Abstract
Herein we present the case of a 9-year-old girl who had an enlarged right lobe of the thyroid gland and sub-clinical hypothyroidism (thyroid stimulating hormone at 9.24 mIU/L). The patient had a history of unintentional exposure to radiation while her mother was receiving radionuclide therapy for diffuse toxic goiter. Ultrasonography of the young girl showed right lobe enlargement with diffuse coarse heterogenous echogenicity, compatible with a microcalcification pattern identified in both lobes of the thyroid gland. Histopathology of the tissue from a thyroidectomy revealed papillary thyroid carcinoma in the right lobe and chronic lymphocytic thyroiditis in the remaining tissue. Molecular pathology demonstrated an RET/PTC1 rearrangement in both tumor and non-tumorous tissue harboring thyroiditis. Considering the history of exposure and the characteristics of the thyroid pathology together, the PTC in this patient was likely a secondary-to-genetic alteration induced by external radiation. This case emphasizes the importance of stringent restrictions when giving radioactive iodine therapy to a patient with small children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is one of the common secondary malignancies occurring in patients with a history of radiation exposure. High-dose external radiation, such as from an atomic bomb or nuclear power plant fallout, is a significant risk factor of PTC. In children, PTC may occur from radiotherapy for cancer [1]. Biologically, the radiation causes chromosomal translocation resulting in genetic lesions that drive neoplastic change. RET/PTC fusions are known genetic rearrangements that can be detected in the majority of radiation-induced PTC cases [2, 3].
Radioactive iodine (Iodine 131) therapy is an effective treatment for patients with diffuse toxic goiter (Grave’s disease). Although emission of radiation from the patient can potentially affect surrounding people, various studies have determined that administration of the treatment is safe and can be done on an ambulatory basis [4–7]. Many countries, including the USA, UK and some European countries, offer outpatient treatment with advice given to minimize subsequent close contact with other individuals [4, 5, 7–10]. Such recommendations are usually based on the amount of radiation exposure in family members or caregivers, which is usually lower than the cut-off level of dose constraints. However, caution is required in individuals who might have higher-than-normal susceptibility such as a patient with young family members, who lives in an area with high background environmental radiation level or who has iodine deficiency.
We report herein a case of PTC in a child who was apparently a victim of third-party therapeutic radiation, and suggest that more restrictions should be considered for at least certain patients receiving outpatient radioactive iodine therapy.
Case report
A 9-year-old female patient presented to our pediatric clinic with an enlarged right thyroid lobe for 3 months. She exhibited no symptoms of hyperthyroidism. The patient was the second child of a mother with a previous history of diffuse toxic goiter who had received radioactive iodine therapy at a total dose of 220 MBq as an ambulatory case when the patient was 4 years old. She was unintentionally in close proximity with the patient during the evenings she had received the treatment and throughout the following several days while her other child was not at home. In an unusual coincidence, the patient had received yet further exposure when she had visited her grandmother who had received 300 MBq of radioactive iodine for toxic goiter, 3 years before the other exposure, at the age of 1 year.
On examination, her thyroid gland was found to be enlarged at the right lobe. The pulse rate was within normal range. The body weight and height were at the 75 and 85 percentile, respectively, according to the Thai national standard growth curve. A thyroid function test reported free T4 at 1.15 ng/dl (normal range 0.70–1.75), free T3 at 3.79 pg/ml (2–4.40) and thyroid stimulating hormone (TSH) at 9.24 mIU/L (0.25–4.00). The anti-thyroglobulin antibody titer was 1:160 and anti-microsomal antibody titer was 1:25,600.
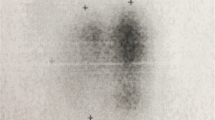
A thyroid scan revealed enlarged thyroid gland with multiple hypofunctioning nodules at the right lobe. Ultrasonography demonstrated a markedly enlarged right thyroid lobe, measuring 2.8 × 2 × 4 cm in width × thickness × height, respectively. The left lobe was 1.5 × 0.8 × 2.8 cm and the isthmus was 0.7 cm thick. Also apparent was diffuse coarse heterogenous echogenicity, compatible with a microcalcification pattern identified in both lobes of the thyroid gland (Fig. 1). A few enlarged adjacent lymph nodes were also noted.
A right thyroid lobectomy was initially performed. A histopathological study revealed papillary structures with typical overlapping and ground glass nuclei, occasional nuclear grooves and the presence of a psammoma body (Fig. 2a). A left lobectomy and isthmectomy together with adjacent cervical nodes dissection were then performed. Pathological testing demonstrated chronic lymphocytic thyroiditis in the uninvolved areas of the thyroid gland, and dense lymphocytic infiltrate with germinal center formation. The psammoma body was also apparent in the contralateral thyroid.
Histopathology of the thyroid gland, a papillary structures with typical overlapping and ground glass nuclei, occasional nuclear grooves and the presence of a psammoma body (arrow) (40× magnification). b Chronic lymphocytic thyroiditis in the uninvolved areas of the thyroid gland, showing dense lymphocytic infiltrate with germinal center formation (10× magnification)
A molecular genetic study was performed, and exon 15 of BRAF gene was analyzed by the direct sequencing method. Rearrangement of RET/PTC1 and RET/PTC3 were studied by reverse-transcription polymerase chain reaction (RT-PCR). To guard against any false positive results, the RT-PCR was performed in 30 cycles. The study showed a wild-type sequence of BRAF and was positive for RET/PTC1 fusion transcript in both tumor tissue and contralateral thyroid tissue without malignancy (Fig. 3).
a On RT-PCR, using tissue from the right lobe of the thyroid gland harboring PTC (T) and uninvolved left lobe (NT), positive bands of the same size were found in both parts. (N-Con negative control, NTC no template control). b Nucleotide sequencing proved the presence of an RET/PTC1 (H4) rearrangement, which was the fusion between RET exon 11 and H4 exon 1. The arrow indicates the break point
The patient received radioactive iodine therapy and was doing well on the last follow-up visit at 10 months post-operatively.
Discussion
The increased risk of papillary thyroid cancer (PTC) in children exposed to ionizating radiation was documented for a wide range of doses, and a linear dose–response relation for doses from 0.1 up to 1–2 Gy has been described [11]. There was a clear peak of thyroid cancer incidence in children, observed in some east European countries, which was related to an uptake of radioiodine following the Chernobyl disaster in 1986 [12, 13]. In these children, the increased incidence started 4–5 years after exposure, reaching its maximum in the mid-1990s, and the disease developed mainly in children <5 years old at the exposure. The child presented here was 4 years of age when exposed to radiation therapy and the cancer was detected at 9 years of age. This shows a similar pattern to the nuclear accident victims. Iodine deficiency has been known to increase cancer risk in radiation-exposed children [11, 13]. However, considering its rarity in southern Thailand [14], the condition was unlikely to be a predisposing factor in our patient.
The thyroid gland in the patient demonstrated a typical pattern of radiation-induced thyroid pathology. Apart from the malignancy, the background histology of chronic lymphocytic thyroiditis provided another clue indicating radiation association. Autoimmune thyroiditis shows increased incidence with environmental radiation exposure [15, 16]. Epidemiological studies of the incidence of autoimmune thyroiditis in children from nuclear contaminated areas or atomic bomb survivors have reported a higher prevalence of positive thyroid autoantibody in exposed children [17–20], although recent long-term cohorts from major fallout areas have not supported the dose–effect relationship [21, 22]. Some authors have speculated that PTC carcinogenesis in the thyroid gland occurs multifocally and that autoimmune thyroiditis presents a “submicroscopic form” of the malignancy. The two conditions are possibly linked through the RET/PTC rearrangement [3].
Our clear observation of genetic changes in the left lobe that harbored no malignancy was in line with the mentioned theory. In addition, we observed that the sonographic finding of microcalcification, which was compatible with the psammoma body on histological study could be well detected in thyroiditis tissue without malignant cells. Microcalcifications are one of the most specific ultrasonographic findings of a thyroid malignancy, with a specificity of up to 95% [23, 24], particularly in PTC. Diffuse microcalcifications in both lobes suggested multifocal tumorigenesis, which is consistent with radiation-induced thyroid cancer.
Radioactive iodine therapy as a day-case service is common practice in nuclear medicine. In general, a patient with toxic goiter decays more radiation than thyroidectomized malignancy cases, despite the latter group receiving higher radiation activity [6]. For thyrotoxicosis patients in our institute, Iodine-131 is given orally at the hospital and a patient is discharged in 4–6 h with a recommendation not to be in close proximity with small children for a period of 1 week. The safety of this type of regimen has been supported by previous studies that evaluated the actual radiation emission from patients or the radiation exposure in caregivers, as compared to the dose constraints accepted in each country [4]. For example, dose constraints in European countries were generally accepted at 3 mSv for relatives, including children, over the age of 10 years, with a higher dose constraint of 15 mSv for relatives over the age of 60. The dose constraint of 1 mSv is set for children under 10 years [8]. Another reference guideline is the recommendation of the International Commission on Radiological Protection (known as ICRP), in which the dose constraint is set at 1 mSv for public and family members unrelated to the patient’s caregiver(s) and 5 mSv for caregivers [10]. A multicenter study of outpatient radioiodine therapy in UK found that 6 of 17 family members aged 3 years or younger who were children of the patients received radiation at an adjusted dose of more than 1 mSv, while almost all adult family members received an adjusted dose within the safety limits [5]. A recent case series from Norway also reported one 2-year-old child who absorbed a radiation dose of 3.04 mSv from the mother despite repeatedly being informed of the safety instructions [7]. It should be noted that the mother of the latter case received only 260 MBq of radioactive iodine, which was comparable with the mother of our patient.
There have been reports of family members who received radiation doses greater than the safety level [5, 7]; however, there has been no report of any patient who developed a thyroid malignancy after third-party iodine-131 exposure. Although strong cause–effect relationship could not be demonstrated on a single-case evidence, our reported case emphasizes that care should be taken in giving ambulatory radioactive iodine therapy to a patient having young family members. Unless arrangements can be made for any small children to stay with an alternative caregiver for 10–14 days [5], inpatient management should be considered.
In summary, we report a case of pediatric PTC, in whom the disease likely developed as a result of third-party radioactive iodine therapy. The evidence suggests more stringent restrictions should be taken after treatment for a patient with young children.
References
Inskip PD (2001) Thyroid cancer after radiotherapy for childhood cancer. Med Pediatr Oncol 36:568–573
Rabes HM (2001) Gene rearrangements in radiation-induced thyroid carcinogenesis. Med Pediatr Oncol 36:574–582
Nikiforov YE (2002) RET/PTC rearrangement in thyroid tumors. Endocr Pathol 13:3–16
Monsieurs M, Thierens H, Dierckx RA et al (1998) Real-life radiation burden to relatives of patients treated with iodine-131: a study in eight centres in Flanders (Belgium). Eur J Nucl Med 25:1368–1376
Barrington SF, O’Doherty MJ, Kettle AG et al (1999) Radiation exposure of the families of outpatients treated with radioiodine (iodine-131) for hyperthyroidism. Eur J Nucl Med 26:686–692
Mathieu I, Caussin J, Smeesters P et al (1999) Recommended restrictions after 131I therapy: measured doses in family members. Health Phys 76:129–136
Cappelen T, Unhjem JF, Amundsen AL et al (2006) Radiation exposure to family members of patients with thyrotoxicosis treated with iodine-131. Eur J Nucl Med Mol Imaging 33:81–86
European Commission (1998) Radiation Protection 97. Radiation protection following Iodine-131 therapy (exposures due to out-patients or discharged inpatients). Office for Official Publications of the European Communities, Luxembourg
Hilditch TE, Connell JM, Davies DL et al (1991) Radiological protection guidance for radioactive patients—new data for therapeutic 131I. Nucl Med Commun 12:485–495
International Commission on Radiological Protection (1991) 1990 Recommendations of the International Commission on Radiological Protection. Ann IRCP 21(1–3):60, Pergamon Press, IRCP Publication, Oxford
Cardis E, Kesminiene A, Ivanov V et al (2005) Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst 18:724–732
Cardis E, Howe G, Ron E et al (2006) Cancer consequences of the chernobyl accident: 20 years on. J Radiol Prot 26:127–140
Ron E (2007) Thyroid cancer incidence among people living in areas contaminated by radiation from the Chernobyl accident. Health Phys 93:502–511
Jaruratanasirikul S, Sopanapikul S, Mo-Suwan L et al (1995) Goiter in Thai schoolchildren: study in Hat Yai, southern Thailand. J Med Assoc Thai 78:449–454
Eheman CR, Garbe P, Tuttle RM (2003) Autoimmune thyroid disease associated with environmental thyroidal irradiation. Thyroid 13:453–464
Volzke H, Werner A, Wallaschofski H et al (2005) Occupational exposure to ionizing radiation is associated with autoimmune thyroid disease. J Clin Endocrinol Metab 90:4587–4592
Nagataki S, Shibata Y, Inoue S et al (1994) Thyroid diseases among atomic bomb survivors in Nagasaki. JAMA 272:364–370
Vykhovanets EV, Chernyshov VP, Slukvin II et al (1997) 131I dose-dependent thyroid autoimmune disorders in children living around Chernobyl. Clin Immunol Immunopathol 84:251–259
Vermiglio F, Castagna MG, Volnova E et al (1999) Post-chernobyl increased prevalence of humoral thyroid autoimmunity in children and adolescents from a moderately iodine-deficient area in Russia. Thyroid 9:781–786
Lyon JL, Alder SC, Stone MB et al (2006) Thyroid disease associated with exposure to the Nevada nuclear weapons test site radiation: a reevaluation based on corrected dosimetry and examination data. Epidemiology 17:604–614
Imaizumi M, Usa T, Tominaga T et al (2006) Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55–58 years after radiation exposure. JAMA 295:1011–1022
Tronko MD, Brenner AV, Olijnyk VA et al (2006) Autoimmune thyroiditis and exposure to iodine 131 in the Ukrainian cohort study of thyroid cancer and other thyroid diseases after the Chernobyl accident: results from the first screening cycle (1998–2000). J Clin Endocrinol Metab 91:4344–4351
Summaria V, Rufini V, Mirk P et al (2000) Diagnostic imaging of differentiated thyroid carcinoma. Rays 25:177–190
Iannuccilli JD, Cronan JJ, Monchik JM (2004) Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med 23:1455–1464
Acknowledgments
The authors thank Wanwisa Maneechay for her assistance in molecular biology techniques. The ultrasonographic pictures were courtesy of Dr. Montanan Rohitopakarn.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sangkhathat, S., Patrapinyokul, S., Chiengkriwate, P. et al. Papillary carcinoma of the thyroid gland in a child of thyrotoxicosis patient receiving radioactive iodine therapy: report of a case. Pediatr Surg Int 24, 747–750 (2008). https://doi.org/10.1007/s00383-008-2151-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-008-2151-7