Abstract
A study on the asymmetric Michael addition of a fluorine containing carbon nucleophile to β-nitrostyrene was carried out to find an easily obtainable cinchona alkaloid derivative, which provides high stereoselectivities, and may be conveniently immobilized over inorganic materials to obtain efficient chiral heterogeneous catalysts. It was shown that high enantioselectivities are reached in the addition of ethyl 2-fluoroacetoacetate catalyzed by β-isocupreidine accompanied by good diastereomeric ratios. This cinchona derivative prepared in one step from quinidine was immobilized by cation-exchange between the layers of an aluminum phyllosilicate, as evidenced by XRD measurements. However, due to the protonation of the tertiary amino group, the material lost its catalytic activity in the Michael addition. The immobilization of the deprotonated alkaloid over the particle surface of an anion exchanger layered double hydroxide resulted in an inorganic–organic hybrid material, with catalytic performance approaching that of the soluble organocatalyst. Upon reuse, gradual deactivation of this heterogeneous catalyst was observed due to the leaching of the organic material. However, using less polar media could increase the lifetime of the hybrid catalyst. The results of catalytic measurements indicated that the Michael addition might occur on the solid surface. Based on the catalytic behavior of the heterogeneous catalyst, bonding possibilities of the cinchona derivative to the surface of the layered hydroxide by electrostatic interactions and hydrogen bonding are suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Asymmetric catalytic methods are convenient procedures for the preparation of optically pure fine chemicals used as intermediates in the production of chiral pharmaceuticals, agrochemicals, flavors and fragrances [1–3]. Besides enantioselective reactions catalyzed by chiral metal complexes, the asymmetric organocatalyzed procedures are used often in the synthesis of chiral chemicals [4–6]. Among the asymmetric organocatalytic reactions, Michael additions catalyzed by chiral organic bases are useful methods to obtain novel C–C bonds enantioselectively and consequently to couple simple molecules in a stereoselective manner [7]. Due to the exceptional versatility of the Michael addition, the development of a large variety of chiral catalysts was necessary to fulfil the requirements imposed by the structural diversity of the various reactants [7–9]. Special interest has been accorded to the reaction of fluorine containing compounds, which may allow tuning the physical, chemical and pharmacological properties of the bioactive chemicals [10, 11]. Accordingly, beside enantioselective hydrogenations of unsaturated fluorinated compounds, which have proved their utility [12–14], asymmetric organocatalytic fluorinations [15–17] and stereoselective catalytic coupling of fluorinated compounds were developed to obtain chiral fluorinated chemicals [15, 18–22]. However, until now, only few publications reported the asymmetric Michael addition of α-fluorinated Michael-donors to nitroolefins [23–25], although this reaction could be applied as the first stereoselective step of asymmetric cascade reactions leading to complex organic synthons bearing multiple chiral centers [26].
Among the chiral catalysts used in these reactions are natural optically pure bases, such as cinchona alkaloids and their derivatives [23, 27]. Natural cinchona alkaloids, besides being used as chiral surface modifiers in heterogeneous enantioselective catalytic hydrogenations [28], also proved to be highly efficient catalysts in various organocatalytic reactions [27]. The functional groups of the cinchona alkaloid molecules allow the modification of their structure in order to obtain finely tuned catalysts for specific applications. Moreover, introducing functional groups designed for the convenient immobilization of the catalytically active molecules on insoluble supports promoted the development of heterogenized chiral catalysts. These materials have numerous advantages compared with their homogeneous counterparts, such as easy separation from the reaction mixture, possible reuse or application in continuously operated reactor systems [29].
The most efficient organocatalysts used in the asymmetric addition of fluorinated carbon nucleophiles to β-nitrostyrenes by Lu and co-workers [23] and Wang and co-workers [24] were laboriously prepared cinchona derivatives. Our aim was to find an easily obtainable derivative able to catalyze the above reaction, which could readily be immobilized by non-covalent bonding over inorganic supports. Based on the outstanding results obtained with cupreine ethers [24, 30], we selected β-isocupreidine (β-iCu) as a promising catalyst, which is a cyclic ether derivative and also possesses a free phenolic –OH group. This compound may be obtained in a one-step synthetic route from quinidine in good yields [31] and proved its outstanding catalytic activity in several asymmetric reactions such as enantioselective Morita–Baylis–Hillman reactions [31, 32] or asymmetric organocatalytic aminations [33]. Our expectations were also supported by the successful use of this cinchona derivative in the asymmetric addition of non-fluorinated nucleophiles to nitrostyrene derivatives [34]. Moreover, this compound bearing both basic and acidic functional groups may be either protonated or deprotonated, thus, could be immobilized either over cation or anion exchanger materials, opening several heterogenization possibilities.
Experimental
Materials and methods
Commercial cinchona alkaloids: cinchonidine (Cd; Alfa Aesar, 99% total base), quinine (Qn; Fluka, ≥98%, contains <5% hydroquinine) and quinidine (Qd; Fluka, ≥98%, contains ~10% hydroquinidine) were used as received. β-iCu was prepared according to the literature procedure, the crude material was purified by flash chromatography followed by two times crystallization from methanol–water mixture [31]. The ion exchangers used as supports, Bentolite H (BH; a bentonite, aluminum phyllosilicate, from Southern Clay Prod. Inc.) and the layered double hydroxide (LD; Pural MG50, aluminum magnesium hydroxyl carbonate with MgO/Al2O3 50/50 produced by Sasol) were also used as received. Reactants trans-β-nitrostyrene (1, 99%, Aldrich) and ethyl 2-fluoroacetoacetate (2, Aldrich) were used without further purifications. Solvents and reagents used in the preparation of catalysts and in catalytic measurements were of analytical grade obtained from commercial sources (Aldrich).
Preparation of immobilized catalysts
Immobilization of β-iCu over BH cation-exchanger
2 g of BH was suspended in 200 mL distilled water by stirring magnetically for 5 h. To this suspension, a solution prepared by dissolving 0.14 g β-iCu in 20 mL distilled water acidified with 0.1 mL 10 wt% aq. HCl was added and was further stirred at 30 °C for 40 h followed by centrifugation and washing with distilled water until the supernatant remained neutral. Drying the solid for 20 h at 50 °C under vacuum resulted in 1.90 g material denoted as BH-β-iCu.
Immobilization of β-iCu over LD anion-exchanger
2 g of LD was suspended in 200 mL distilled water by magnetic stirring for 5 h. To this suspension, a solution prepared by dissolving 0.14 g β-iCu in 20 mL distilled water and 5 mL 25 wt% aqueous NH3 was added. The obtained suspension was stirred at 30 °C for 66 h followed by centrifugation and washing with distilled water until the supernatant remained neutral. Drying the solid for 20 h at 50 °C under vacuum resulted in 2.15 g material denoted as LD-β-iCu. In a separate experiment 0.67 g LD was heated at 500 °C in air for 5 h to obtain 0.41 g LDh, which was suspended in 50 mL distilled water by stirring magnetically for 5 h. To this suspension, a solution prepared by dissolving 0.05 g β-iCu in 12.5 mL distilled water and 2.5 mL 25 wt% aqueous NH3 was added. Stirring at 30 °C for 66 h followed by centrifugation and washing with distilled water until the supernatant remained neutral and finally drying the solid for 20 h at 50 °C under vacuum resulted in 0.60 g material denoted as LDh-β-iCu.
Characterization of the inorganic–organic hybrid materials
Fourier transform-infrared (FT-IR) spectra of the solid materials were recorded on a Bio-Rad Digilab FTS-65A/896 FT-IR spectrometer operated in diffuse reflectance mode (DRIFT). The spectra were recorded in the 400–4000 cm−1 wavelength range using 2 cm−1 resolution and averaging 256 scans. The X-ray diffractograms (XRD) of the materials were taken on a Rigaku Miniflex-II diffractometer using Cu Kα radiation source.
General procedure for the asymmetric Michael addition
Michael additions
Reactions were carried out in closed glass vials of 4 mL using magnetic stirring with a stirrer placed in a refrigerator set to the desired temperature. The reaction conditions were set based on results of a short optimization using β-iCu as catalyst. In a typical experiment, the given amount of catalyst was dissolved or suspended in 1 mL solvent followed by addition of 0.25 mmol trans-β-nitrostyrene (1). The solution was cooled to −20 °C and 0.5 mmol ethyl 2-fluoroacetoacetate (2) was added. The reaction started by turning on the stirrer (800 rpm). After the given reaction time the soluble catalyst was removed by washing with 1 mL 10 wt% aq. HCl solution. The aqueous solution was washed twice with 1 mL organic solvent and the unified organic solution was dried over desiccated Na2SO4. The products were analyzed by gas chromatography. The solid catalysts were initially centrifuged, the material was washed twice with 1 mL organic solvent and the unified organic solution was washed with 1 mL 10 wt% aq. HCl to remove the organic catalyst leached to the solution. After drying of the organic phase over Na2SO4, the products were analyzed by gas chromatography. The remaining solid material was dried at room temperature and reused in a successive run.
Product analysis
The products of the catalytic reactions were identified based on their mass spectra measured on Agilent Techn. 6890N GC-5973 inert MSD system (GC-MSD) equipped with HP-1MS 60 m × 0.25 mm i.d. capillary column. Only two diastereomeric Michael addition products having identical mass spectra were detected in the product mixture: m/z (rel. int.): 297 (M+, 1), 207 (23), 194 (12), 177 (16), 159 (17), 149 (13), 131 (31), 115 (23), 104 (28), 91 (20), 77 (10), 43 (100), 29 (10).
Quantitative analysis was carried out using gas chromatography with Agilent 7890A GC System equipped with flame ionization detector (FID) and Hydrodex-g-TBDAc 30 m × 0.25 mm (manufactured by Macherey–Nagel GmbH) chiral capillary column, which allowed the separation of all four ethyl 2-acetyl-2-fluoro-4-nitro-3-phenylbutanoate (3) Michael adduct stereoisomers. The peaks of the individual stereoisomers were assigned by comparison of the results obtained with previously published data (a gas chromatogram including the assignments of the peaks is shown in the Supplementary material) [24]. Based on chromatographic results, the conversion, the diastereomeric ratio and the enantioselectivities were calculated using the following formulae:
Here c 0 (1) and c t (1) are the initial concentration of 1 and the concentration of 1 at time t; c(3A) = c(S, S − 3) + c(R, R − 3), c(3B) = c(R, S − 3) + c(S, R − 3) and c(S, S − 3), c(R, R − 3), c(R, S − 3), c(S, R − 3) are the concentrations of the individual enantiomers of the Michael adduct 3. No significant quantities of other products except 3 were detected in the crude mixtures, thus the chemoselectivity was always close to 100%. Experiments repeated at least three times showed that the reproducibility of product composition was within ±1%.
Product 3 obtained in some reactions was also isolated (as mixture of stereoisomers) by flash chromatography over silica gel using hexane isomer mixture/ethyl acetate 10/1 as eluent. Isolated yields of 3, where available, are included in tables and figures. The separation of the diastereomers was not possible under the applied chromatographic conditions, product 3A could be isolated. However, the 3B isomers formed in smaller quantities always contained various amounts of 3A. The purified compounds were characterized by their 1H and 13C NMR spectra recorded on a Bruker AVANCE DRX 400 NMR spectrometer in CDCl3 solution. The 1H and 13C NMR spectra of pure 3A are given in the Supplementary material, which were in accordance with those published in previous studies [24].
Results and discussion
Homogeneous asymmetric Michael addition of the fluorinated nucleophile 2 catalyzed by cinchona alkaloids
Motivated by the importance of the fluorine containing chiral building blocks, we chose to study the asymmetric Michael addition of a nucleophile bearing a fluorine substituent on the activated carbon atom. The addition of this molecule to 1 may lead to the formation of four stereoisomers as shown in Scheme 1. Excellent results were obtained in this reaction using a cinchona alkaloid derivative applied initially in the asymmetric addition of non-fluorinated nucleophiles, i.e., cupreine 9-phenanthryl ether (4), which may be prepared in two synthetic steps from Qn [35]. Our attempt to replace the cupreine ether 4 with a similarly efficient derivative, such as β-iCu prepared in a single step in good yield from Qd, was successful, as shown by the results summarized in Table 1. The structures of the cinchona alkaloids used in this study are shown in Fig. 1. The published results obtained using catalyst 4 were also included in the table for comparison [24].
Although the natural cinchona alkaloids proved to be efficient catalysts in this reaction, the stereoselectivities obtained with these were poor. As expected, the sense of the ees were determined by the configuration of the C9 and C8 chiral centers, the same excess enantiomers were obtained with Cd and Qn and opposite with Qd.
Interestingly, the diastereomers formed in excess with the natural cinchona alkaloids were influenced by the C6′ substituent. This influence of the C6′ substituent on the dr was also observed using 4 or β-iCu catalysts, which gave identical diastereomers in excess (the same as Cd) although had opposite configuration of the C9–C8 chiral centers. Both C6′–OH derivatives were highly efficient and stereoselective. However, the β-iCu catalyst provided better dr and slightly higher ee (up to 98%), beside a close to complete transformation of 1 in less time (6 h). However, one must not overlook that the reaction conditions differed in the experiments using 4 and β-iCu catalysts. Interestingly, the direction of the enantioselectivities were the opposite of those obtained using the corresponding parent natural cinchona alkaloids, i.e., the C6′–OH group had a crucial directing role in this reaction. Further studies on the effect of the cinchona alkaloid structure will be reported elsewhere. Altogether, we found that β-iCu seems to be a better catalyst of this asymmetric Michael addition than 4, thus we attempted to prepare a heterogeneous catalyst by immobilizing this compound over inorganic ion exchangers.
Immobilization of β-iCu over inorganic ion-exchangers
The advantages of the heterogeneous catalysts compared to their soluble counterparts motivated our efforts of immobilizing the β-iCu over solid supports. Besides maintaining the activity and selectivity of the catalyst a key issue is to find a simple immobilization method, which does not require tedious functionalization either of the support or the catalytically active molecule. Thus, our choice was to attempt the heterogenization by ionic bonding to inorganic ion exchangers. The tertiary amine and the acidic hydroxyl group on the aromatic moiety may be transformed either in cationic or anionic functionalities, thus, may allow the immobilization over cation or anion exchanger layered materials. The introduction of the catalysts between the layer of layered inorganic solids may also assure a favorable environment for the reaction, beside preventing leaching of the active species and deactivation of the heterogenized catalyst.
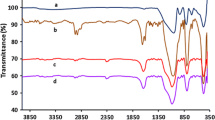
The hydrochloride salt of β-iCu obtained in aq. HCl was ion exchanged in a cation exchanger aluminum phyllosilicate (BH), whereas the β-iCu deprotonated by aqueous NH3 was used in the attempt to immobilize over an anion-exchanger layered double hydroxide (LD). Due to the difficulties of replacing the carbonate anion in the latter material, LD was also used following calcination at 500 °C, as the layered structured may be restored by the rehydration of the decarbonated material simultaneously with the introduction of an organic anion [36, 37]. We have examined the obtained solids by infrared spectroscopy to ascertain that the organic molecule has been deposited on the inorganic materials (Fig. 2). The appearance of novel IR absorption bands in the spectra (1200–1600 and 2500–3200 1/cm), characteristic of vibrations of the organic molecule and changes of the position and intensity of the ν(O–H) bands (between 3000 and 3700 1/cm) proved that these materials are inorganic–organic hybrids.
The position of the organic molecule was deduced based on the XRD patterns of the hybrids, shown in Fig. 3. The diffractograms showed no change in the position of the peak characteristic to the (003) reflections in the LD based hybrid materials as compared with the pristine LD. Accordingly, no intercalation in the interlamellar space occurred and the organic material was deposited on the surface of the particles. On the contrary, the significant increase in the interlamellar distance of BH, deduced from the shift of the (001) reflection indicated that the protonated cinchona alkaloid was successfully introduced between the lamellae of BH by cation-exchange.
Catalytic performance of the hybrid materials in the asymmetric Michael addition
The prepared hybrid materials were tested as catalysts in the asymmetric Michael addition (Scheme 1). Characteristic results are summarized in Table 2. The material obtained by immobilization over BH by cation-exchange gave similarly low conversion as the parent solid material, due to the transformation of the catalytically active tertiary amine group by protonation. Accordingly, the racemic product was obtained using BH-β-iCu, as presumably only the support catalyzed the reaction.
In contrast, the LD supported cinchona alkaloid was active and stereoselective in the studied reaction, although both the dr and the enantioselectivities were lower as compared to the reaction under homogeneous conditions (see Table 1, entries 4, 5). The worse stereoselectivities may be explained if one takes into account that the support also catalyzed the addition, obviously unselectively (entry 3). Thus, competition between the selective and unselective reaction occurred using these materials. However, the obtained good stereoselectivities showed that the cinchona catalyzed asymmetric reaction was faster. Moreover, the increase in the dr and ees obtained by decreasing the catalyst amount (compare entries 4, 6, 7) showed that the presence of less support is favorable, which also contains sufficient cinchona alkaloid to catalyze the asymmetric reaction.
Based on these observations, one may suspect that the addition may also occur in solution catalyzed by leached β-iCu. However, some results suggest that the asymmetric reaction partially took place on the surface. Thus, the hybrid material obtained using calcined and rehydrated LD was less selective than LD-β-iCu (entry 5) though the former contained more organic compound based on the FT-IR spectra (Fig. 2), which may lead to higher or at least equal amount of dissolved cinchona alkaloid. By the addition of LD to the homogeneous reaction mixture, the stereoselectivities were much better than with the same amount of LD-β-iCu (compare entries 4 and 8). Finally, in a nonpolar solvent such as n-heptane (entries 9, 10), in which the solubility of β-iCu is lower than in DCE, similar ees were obtained as in DCE, only the dr decreased significantly.
In continuation, the recovery and reuse of the LD-β-iCu catalyst was attempted. Due to the difficult manipulation of low amount of catalysts these experiments were carried out using 50 mg solids. The results obtained both in DCE and n-heptane are presented in Fig. 4.
Results obtained by reuse of LD-β-iCu in DCE (a) and n-heptane (b); Conv. solid bars, dr white bars, ees dark and white lines. For reaction conditions, see Table 2 entries 4 and 9; isolated yields of the second runs: 80% (a) and 84% (b)
In DCE, the catalyst almost completely lost its activity following 8 uses, whereas in n-heptane the activity of the material was maintained after the same recycle number. Interestingly, increased stereoselectivities (both dr and ees) were obtained in the first reuse of the catalyst in both solvents as compared with the results reached over freshly used materials. In further reactions, these values decreased. However, in n-heptane the ee 3A could be maintained at relatively high level even in the 8th use of the catalyst. One may assume that the surface layer of cinchona alkaloid is restructured during the first reaction by leaching the excess of adsorbed β-iCu and/or by rearrangement of the surface bonded molecules. Accordingly, the increase of the stereoselectivities in the first reuse indicates that the reaction is catalyzed mainly by the cinchona alkaloid bonded to the surface.
Based on the characterization data of the heterogeneous catalysts and the results of the catalytic measurements, we assume that the cinchona alkaloid is bonded by electrostatic interactions and/or by hydrogen bonding to the surface, the latter complementing the former as illustrated in Fig. 5. Thus, β-iCu molecules will bond to the surface with different strengths and geometry in the hybrid material, which may give a possible explanation of its catalytic performance observed upon reuse. The weakly bonded cinchona alkaloid will leach to the solution easily during the reactions, leading to the formation of an appropriately covered surface, which by further use will gradually deteriorate.
Conclusions
The asymmetric Michael addition of an α-fluoro-β-ketoester to trans-β-nitrostyrene was studied in order to find a cinchona alkaloid derivative, which may be easily synthetized and affords similar results as the tediously obtained derivatives used as catalysts in these reactions. We found that the β-isocupreidine prepared in a one-step procedure from the natural quinidine is more efficient catalyst than the cupreine 9-phenanthryl ether, employed in a previous study. The immobilization by ion exchange over cation and anion exchanger inorganic layered materials of the former cinchona alkaloid was attempted, using the basic or acidic functional groups of the molecule, in order to prepare inorganic–organic hybrid materials. Although the protonated cinchona alkaloid could be introduced by cation-exchange between the interlamellar space of an aluminum phyllosilicate, due to the transformation of the tertiary amine group, the molecule lost its catalytic activity in the Michael addition. The introduction of the deprotonated cinchona alkaloid by anion-exchange between the lamellae of a layered double hydroxide was not achieved, both using the as-received or the decarbonated and rehydrated material, as evidenced by XRD measurements. However, the cinchona alkaloid bonded to the outer surface of the particles, as shown by the FT-IR spectra and the catalytic activity of the materials. The stereoselectivities obtained with this heterogeneous catalyst approached those obtained in reactions in solution using the parent cinchona derivative. However, leaching of the catalytically active species from the surface was indicated by the decrease in the activity and the stereoselectivities of the heterogenized catalyst upon several reuses. Leaching could be prevented or delayed by carrying out the reaction in nonpolar media. It is suggested that in the latter material the cinchona alkaloid is bonded by electrostatic interactions and/or by hydrogen bonding to the surface, with different binding strengths and geometry, which may explain its catalytic performance observed upon the first reuse. Thus, the weakly bonded cinchona alkaloid will leach easier into the solution, leading to the formation of an appropriately covered surface, which provides better results during the first reuse of the catalyst. However, in further reactions, the active surface will slowly deteriorate.
References
Mikami K, Lautens M (eds) (2007) New frontiers in asymmetric catalysis. Wiley, Hoboken
Ojima I (ed) (2010) Catalytic asymmetric synthesis, 3rd edn. Wiley, Hoboken
Gruttadauria M, Giacalone F (eds) (2011) Catalytic methods in asymmetric synthesis: advanced materials, techniques, and applications. Wiley, Hoboken
Berkessel A, Gröger H (2005) Asymmetric organocatalysis—from biomimetic concepts to applications in asymmetric synthesis. Wiley-VCH, Weinheim
Dalko PI (ed) (2007) Enantioselective organocatalysis. Wiley-VCH, Weinheim
Pellissier H (2010) Recent developments in asymmetric organocatalysis. RSC Catalysis Series No. 3, RSC Publishing, Cambridge
Quintard A, Alexakis A (2013) In: Andrushko V, Andrushko N (eds) Stereoselective synthesis of drugs and natural products, 1st edn, vol 1, chap 11. Wiley, Hoboken, pp 319–346
Tsogoeva SB (2007) Eur J Org Chem 2007:1701–1716
Almaşi D, Alonso DA, Nájera C (2007) Tetrahedron Asymmetry 18:299–365
Müller K, Faeh C, Diederich F (2007) Science 317:1881–1886
Begue JP, Bonnet-Delpon D (2008) Bioorganic and medicinal chemistry of fluorine. Wiley, Hoboken
Kuroki Y, Sakamaki Y, Iseki K (2001) Org Lett 3:457–459
Szőri K, Szőllősi G, Bartók M (2006) Adv Synth Catal 348:515–522
Jiang J, Lu W, Lv H, Zhang X (2015) Org Lett 17:1154–1156
González GV, Montaner XC, Torres RR (2011) Chem Eur J 17:2018–2037
Yang X, Phipps RJ, Toste FD (2014) J Am Chem Soc 136:5225–5228
Shibatomi K, Okimi T, Abe Y, Narayama A, Nakamura N, Iwasa S (2014) Beilstein J Org Chem 10:323–331
Zhong G, Fan J, Barbas CF III (2004) Tetrahedron Lett 45:5681–5684
London G, Szőllősi G, Bartók M (2007) J Mol Catal A Chem 267:98–101
Ullah F, Zhao G-L, Deiana L, Zhu M, Dziedzic P, Ibrahem I, Hammar P, Sun J, Córdova A (2009) Chem Eur J 15:10013–10017
Jiang Z, Pan Y, Zhao Y, Ma T, Lee R, Yang Y, Huang K-W, Wong MW, Tan C-H (2009) Angew Chem Int Ed 48:3627–3631
Wen L, Yin L, Shen Q, Lu L (2013) ACS Catal 3:502–506
Han X, Luo J, Liu C, Lu Y (2009) Chem Commun 15:2044–2046
Li H, Zhang S, Yu C, Song X, Wang W (2009) Chem Commun 16:2136–2138
Oh Y, Kim SM, Kim DY (2009) Tetrahedron Lett 50:4674–4676
Huang X, Pham K, Zhang X, Yi W-B, Hyatt JH, Tran AP, Jasinski JP, Zhang W (2015) RSC Adv 5:71071–71075
Song CEPI (ed) (2009) Cinchona alkaloids in synthesis and catalysis, ligands, immobilization and organocatalysis. Wiley-VCH, Weinheim
Mallat T, Orglmeister E, Baiker T (2007) Chem Rev 107:4863–4890
Mándity IM, Ötvös SB, Szőllősi G, Fülöp F (2016) Chem Rec 16:1018–1033
Li H, Zu L, Xie H, Wang W (2009) Synthesis 2009:1525–1530
Iwabuchi Y, Nakatani M, Yokoyama N, Hatakeyama S (1999) J Am Chem Soc 121:10219–10220
Shi M, Xu Y-M (2002) Angew Chem Int Ed 41:4507–4510
Saaby S, Bella M, Jørgensen KA (2004) J Am Chem Soc 126:8120–8121
Li H, Wang Y, Tang L, Wu F, Liu X, Guo C, Foxman BM, Deng L (2005) Angew Chem Int Ed 44:105–108
Li H, Wang Y, Tang L, Deng L (2004) J Am Chem Soc 126:9906–9907
You Y, Zhao H, Vance GF (2002) Appl Clay Sci 21:217–226
Extremera R, Pavlovic I, Pérez MR, Barriga C (2012) Chem Eng J 213:392–400
Acknowledgements
Financial support of the Hungarian National Science Foundation (OTKA Grant K 109278) is appreciated. The work was supported by the ÚNKP-16-2-II. New National Excellence Program of the Ministry of Human Capacities (V.K. and V.J.K.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Szőllősi, G., Kovács, L., Kozma, V. et al. Asymmetric Michael addition catalyzed by a cinchona alkaloid derivative non-covalently immobilized on layered inorganic supports. Reac Kinet Mech Cat 121, 293–306 (2017). https://doi.org/10.1007/s11144-017-1144-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1144-8