Abstract
The kinetics of the oxidation of formaldehyde by octacyanomolybdate(V) ions has been studied in aqueous alkaline medium. A formaldehyde-dependent and -independent reaction has been observed. The HCHO-dependent reaction displays first order kinetics in [Mo(CN)8 3−], [HCHO], [OH−] and alkali metal ion catalysis was observed. The rate law is consistent with: \( \frac{{ - {\text{d}}[{\text{Mo}}\left( {\text{CN}} \right)_{ 8}^{ 3- } ]}}{\text{dt}} = { 2}K_{\text{h}} K_{ 1} K_{ 2} k_{ 3} \left[ {{\text{Mo}}\left( {\text{CN}} \right)_{ 8}^{ 3- } } \right] \, \left[ {\text{HCHO}} \right] \, \left[ {{\text{OH}}^{ - } } \right] \, \left[ {{\text{A}}^{ + } } \right]. \) Activation parameters for both the formaldehyde-dependent and -independent reaction have been obtained. The activation parameters for the HCHO-independent reaction provide evidence for the identification of the reaction. A reaction mechanism is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The carbonyl group, –C=O, is a structural characteristic of a wide range of compounds. The reactivity of this functional group is mainly due to the difference in the electronegativity between the carbon and oxygen atoms. Formaldehyde belongs to this group of compounds. Hydration of formaldehyde is a spontaneous process.
In aqueous medium, formaldehyde exists more than 99% in the hydrated form [1, 2] with a K h value of 2 × 103.
Ferricyanide (E° = 0.36 V [3]) and potassium permanganate (E° = 0.57 V [3]) are the most common reagents used as oxidants for organic functional groups in alkaline medium. Speakman and Waters [4] reported the oxidation of aldehydes, ketones and nitroparaffins with ferricyanide while Holluta and Mutshin [5] reported the oxidation of formaldehyde by neutral permanganate and found the reaction to be base catalyzed.
W(CN) 3−8 (E° = 0.54 V [6]) and Mo(CN)8 3− (E° = 0.8 V [6]) exhibit approximately similar redox potentials as the above mentioned oxidants. Furthermore, these M(CN) 3−8 (M=W, Mo) complexes are relatively substitution inert. This prompted the kinetic study of these complexes with some organic compounds.
Kinetic studies of the oxidation of formaldehyde (HCHO) with ferricyanide (Fe(CN) 3−6 ) [7] and octacyanotungstate(V) (W(CN) 3−8 ) [8] have been reported. In both studies, the reactions are first order with respect to the metal cyanide complex, formaldehyde and the hydroxide ion and correspond to the rate law:
Experimental
Cesium octacyanomolybdate(V)dihydrate, Cs3Mo(CN)8·2H2O, was prepared as described by Leipoldt et al. [9–11] and was used as primary standard [12] after recrystallization. A stock solution of formaldehyde was prepared from Merck ‘‘Pro Analisi” 35% formaldehyde solution and was standardized by the method of Kolthoff and Belcher [13]. A stock solution of carbonate free sodium hydroxide was also prepared and standardized with potassium phthalate [14, 15]. All other reagents used were analytical grade and redistilled water was used throughout.
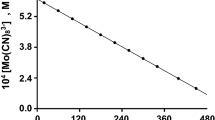
Kinetic measurements were made at constant ionic strength using sodium chloride as an electrolyte. Experiments were carried out in diffuse laboratory light because Mo(CN)8 3− is sensitive to light [16], although in the dark at a pH between 1 and 12 the radiation effect is insignificant [17]. Rate data have been obtained by monitoring the absorbance decrease of Mo(CN)8 3− on a Pye Unicam SP 1700 double beam spectrophotometer at 390 nm (Fig. 1). The temperature of reaction mixtures was controlled within 0.1 °C.
The extinction coefficients of Mo(CN)8 3− and Mo(CN)8 4− at 390 nm are 1,400 M−1cm−1 and 140 M−1cm−1, respectively [18]. Both the reagent, Mo(CN)8 3−, and the product, Mo(CN)8 4−, absorbed light at this wavelength. Beer’s law thus cannot be used directly to determine the concentration of Mo(CN)8 3− at a time t. If the initial concentration of the Mo(CN)8 3− equals “a” mol dm−3 then the total absorbance at a time t is given by:
and
This equation was used throughout to calculate [Mo(CN)8 3−] at a time t.
Results and discussion
Pseudo-first order plots of ln[Mo(CN)8 3−] versus time were linear for more than two half-lives of the reaction. This as well as the results in Table 1 indicate that the reaction is first order with respect to [Mo(CN)8 3−], [HCHO], and [OH−]. The plot of k obsd verus [HCHO] (Fig. 2) for the variation of the formaldehyde concentration in the reaction mixture yields a non-zero intercept. This is indicative of a second reaction independent of the [HCHO] taking place. The hydroxide ion concentration range for this study could have the consequence that the Mo(CN)8 3− oxidation of the hydroxide ion [19] become significant.
Alkali metal ion catalysis was significant in previous redox studies [20, 21] of the Mo(CN)8 3− complex ion. The influence of added alkali metal ions on the reaction rate has also been studied by varying the alkali metal ion concentration with the addition of the alkali metal chloride to the reaction mixture and the ionic strength of the reaction mixture was kept constant with tetramethylammonium chloride. The plot of the first order rate constant versus alkali metal ion concentration is linear (Fig. 3) and indicates a very strong alkali metal ion catalysis and also that the reaction is first order in alkali metal ion concentration ([A+]). The effectivity of the A+ ion on the reaction rate is in the order Na+<K+<Rb+<Cs+.
First order rate constant, k obsd, versus [Alkali metal ion]. [Mo(CN)8 3−] = 5.0 × 10−4 mol dm−3; [HCHO] = 0.10 mol dm−3; [OH−] = 0.050 mol dm−3; T = 25 ± 0.1 °C; μ = 0.6 mol dm−3. The intercept value is the first order rate constant without added alkali metal ions plus the first order rate constant of the formaldehyde independent reaction under the reaction conditions
The observed first order dependence of k obsd in [Mo(CN)8 3−], [HCHO], [OH−] and [A+] show the rate law to be of the form:
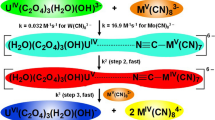
The experimental results may be explained by the reaction mechanism:
The equillibria, Eqs. 3 [1, 2] and 5 [21] exist in solution and equilibrium 4 explains the OH− dependence. The results of the variation of the alkali metal ion in the reaction mixture (Fig. 3) show a strong alkali metal ion catalysis and that the cation (A+) should be a constituent of the activated complex. The formation of the activated complex (Eq. 6) is the rate determining step followed by a fast decomposition for the formation of the products. Tests to establish the formation of a free radical intermediate using vinyl acetate were negative. This is in agreement with previous ferricyanide oxidations of similar functional groups, where it is concluded that the free radical had no independent existence [7].
With Eq. 6 proposed as the rate determining step the rate of decrease of the Mo(CN)8 3− is given by:
Steady state treatment to H2C(OH)2, H2CO−(OH), AMo(CN)8 2− and [(CN)7MoCN:A:OC(OH)H2]3− yield the rate law:
The total rate law under experimental conditions, as a HCHO-dependent reaction and a HCHO-independent reaction was observed (Fig. 2), is thus given by:
The rate constant for the HCHO-dependent reaction has been determined as. R = 0.91 ± 0.02 M−3s−1 and for the HCHO-independent reaction R ′ = 3.32 ± 0.06 × 10−3 M−1s−1.
The activation parameters for both the HCHO-dependent and -independent reactions were obtained by variation of [HCHO] at various temperatures between 22 and 35 °C (Table 2). The parameters ∆H # and ∆S* were obtained from the Eyring equation:
The equation converted to the logarithmic form yield a linearized form, [22]
where ∆H # can be obtained from the slope, \( \frac{{ - \Updelta H^{\# } }}{R} \), and ∆S* from the intercept
Non-linear least-squares fits of the results in Table 2 to Eqs. 10 and 11 yield an activation enthalpy, ∆H # = 54 ± 3 kJ mol−1, and an activation entropy, ∆S * = −110 ± 9 J K−1 mol−1, for the HCHO-dependent reaction and an activation enthalpy, ∆H # = 21 ± 4 kJ mol−1, and an activation entropy, ∆S * = −245 ± 15 J K−1 mol−1, for the HCHO-independent reaction. All the obtained values have been validated by using: [22]
The activation parameters for the HCHO-independent reaction is similar to that of the Mo(CN)8 3− oxidation of the hydroxide ion [19]. This is a means of identification for the HCHO-independent reaction.
References
Sutton AC, Downes TM (1972) Chem Comm 1:1
Bell RP (1966) Advan Phys Org Chem 1:4
Dobos D (1975) Electrochemical data. Elsevier, New York
Speakman PT, Waters WA (1955) J Chem Soc, Part 1, 40
Holluta J, Mutshin A (1930) Z Phys Chem 150:381
Campion RJ, Purdie N, Sutin N (1964) Inorg Chem 3:1091
Singh VN, Gangwar MC, Saxena BBL, Singh MP (1969) Can J Chem 47:1051
Basson SS, Bok LDC, Leipoldt JG, Grobler SR (1977) J Inorg Nucl Chem 39:376
Leipoldt JG, Bok LDC, Cilliers PJ (1974) Z Anorg Allg Chem 409:343
Bok LDC, Leipoldt JG, Basson SS (1975) Z Anorg Allg Chem 415:81
Dennis CR, van Wyk AJ, Basson SS, Leipoldt JG (1992) Trans Met Chem 17:471
Basson SS, Bok LDC, Grobler SR (1974) Z Anal Chem 268:287
Kolthoff IM, Belcher R (1957) Volumetric analysis, vol 3. Interscience Inc, New York
Meitz L (1963) Handbook of analytical chemistry, 1st edn. McGraw-Hill, New York
Vogel AI (1972) A textbook of quantitative inorganic analysis, 3rd edn. Longman, London
Gray GW, Spence JT (1971) Inorg Chem 10:2751
Adamson AW, Walker JP, Volpe M (1959) J Am Chem Soc 17:4030
Dennis CR (1975) A kinetic study of the octacyanomolybdate(V) ion oxidation of thiourea and arsenic(lll). MSc Dissertation. University of the Orange Free State, Bloemfontein
Dennis CR, Potgieter IM, Basson SS (2010) React Kinet Mech Cat 99:63
Leipoldt JG, Bok LDC, Dennis CR (1976) J Inorg Nucl Chem 38:1655
Dennis CR, Basson SS, Leipoldt JG (1983) Polyhedron 2:1357
Lente G, Fabian I, Poe J (2005) New J Chem 29:759
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robert Dennis, C., Potgieter, I.M. & Basson, S.S. A kinetic study of the oxidation of formaldehyde by the octacyanomolybdate(V) ion in aqueous alkaline medium. Reac Kinet Mech Cat 104, 1–7 (2011). https://doi.org/10.1007/s11144-011-0342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0342-z