Chromosomal breakpoint positions suggest a direct role for radiation in inducing illegitimate recombination between the ELE1 and RET genes in radiation-induced thyroid carcinomas. Oncogene. 1999;18:6330–4. https://doi.org/10.1038/SJ.ONC.1203019.
Nikiforov YE, Nikiforova M, Fagin JA. Prevalence of minisatellite and microsatellite instability in radiation-induced post-Chernobyl pediatric thyroid carcinomas. Oncogene. 1998;17:1983–8. https://doi.org/10.1038/SJ.ONC.1202120.
Nikiforov YE, Erickson LA, Nikiforova MN, Caudill CM, Lloyd RV. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am J Surg Pathol. 2001;25:1478–84. https://doi.org/10.1097/00000478-200112000-00002.
Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–4.
Oishi N, Kondo T, Nakazawa T, Mochizuki K, Inoue T, Kasai K, et al. Frequent BRAF V600E and absence of TERT promoter mutations characterize sporadic pediatric papillary thyroid carcinomas in Japan. Endocr Pathol. 2017;28:103–11. https://doi.org/10.1007/S12022-017-9470-Y.
Oliveira G, Polónia A, Cameselle-Teijeiro JM, Leitão D, Sapia S, Sobrinho-Simões M, et al. EWSR1 rearrangement is a frequent event in papillary thyroid carcinoma and in carcinoma of the thyroid with Ewing family tumor elements (CEFTE). Virchows Arch. 2017;470:517–25. https://doi.org/10.1007/S00428-017-2095-1.
Onder S, Ozturk Sari S, Yegen G, Sormaz IC, Yilmaz I, Poyrazoglu S, et al. Classic architecture with multicentricity and local recurrence, and absence of TERT promoter mutations are correlates of BRAF (V600E) harboring pediatric papillary thyroid carcinomas. Endocr Pathol. 2016;27:153–61. https://doi.org/10.1007/S12022-016-9420-0.
Ory C, Ugolin N, Levalois C, Lacroix L, Caillou B, Bidart JM, et al. Gene expression signature discriminates sporadic from post-radiotherapy-induced thyroid tumors. Endocr Relat Cancer. 2011;18:193–206. https://doi.org/10.1677/ERC-10-0205.
Papotti M, Volante M, Giuliano A, Fassina A, Fusco A, Bussolati G, et al. RET/PTC activation in hyalinizing trabecular tumors of the thyroid. Am J Surg Pathol. 2000;24:1615–21. https://doi.org/10.1097/00000478-200012000-00004.
Pelizzo MR, Boschin IM, Barollo S, Pennelli G, Toniato A, Zambonin L, et al. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor. A mono-institutional experience CCLM. 2011;49:325–9. https://doi.org/10.1515/CCLM.2011.031.
Penko K, Livezey J, Fenton C, Patel A, Nicholson D, Flora M, et al. BRAF mutations are uncommon in papillary thyroid cancer of young patients. Thyroid. 2005;15:320–5. https://doi.org/10.1089/THY.2005.15.320.
Pessôa-Pereira D, Medeiros MF da S, Lima VMS, Silva JC da, Cerqueira TL de O, Silva IC da, et al. Association between BRAF (V600E) mutation and clinicopathological features of papillary thyroid carcinoma: a Brazilian single-centre case series. Arch Endocrinol Metab. 2019;63:97–106. https://doi.org/10.20945/2359-3997000000120.
Pfeifer A, Rusinek D, Żebracka-Gala J, Czarniecka A, Chmielik E, Zembala-Nożyńska E, et al. Novel TG-FGFR1 and TRIM33-NTRK1 transcript fusions in papillary thyroid carcinoma. Genes Chromosomes Cancer. 2019;58:558–66. https://doi.org/10.1002/GCC.22737.
Pisarchik AV, Ermak G, Fomicheva V, Kartel NA, Figge J. The ret/PTC1 rearrangement is a common feature of Chernobyl-associated papillary thyroid carcinomas from Belarus. Thyroid. 1998;8:133–9. https://doi.org/10.1089/THY.1998.8.133.
Pisarchik AV, Ermak G, Demidchik EP, Mikhalevich LS, Kartel NA, Figge J. Low prevalence of the ret/PTC3r1 rearrangement in a series of papillary thyroid carcinomas presenting in Belarus ten years post-Chernobyl. Thyroid. 1998;8:1003–8. https://doi.org/10.1089/THY.1998.8.1003.
Powell N, Jeremiah S, Morishita M, Dudley E, Bethel J, Bogdanova T, et al. Frequency of BRAF T1796A mutation in papillary thyroid carcinoma relates to age of patient at diagnosis and not to radiation exposure. J Pathol. 2005;205:558–64. https://doi.org/10.1002/PATH.1736.
Prasad ML, Vyas M, Horne MJ, Virk RK, Morotti R, Liu Z, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–107. https://doi.org/10.1002/CNCR.29887.
Rogounovitch TI, Mankovskaya SV, Fridman MV, Leonova TA, Kondratovitch VA, Konoplya NE, et al. Major oncogenic drivers and their clinicopathological correlations in sporadic childhood papillary thyroid carcinoma in belarus. Cancers (Basel). 2021;13. https://doi.org/10.3390/CANCERS13133374.
Romanelli K, Wells J, Patel A, Mendonca Torres M, Costello J, Jensen K, et al. Clinical and molecular characterization of thyroid cancer when seen as a second malignant neoplasm. Ther Adv Endocrinol Metab. 2021;12. https://doi.org/10.1177/20420188211058327.
Romitti M, Wajner SM, Zennig N, Goemann IM, Bueno AL, Meyer ELS, et al. Increased type 3 deiodinase expression in papillary thyroid carcinoma. Thyroid. 2012;22:897–904. https://doi.org/10.1089/THY.2012.0031.
Rossi ED, Bizzarro T, Martini M, Capodimonti S, Cenci T, Fadda G, et al. Morphological features that can predict BRAFV600E -mutated carcinoma in paediatric thyroid cytology. Cytopathology. 2016;28:55–64. https://doi.org/10.1111/CYT.12350.
Sangkhathat S, Patrapinyokul S, Chiengkriwate P, Kritsaneepaiboon S, Kayasut K, Pramphapa T, et al. Papillary carcinoma of the thyroid gland in a child of thyrotoxicosis patient receiving radioactive iodine therapy: report of a case. Pediatr Surg Int. 2008;24:747–50. https://doi.org/10.1007/S00383-008-2151-7.
Santoro M, Thomas GA, Vecchio G, Williams GH, Fusco A, Chiappetta G, et al. Gene rearrangement and Chernobyl related thyroid cancers. Br J Cancer. 2000;82:315–22. https://doi.org/10.1054/BJOC.1999.0921.
Sheu SY, Schwertheim S, Worm K, Grabellus F, Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol. 2007;20:779–87. https://doi.org/10.1038/MODPATHOL.3800797.
Stosic A, Fuligni F, Anderson ND, Davidson S, de Borja R, Acker M, et al. Diverse oncogenic fusions and distinct gene expression patterns define the genomic landscape of pediatric papillary thyroid carcinoma. Cancer Res. 2021;81:5625–37. https://doi.org/10.1158/0008-5472.CAN-21-0761.
Swierniak M, Pfeifer A, Stokowy T, Rusinek D, Chekan M, Lange D, et al. Somatic mutation profiling of follicular thyroid cancer by next generation sequencing. Mol Cell Endocrinol. 2016;433:130–7. https://doi.org/10.1016/J.MCE.2016.06.007.
Unger K, Zitzelsberger H, Salvatore G, Santoro M, Bogdanova T, Braselmann H, et al. Heterogeneity in the distribution of RET/PTC rearrangements within individual post-Chernobyl papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4272–9. https://doi.org/10.1210/JC.2003-031870.
van der Tuin K, Ventayol Garcia M, Corver WE, Khalifa MN, Ruano Neto D, Corssmit EPM, et al. Targetable gene fusions identified in radioactive iodine refractory advanced thyroid carcinoma. Eur J Endocrinol. 2019;180:235–41. https://doi.org/10.1530/EJE-18-0653.
Vanden Borre P, Schrock AB, Anderson PM, Morris JC, Heilmann AM, Holmes O, et al. Pediatric, adolescent, and young adult thyroid carcinoma harbors frequent and diverse targetable genomic alterations. Including Kinase Fusions Oncologist. 2017;22:255–63. https://doi.org/10.1634/THEONCOLOGIST.2016-0279.
Wasserman JD, Sabbaghian N, Fahiminiya S, Chami R, Mete O, Acker M, et al. DICER1 mutations are frequent in adolescent-onset papillary thyroid carcinoma. J Clin Endocrinol Metab. 2018;103:2009–15. https://doi.org/10.1210/JC.2017-02698.
Williams GH, Rooney S, Thomas GA, Cummins G, Williams ED. RET activation in adult and childhood papillary thyroid carcinoma using a reverse transcriptase-n-polymerase chain reaction approach on archival-nested material. Br J Cancer. 1996;74:585–9. https://doi.org/10.1038/BJC.1996.405.
Woodford RL, Nikiforov YE, Hunt JL, Bellizzi AM, Zhang X, Mills SE, et al. Encapsulated papillary oncocytic neoplasms of the thyroid: morphologic, immunohistochemical, and molecular analysis of 18 cases. Am J Surg Pathol. 2010;34:1582–90. https://doi.org/10.1097/PAS.0B013E3181F2D820.
Yuan X, Mu N, Wang N, Strååt K, Sofiadis A, Guo Y, et al. GABPA inhibits invasion/metastasis in papillary thyroid carcinoma by regulating DICER1 expression. Oncogene. 2019;38:965–79. https://doi.org/10.1038/S41388-018-0483-X.
Zheng X, Wang C, Xu M, Yu Y, Yun X, Jia Y, et al. Progression of solitary and multifocal papillary thyroid carcinoma - a retrospective study of 368 patients. Chin Med J (Engl). 2012;125:4434–9.
Zheng X, Xia T, Lin L, Gao S, Lee Y, Yu Y, et al. BRAFV600E status and clinical characteristics in solitary and multiple papillary thyroid carcinoma: experience of 512 cases at a clinical center in China. World J Surg Oncol. 2012;10. https://doi.org/10.1186/1477-7819-10-104.
Zurnadzhy L, Bogdanova T, Rogounovitch TI, Ito M, Tronko M, Yamashita S, et al. The BRAFV600E mutation is not a risk factor for more aggressive tumor behavior in radiogenic and sporadic papillary thyroid carcinoma at a young age. Cancers (Basel). 2021;13. https://doi.org/10.3390/CANCERS13236038.
Zurnadzhy L, Bogdanova T, Rogounovitch TI, Ito M, Tronko M, Yamashita S, et al. Clinicopathological implications of the BRAF V 600 E mutation in papillary thyroid carcinoma of ukrainian patients exposed to the chernobyl radiation in childhood: A study for 30 years after the accident. Front Med (Lausanne). 2022;9. https://doi.org/10.3389/FMED.2022.882727.
Pisarchik AV, Ermak G, Kartei NA, Figge J. Molecular alterations involving p53 codons 167 and 183 in papillary thyroid carcinomas from chernobyl-contaminated regions of belarus. Thyroid. 2000;10:25–30. https://doi.org/10.1089/THY.2000.10.25.
Lima J, Trovisco V, Soares P, Máximo V, Magalhães J, Salvatore G, et al. BRAF mutations are not a major event in post-chernobyl childhood thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4267–71. https://doi.org/10.1210/JC.2003-032224.
Elisei R, Romei C, Vorontsova T, Cosci B, Veremeychik V, Kuchinskaya E, et al. RET/PTC rearrangements in thyroid nodules: Studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab. 2001;86:3211–6. https://doi.org/10.1210/JCEM.86.7.7678.
Sisdelli L, Cordioli MICV, Vaisman F, Moraes L, Colozza-Gama GA, Alves PAG, et al. AGK-BRAF is associated with distant metastasis and younger age in pediatric papillary thyroid carcinoma. Pediatr Blood Cancer. 2019;66. https://doi.org/10.1002/PBC.27707.
Unger K, Zurnadzhy L, Walch A, Mall M, Bogdanova T, Braselmann H, et al. RET rearrangements in post-Chernobyl papillary thyroid carcinomas with a short latency analysed by interphase FISH. Br J Cancer. 2006;94:1472–7. https://doi.org/10.1038/SJ.BJC.6603109.
Alzahrani AS, Murugan AK, Qasem E, Al-Hindi H. HABP2 gene mutations do not cause familial or sporadic non-medullary thyroid cancer in a highly inbred middle eastern population. Thyroid. 2016;26:667–71. https://doi.org/10.1089/THY.2015.0537.
Alzahrani AS, Murugan AK, Qasem E, Alswailem MM, AlGhamdi B, Moria Y, et al. Absence of EIF1AX, PPM1D, and CHEK2 mutations reported in Thyroid Cancer Genome Atlas (TCGA) in a large series of thyroid cancer. Endocrine. 2019;63:94–100. https://doi.org/10.1007/S12020-018-1762-6.
Alzahrani AS, Alswailem M, Alswailem AA, Al-Hindi H, Goljan E, Alsudairy N, et al. Genetic alterations in pediatric thyroid cancer using a comprehensive childhood cancer gene panel. J Clin Endocrinol Metab. 2020;105:3324–34. https://doi.org/10.1210/CLINEM/DGAA389.
Bae JS, Jung SH, Hirokawa M, Bychkov A, Miyauchi A, Lee S, et al. High prevalence of DICER1 mutations and low frequency of gene fusions in pediatric follicular-patterned tumors of the thyroid. Endocr Pathol. 2021;32:336–46. https://doi.org/10.1007/S12022-021-09688-9.
Ballester LY, Sarabia SF, Sayeed H, Patel N, Baalwa J, Athanassaki I, et al. Integrating molecular testing in the diagnosis and management of children with thyroid lesions. Pediatr Dev Pathol. 2016;19:94–100. https://doi.org/10.2350/15-05-1638-OA.1.
Chakraborty D, Shakya S, Ballal S, Agarwal S, Bal C. BRAF V600E and TERT promoter mutations in paediatric and young adult papillary thyroid cancer and clinicopathological correlation. J Pediatr Endocrinol Metab. 2020;33:1465–74. https://doi.org/10.1515/JPEM-2020-0174.
Ciampi R, Knauf JA, Kerler R, Gandhi M, Zhu Z, Nikiforova MN, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. https://doi.org/10.1172/JCI23237.
Eloy C, Santos J, Soares P, Sobrinho-Simões M. Intratumoural lymph vessel density is related to presence of lymph node metastases and separates encapsulated from infiltrative papillary thyroid carcinoma. Virchows Arch. 2011;459:595–605. https://doi.org/10.1007/S00428-011-1161-3.
Espadinha C, Santos JR, Sobrinho LG, Bugalho MJ. Expression of iodine metabolism genes in human thyroid tissues: evidence for age and BRAFV600E mutation dependency. Clin Endocrinol (Oxf). 2009;70:629–35. https://doi.org/10.1111/J.1365-2265.2008.03376.X.
Fehér LZ, Pocsay G, Krenács L, Zvara Á, Bagdi E, Pocsay R, et al. Amplification of thymosin beta 10 and AKAP13 genes in metastatic and aggressive papillary thyroid carcinomas. Pathol Oncol Res. 2012;18:449–58. https://doi.org/10.1007/S12253-011-9467-7.
Fenton C, Anderson J, Lukes Y, Welch Dinauer CA, Tuttle RM, Francis GL. Ras mutations are uncommon in sporadic thyroid cancer in children and young adults. J Endocrinol Invest. 1999;22:781–9. https://doi.org/10.1007/BF03343644.
Franco AT, Ricarte-Filho JC, Isaza A, Jones Z, Jain N, Mostoufi-Moab S, et al. Fusion oncogenes are associated with increased metastatic capacity and persistent disease in pediatric thyroid cancers. J Clin Oncol. 2022;40:1081–90. https://doi.org/10.1200/JCO.21.01861.
Galuppini F, Vianello F, Censi S, Barollo S, Bertazza L, Carducci S, et al. Differentiated thyroid carcinoma in pediatric age: Genetic and clinical scenario. Front Endocrinol (Lausanne). 2019;10. https://doi.org/10.3389/FENDO.2019.00552.
Geng J, Liu Y, Guo Y, Wang H, Tai J, Jin Y, et al. Correlation between TERT C228T and clinic-pathological features in pediatric papillary thyroid carcinoma. Sci China Life Sci. 2019;62:1563–71. https://doi.org/10.1007/S11427-018-9546-5.
Geng J, Wang H, Liu Y, Tai J, Jin Y, Zhang J, et al. Correlation between BRAF V600E mutation and clinicopathological features in pediatric papillary thyroid carcinoma. Sci China Life Sci. 2017;60:729–38. https://doi.org/10.1007/S11427-017-9083-8.
Gertz RJ, Nikiforov Y, Rehrauer W, McDaniel L, Lloyd RV. Mutation in BRAF and other members of the MAPK pathway in papillary thyroid carcinoma in the pediatric population. Arch Pathol Lab Med. 2016;140:134–9. https://doi.org/10.5858/ARPA.2014-0612-OA.
Şenyürek YG, İşcan Y, Sormaz İC, Poyrazoğlu Ş, Tunca F. The role of American thyroid association pediatric thyroid cancer risk stratification and BRAFV600E mutation in predicting the response to treatment in papillary thyroid cancer patients ≤18 years old. J Clin Res Pediatr Endocrinol. 2022;14:196. https://doi.org/10.4274/JCRPE.GALENOS.2022.2021-10-4.
Givens DJ, Buchmann LO, Agarwal AM, Grimmer JF, Hunt JP. BRAF V600E does not predict aggressive features of pediatric papillary thyroid carcinoma. Laryngoscope. 2014;124. https://doi.org/10.1002/LARY.24668.
Hieber L, Huber R, Bauer V, Schäffner Q, Braselmann H, Thomas G, et al. Chromosomal rearrangements in post-Chernobyl papillary thyroid carcinomas: evaluation by spectral karyotyping and automated interphase FISH. J Biomed Biotechnol. 2011;2011. https://doi.org/10.1155/2011/693691.
Hillebrandt S, Streffer C, Demidchik EP, Biko J, Reiners C. Polymorphisms in the p53 gene in thyroid tumours and blood samples of children from areas in Belarus. Mutat Res. 1997;381:201–7. https://doi.org/10.1016/S0027-5107(97)00169-3.
Jie Y, Ruan J, Cai Y, Luo M, Liu R. Comparison of ultrasonography and pathology features between children and adolescents with papillary thyroid carcinoma. Heliyon. 2023;9. https://doi.org/10.1016/J.HELIYON.2023.E12828.
Lee YJ, Cho YJ, Heo YJ, Chung EJ, Choi YH, Kim J Il, et al. Thyroid nodules in childhood-onset Hashimoto’s thyroiditis: Frequency, risk factors, follow-up course and genetic alterations of thyroid cancer. Clin Endocrinol (Oxf). 2021;95:638–48. https://doi.org/10.1111/CEN.14490.
Lee YA, Lee H, Im SW, Song YS, Oh DY, Kang HJ, et al. NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J Clin Invest. 2021;131. https://doi.org/10.1172/JCI144847.
Macerola E, Proietti A, Poma AM, Ugolini C, Torregrossa L, Vignali P, et al. Molecular alterations in relation to histopathological characteristics in a large series of pediatric papillary thyroid carcinoma from a single institution. Cancers (Basel). 2021;13. https://doi.org/10.3390/CANCERS13133123.
Mclver B, Grebe SKG, Wang L, Hay ID, Yokomizo A, Liu W, et al. FHIT and TSG101 in thyroid tumours: Aberrant transcripts reflect rare abnormal RNA processing events of uncertain pathogenetic or clinical significance. Clin Endocrinol (Oxf). 2000;52:749–57. https://doi.org/10.1046/j.1365-2265.2000.01009.x.
Mostoufi-Moab S, Labourier E, Sullivan L, Livolsi V, Li Y, Xiao R, et al. Molecular testing for oncogenic gene alterations in pediatric thyroid lesions. Thyroid. 2018;28:60–7. https://doi.org/10.1089/THY.2017.0059.
Naito H, Pairojkul C, Kitahori Y, Yane K, Miyahara H, Konishi N, et al. Different ras gene mutational frequencies in thyroid papillary carcinomas in Japan and Thailand. Cancer Lett. 1998;131:171–5. https://doi.org/10.1016/S0304-3835(98)00149-9.
Nicolson NG, Murtha TD, Dong W, Paulsson JO, Choi J, Barbieri AL, et al. Comprehensive genetic analysis of follicular thyroid carcinoma predicts prognosis independent of histology. J Clin Endocrinol Metab. 2018;103:2640–50. https://doi.org/10.1210/JC.2018-00277.
Nikita ME, Jiang W, Cheng SM, Hantash FM, McPhaul MJ, Newbury RO, et al. Mutational analysis in pediatric thyroid cancer and correlations with age, ethnicity, and clinical presentation. Thyroid. 2016;26:227–34. https://doi.org/10.1089/THY.2015.0401.
Park HJ, Baek I, Cheang G, Solomon JP, Song W. Comparison of RNA-based next-generation sequencing assays for the detection of NTRK gene fusions. J Mol Diagn. 2021;23:1443–51. https://doi.org/10.1016/J.JMOLDX.2021.07.027.
Passon N, Bregant E, Sponziello M, Dima M, Rosignolo F, Durante C, et al. Somatic amplifications and deletions in genome of papillary thyroid carcinomas. Endocrine. 2015;50:453–64. https://doi.org/10.1007/S12020-015-0592-Z.
Pauws E, Tummers RFHM, Ris-Stalpers C, De Vijlder JJM, Vote T. Absence of activating mutations in ras and gsp oncogenes in a cohort of nine patients with sporadic pediatric thyroid tumors. Med Pediatr Oncol. 2001;36:630–4. https://doi.org/10.1002/MPO.1140.
Pekova B, Dvorakova S, Sykorova V, Vacinova G, Vaclavikova E, Moravcova J, et al. Somatic genetic alterations in a large cohort of pediatric thyroid nodules. Endocr Connect. 2019;8:796–805. https://doi.org/10.1530/EC-19-0069.
Picarsic JL, Buryk MA, Ozolek J, Ranganathan S, Monaco SE, Simons JP, et al. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next-generation sequencing assay. Pediatr Dev Pathol. 2016;19:115–22. https://doi.org/10.2350/15-07-1667-OA.1.
Port M, Boltze C, Wang Y, Röper B, Meineke V, Abend M. A radiation-induced gene signature distinguishes post-Chernobyl from sporadic papillary thyroid cancers. Radiat Res. 2007;168:639–49. https://doi.org/10.1667/RR0968.1.
Poyrazoğlu Ş, Bundak R, Baş F, Yeğen G, Şanlı Y, Darendeliler F. Clinicopathological characteristics of papillary thyroid cancer in children with emphasis on pubertal status and association with BRAFV600E mutation. J Clin Res Pediatr Endocrinol. 2017;9:185–93. https://doi.org/10.4274/JCRPE.3873.
Rabes HM, Demidchik EP, Sidorow JD, Lengfelder E, Beimfohr C, Hoelzel D, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: Biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;6:1093–103.
Ricarte-Filho JC, Halada S, O’Neill A, Casado-Medrano V, Laetsch TW, Franco AT, et al. The clinical aspect of NTRK-fusions in pediatric papillary thyroid cancer. Cancer Genet. 2022;262–263:57–63. https://doi.org/10.1016/J.CANCERGEN.2022.01.002.
Ricarte-Filho JC, Li S, Garcia-Rendueles MER, Montero-Conde C, Voza F, Knauf JA, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123:4935–44. https://doi.org/10.1172/JCI69766.
Ricarte-Filho JC, Casado-Medrano V, Reichenberger E, Spangler Z, Scheerer M, Isaza A, et al. DICER1 RNase IIIb domain mutations trigger widespread miRNA dysregulation and MAPK activation in pediatric thyroid cancer. Front Endocrinol (Lausanne). 2023;14. https://doi.org/10.3389/FENDO.2023.1083382/FULL.
Richter HE, Lohrer HD, Hieber L, Kellerer AM, Lengfelder E, Bauchinger M. Microsatellite instability and loss of heterozygosity in radiation-associated thyroid carcinomas of Belarussian children and adults. Carcinogenesis. 1999;20:2247–51. https://doi.org/10.1093/CARCIN/20.12.2247.
Rosenbaum E, Hosler G, Zahurak M, Cohen Y, Sidransky D, Westra WH. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Mod Pathol. 2005;18:898–902. https://doi.org/10.1038/MODPATHOL.3800252.
Rusinek D, Pfeifer A, Cieslicka M, Kowalska M, Pawlaczek A, Krajewska J, et al. TERT promoter mutations and their impact on gene expression profile in papillary thyroid carcinoma. Cancers (Basel). 2020;12:1–20. https://doi.org/10.3390/CANCERS12061597.
Smida J, Zitzelsberger H, Kellerer A, Lehmann L, Minkus G, Negele T, et al. p53 mutations in childhood thyroid tumors from Belarus and in theyroid tumors without radiation history. Int J Cancer. 1997. https://doi.org/10.1002/(SICI)1097-0215(19971210)73:6.
Thomas GA, Bunnell H, Cook HA, Williams ED, Nerovnya A, Cherstvoy ED, et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab. 1999;84:4232–8. https://doi.org/10.1210/JCEM.84.11.6129.
Tian T, Huang S, Dai H, Qi M, Liu B, Huang R. Radioactive iodine-refractory pulmonary metastases of papillary thyroid cancer in children, adolescents, and young adults. J Clin Endocrinol Metab. 2023;108:306–14. https://doi.org/10.1210/CLINEM/DGAC600.
Unger K, Malisch E, Thomas G, Braselmann H, Walch A, Jackl G, et al. Array CGH demonstrates characteristic aberration signatures in human papillary thyroid carcinomas governed by RET/PTC. Oncogene. 2008;27:4592–602. https://doi.org/10.1038/ONC.2008.99.
Vinagre J, Almeida A, Pópulo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4. https://doi.org/10.1038/NCOMMS3185.
Zhao X, Kotch C, Fox E, Surrey LF, Wertheim GB, Baloch ZW, et al. NTRK fusions identified in pediatric tumors: The frequency, fusion partners, and clinical outcome. JCO Precis Oncol. 2021;1:204–14. https://doi.org/10.1200/PO.20.00250.
Kuick CH, Chen WW, Chen H, Chang KTE, Mok Y. Validation of long mononucleotide repeat markers for microsatellite instability testing in paediatric tumours. J Clin Pathol. 2023;76:214–6. https://doi.org/10.1136/JCP-2022-208692.
Smida J, Salassidis K, Hieber L, Zitzelsberger H, Kellerer AM, Demidchik EP, et al. Distinct frequency of ret rearrangements in papillary thyroid carcinoma of children and adults from Belarus. J Cancer. 1999;80:32–8. https://doi.org/10.1002/(SICI)1097-0215(19990105)80:1.
Sassolas G, Hafdi-Nejjari Z, Ferraro A, Decaussin-Petrucci M, Rousset B, Borson-Chazot F, et al. Oncogenic alterations in papillary thyroid cancers of young patients. Thyroid. 2012;22:17–26. https://doi.org/10.1089/THY.2011.0215.
Onder S, Mete O, Yilmaz I, Bayram A, Bagbudar S, Altay AY, et al. DICER1 mutations occur in more than one-third of follicular-patterned pediatric papillary thyroid carcinomas and correlate with a low-risk disease and female gender predilection. Endocr Pathol. 2022;33:437–45. https://doi.org/10.1007/S12022-022-09736-Y.
Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19:705–14. https://doi.org/10.1016/S1470-2045(18)30119-0.
Bauer AJ. Molecular genetics of thyroid cancer in children and adolescents. Endocrinol Metab Clin North Am. 2017;46:389–403. https://doi.org/10.1016/J.ECL.2017.01.014.
Cherella CE, Wassner AJ. Pediatric thyroid cancer: Recent developments. Best Pract Res Clin Endocrinol Metab. 2023;37. https://doi.org/10.1016/J.BEEM.2022.101715.
Jargin SV. On the RET rearrangements in chernobyl-related thyroid cancer. J Thyroid Res. 2012;2012. https://doi.org/10.1155/2012/373879.
Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones. 2007;6:200–9.
Nikiforov YE. Radiation-induced thyroid cancer: what we have learned from chernobyl. Endocr Pathol. 2006;17:307–18. https://doi.org/10.1007/S12022-006-0001-5.
Pacini F, Vorontsova T, Molinaro E, Shavrova E, Agate L, Kuchinskaya E, et al. Thyroid consequences of the Chernobyl nuclear accident. Acta Paediatr Suppl. 1999;88:23–7. https://doi.org/10.1111/J.1651-2227.1999.TB14399.X.
Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes (Basel). 2019;10. https://doi.org/10.3390/GENES10090723.
Poorten V Vander, Hens G, Delaere P. Thyroid cancer in children and adolescents. Curr Opin Otolaryngol Head Neck Surg. 2013;21:135–42. https://doi.org/10.1097/MOO.0B013E32835E15D9.
Papadopoulou F, Efthimiou E. Thyroid cancer after external or internal ionizing irradiation. Hell J Nucl Med. 2009;12:266–70.
Thomas G. Radiation and thyroid cancer - an overview. Radiat Prot Dosimetry. 2018;182:53–7. https://doi.org/10.1093/RPD/NCY146.
Kotanidou EP, Giza S, Tsinopoulou VR, Margaritis K, Papadopoulou A, Sakellari E, et al. The prognostic significance of BRAF gene analysis in children and adolescents with papillary thyroid carcinoma: A systematic review and meta-analysis. Diagnostics (Basel). 2023;13. https://doi.org/10.3390/DIAGNOSTICS13061187.
Satapathy S, Bal C. Genomic landscape of sporadic pediatric differentiated thyroid cancers: a systematic review and meta-analysis. J Pediatr Endocrinol Metab. 2022;35:749–60. https://doi.org/10.1515/JPEM-2021-0741.
Cordioli MICV, Moraes L, Carvalheira G, Sisdelli L, Alves MTS, Delcelo R, et al. AGK-BRAF gene fusion is a recurrent event in sporadic pediatric thyroid carcinoma. Cancer Med. 2016;5:1535–41. https://doi.org/10.1002/CAM4.698.
Acknowledgements
This work was supported by research grant 406952/2022-1 from the Ministry of Health, Brazil, and research grants 2014/06570-6 and 2022/09713-9 from the São Paulo State Research Foundation (FAPESP). INN and YPC are CAPES scholars. MSAS is a Brazilian Research Council (CNPq) scholar. JMC is a CNPq investigator. The authors thank Mabel F Figueiró for supporting protocol development and validation, and systematic review methodology revision.
Author information
Authors and Affiliations
Contributions
MSAS and JMC made substantial contributions to the conception or design of the work; MSAS, INN, YPC, LS and JMC made substantial contributions to the acquisition, analysis, or interpretation of data; MSAS, INN, YPC, LS and JMC drafted the work or revised it critically for important intellectual content; all authors approved the version to be published; and MSAS, INN, YPC, LS and JMC agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interest
All authors declare no direct or indirect financial or non-financial competing interests that are directly or indirectly related to the work submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Sousa, M.S.A., Nunes, I.N., Christiano, Y.P. et al. Genetic alterations landscape in paediatric thyroid tumours and/or differentiated thyroid cancer: Systematic review. Rev Endocr Metab Disord 25, 35–51 (2024). https://doi.org/10.1007/s11154-023-09840-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09840-2