Abstract
The oxidation of the tetrakisoxalatouranate(IV) ion by the octacyanometalate(V) ion of tungsten and molybdenum was studied in an oxalate buffer medium. The reaction was first order in both UIV(C2O4)44− and M(CN)83− (M = W, Mo), and a second order rate constant of k2 = 0.032 ± 0.002 M−1s−1 at pH 4.27 and 24.8 °C was found for the W(CN)83− reaction. For the Mo(CN)83− reaction the second order rate constant is k2 = 16.9 ± 0.1 M−1s−1 at pH 4.77 and 25.3 °C. The reactions are inversely proportional to [H+] and an equilibrium constant of Ka ≈ 1.3 × 10–6 M−1 (pKa ≈ 5.89) at 25 °C was found for the deprotonation of UIV(C2O4)3(H2O)22−. Activation parameters have been obtained by a least squares fit of temperature data directly to the Eyring equation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The stereochemical configuration of the octacyanotungstate(V) ion and the octacyanomolybdate(V) ion in solution has been reported to be square antiprismatic and dodecahedral [1,2,3,4]. The exchange of a cyanide ligand in aqueous solution is very slow in light, but have not been detected in diffuse light between pH 4 and pH 12 [5]. The reduction potential for W(CN)83– is E° = 0.52 V and for Mo(CN)83– is E° = 0.76 V vs NHE in 0.1 M NaClO4 at 25 °C [6, 7]. Both W(CN)83– and Mo(CN)83– is one equivalent oxidants and have been used extensively in oxidation–reduction kinetic studies in alkaline medium [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22].
Uranium(IV) octahydrate is a relative stable species in aqueous solution [23]. In aqueous solution the polar H2O molecule compete with acidic substituted ligands by displacement reactions. Uranium(IV) cations hydrolyze in aqueous medium to form hydroxo or hydrated species which dissociate as a Brønsted acid [24]. Kraus and Nelson [25, 26] indicated that uranium(IV) cations hydrolyze in perchloric acid solution < 1.0 M to form U(H2O)84+ which dissociate as in Eq. 1.
Kinetic oxidation studies of U(IV) substrates with several multi-electron oxidizing agents have been reported [25,26,27,28,29,30,31,32]. The reaction mechanisms of these reactions are complicated, since both the U(IV) and the oxidizing agent hydrolyze in aqueous medium. This could influence the interpretation of hydrogen ion dependence results of these reactions. In addition, the use of two- or more-electron oxidants does not clearly indicate whether a U(V) species is formed as an intermediate when U(IV) is oxidized to U(VI). This problem may be addressed when oxidation is performed by one-electron oxidants such as Fe(CN)63−, Mo(CN)83– or W(CN)83–.
The oxidation of U(IV) hydroxo species by mono-equivalent oxidants FeIII(CN)63– [33] and IrIVCl62– [34] have also been reported. These studies were performed in HClO4/NaClO4 mediums. These mono-equivalent oxidants do not hydrolyze in aqueous medium. The initial stage of these reactions is relatively fast, corresponding to the formation of a binuclear intermediate complex [33, 34]. An important aspect of all the kinetic studies of U(IV) is that the reactions are first order in both the oxidant and reductant.
The affinity of U(IV) for oxygen is the main reason for uranium(IV) complexes containing carboxylic acid groups to be the most stable uranium(IV) chelates [24]. The only kinetic study we know of where uranium(IV) oxalate complexes have been oxidized to an uranium(VI) species is the reaction between UIV(C2O4)44– and the one-electron oxidant FeIII(CN)63– in an acidic oxalate buffer medium [35]. The UIV(C2O4)44– complex hydrolyzes only a little in aqueous acidic solution [25] which makes it suitable for kinetic oxidation studies by relatively strong oxidizing agents. In this article, the kinetics of oxidation of U(C2O4)44− with the one-electron oxidants W(CN)83– and Mo(CN)83– are reported. Activation parameters have been determined and a mechanism is proposed.
Experimental
Cesium octacyanomolybdate(V)dihydrate, Cs3Mo(CN)8·2H2O, and cesium octacyanotungstate(V)dihydrate, Cs3W(CN)8·2H2O, were synthesized as previously described [36,37,38] and were used as a primary standard [39] after recrystallization. Potassium tetrakisoxalatouranate(IV), K4U(C2O4)4·5H2O was synthesized as described [40] previously and it’s purity was determined as 98% by a permanganate titration.
To find a suitable buffer system for the oxidation of K4UIV(C2O4)4 by WV(CN)83– and MoV(CN)83– had its difficulty. U(C2O4)4 precipitate from a buffer solution containing a mineral acid. Several buffer systems were tested and only a carbonate/bicarbonate buffer and an oxalate/oxalic acid buffer did not show precipitation. The carbonate/bicarbonate could displace the oxalate ions from the uranium coordination sphere and thus only the oxalate/oxalic acid buffer was used in this kinetic study. This, however, means that the pH variation of the reaction mixture was limited to between pH 1.3 and pH 5.5. The oxalate buffer has been prepared by adding a KOH solution to a 0.05 M oxalic acid solution until the required pH was reached. The pH was measured with a T & C model 1002 pH meter using a glass pH electrode. The concentration of the oxalate in the reaction mixture was 0.05 M. Doubly distilled water was used throughout.
The stoichiometry of the reactions was determined spectrophotometrically by treating MV(CN)83– (M = Mo, W) solutions with a known excess of K4U(C2O4)4 and then measuring the excess K4U(C2O4)4 at 665 nm on completion of the reaction. In both cases very good values of a 1:2 stoichiometry, U(IV):MV(CN)83–, has been obtained.
Kinetic measurements were made under pseudo first order conditions with the U(IV) species in excess and at constant ionic strength (µ) using NaCl as electrolyte, see Tables S1–S5 in Supporting Information for exact conditions. Experiments were carried out in diffuse laboratory light because the Mo(CN)83– ion in solution is sensitive to light [41]. In diffuse light at pH 1–12, the radiation effect is undetectable [5].
The oxidation of the U(C2O4)44– by WV(CN)83– and MoV(CN)83– was monitored on a Pye Unicam SP 1700 double beam spectrophotometer connected to a Fryca-Kaltetechnik KB 300 water bath with a Thermomix 1440 thermostat for temperature control. The decrease in the absorption of the WV(CN)83– and MoV(CN)83– was measured at 357 nm and 388 nm, respectively [6, 7]. From UV spectra (Fig. 1), the molar extinction coefficients of the WV(CN)83– and WIV(CN)84– was determined as 1750 and 230 M−1cm−1, respectively. The extinction coefficients of MoV(CN)83– and MoIV(CN)84– were determined as 1400 M−1cm−1 and 140 M−1cm−1, respectively. The extinction coefficient of the U(C2O4)44– ion as well as of the UVI product were negligibly small at 357 nm and 388 nm, respectively [35].
Both the reagent WV(CN)83– and it’s product, WIV(CN)84−, and also MoV(CN)83− and it’s product, MoIV(CN)84− absorbed light at the wavelength (λmax) where the reactions were monitored. The Beer–Lambert law thus cannot be used directly to determine the concentration of M(CN)83− (M = W, Mo) at any time t. If the initial concentration of the M(CN)83− equals “a” mol dm−3 and if at any time t a quantity “x” has reacted to form x mol dm−3 M(CN)84−, then the total absorbance at a time t is given by
The concentration of M(CN)83− at time t may be calculated through Eq. 3.
Activation parameters were obtained by performing reactions at various temperatures. The parameters \(\Delta {\mathrm{H}}^{\#}\) and \(\Delta {\mathrm{S}}^{\#}\), applicable only to the experimental conditions, have been obtained from the Eyring equation (Eq. 4) where kB and h are the Boltzman and Planck constants:
Equation 4 converted to the logarithmic form yields a linearized Eyring equation [42] (Eq. 5),
Here \(\Delta {\mathrm{H}}^{\#}\) can be obtained from the slope, \(\frac{-\Delta {\mathrm{H}}^{\#}}{\mathrm{R}}\), and \(\Delta {\mathrm{S}}^{\#}\) from the intercept, \(\frac{{\Delta \mathrm{S}}^{\#}}{\mathrm{R}}+\mathrm{ln}\left(\frac{{\mathrm{k}}_{\mathrm{B}}}{\mathrm{h}}\right)\), of a plot of ln \(\left(\frac{\mathrm{k}}{\mathrm{T}}\right)\) versus \(\frac{1}{\mathrm{T}}\).
Least squares fittings of measured data were carried out by using the general fitting software Micro Math “Scientist” [43].
Results and discussion
The oxidation of the U(C2O4)42− ion by MV(CN)83– ions (M = Mo, W) was performed under pseudo-order conditions with the MV(CN)83– ion as the limiting reagent. The [U(C2O4)42−] was in excess of 6–20-fold. Lente provided evidence in his book [44] that an excess of fourfold is enough for a flooding (pseudo-first order) process in chemical kinetics. This was also recently experimentally confirmed [35]. In this study, the cases where the flooding excess is less than tenfold (but still larger or equal to 4), i.e. the sixfold and eightfold excess data, were also found to be acceptable for pseudo first order reaction conditions by virtue of the linearity of the kobsd versus [K4UIV(C2O4)4] graphs (Fig. 3).
The non-linear least squares fit [43] of the data from the reaction traces, Fig. 2, to the first order rate expression, Eq. 6 [45], indicate that the reactions are first order in MV(CN)83– (M = Mo, W). Rate constants for these reactions are summarised in Supplementary Information Tables S1 (WV(CN)83– reaction, entries 1–6) and S2 (MoV(CN)83– reaction, entries 1–12)
Variation of [K4U(C2O4)4] in the WV(CN)83– reaction mixtures (Fig. 3, left, data from Supplementary Information Table S1, entries 7–12) as well as MoV(CN)83– reaction mixtures (Fig. 3, right, data from Table S2, entries 13–18 at pH 1.61 and 19–25 at pH 4.93) yield a linear relationship with a direct dependence of kobsd on [K4U(C2O4)4]. The gradients of the Fig. 3 plots yield second order rate constant, k2, for the WV(CN)83– oxidation of UIV(C2O4)44– as k2(WV(CN)83– reaction) = 0.100 ± 0.001 M−1s−1 at pH 4.27. The second order rate constant for the MoV(CN)83– oxidation of U(C2O4)44– is k2(MoV(CN)83– reaction) = 22.7 ± 0.2 M−1s−1 at pH 4.93.
Variation of the reduction product WIV(CN)84– and MoIV(CN)84–in the reactions, do not have an effect on the reaction rate (Supplementary Information Table S3 entries 1–3 and Table S4, entries 1–4) indicating the rate determining step of the reaction is not an equilibrium involving the reduced products WIV(CN)84– or MoIV(CN)84–.
Variation of the specific cation in the reaction medium, K+ in the W(CN)83− reaction and Na+ in the Mo(CN)83− reaction, do not have a direct proportional effect on the reaction rate. Although the alkali metal cation concentration was in large excess over both UIV(C2O4)44– and MV(CN)83− (M = W, Mo), plots of kobsd versus [alkali metal cation] was not linear (Supplementary Information Fig. S1). This implies the effect that these alkali metals have on the reaction rate is non-specific and not pseudo-first order. Secondly, plots of log (kobsd) versus log [alkali metal cation] (Supplementary Information Fig. S2) are linear but the slopes are not one. Slopes were found to be 0.47 ± 0.01 for K+ in the W(CN)83– reaction and 0.34 ± 0.02 for Na+ in the Mo(CN)83– reaction. These small slopes confirm that the interactions of alkali metal cations with reactants in the reactions are non-specific, i.e. they do not take part in the rate determining step of the reaction mechanism.
A theory for the influence of the ionic strength of the reaction mixture on the reaction rate was formulated by Brønsted and Bjerrum [46]. The logarithmic form of the equation, where ∂ is the Debye–Hückel solvent constant, is
The Debye–Hückel solvent constant (∂) for water is ~ 0.51 at 25 °C which simplifies the equation to:
Should the slope of a plot of log k versus \(\left( {\frac{{{\text{I}}^{\raise.5ex\hbox{$\scriptstyle 1$}\kern-.1em/ \kern-.15em\lower.25ex\hbox{$\scriptstyle 2$} } }}{{1 + {\text{I}}^{\raise.5ex\hbox{$\scriptstyle 1$}\kern-.1em/ \kern-.15em\lower.25ex\hbox{$\scriptstyle 2$} } }}} \right)\) have a positive value it follows that ZA and ZB have the same charge (i.e. both positive or both negative). The data for varying the ionic strength (µ) of the reaction mixture for the M(CN)83– (M = W, Mo) oxidation of U(C2O4)44– (Supplementary Information Table S3, entries 4–8, and Table S4, entries 5–11), highlights an increase in the reaction rate with an increase in ionic strength (µ). This positive effect (slope) is also demonstrated in Fig. 4 and indicates ions of like charge react in the rate determining step. This criteria is met in the second step of the reaction mechanism, Scheme 1, where MV(CN)83– react with UIV(C2O4)3(H2O)(OH)3– to form a binuclear intermediate complex.
Brønsted–Bjerrum plot of log kobsd vs. \(\frac{{{\text{I}}^{\raise.5ex\hbox{$\scriptstyle 1$}\kern-.1em/ \kern-.15em\lower.25ex\hbox{$\scriptstyle 2$} } }}{{1 + {\text{I}}^{\raise.5ex\hbox{$\scriptstyle 1$}\kern-.1em/ \kern-.15em\lower.25ex\hbox{$\scriptstyle 2$} } }}\). [WV(CN)83–] = 5.0 × 10–4 M (red plot, I was controlled with KCl), [MoV(CN)83–] = 5.0 × 10–4 M (black plot, I was controlled with NaCl). For both, [U(C2O4)44–] = 5.0 × 10–3 M, [C2O42–] = 0.05 M. (Color figure online)
It has been shown before [47, 48] that in the presence of oxalate ions, K4U(C2O4)4 exists in solution as a mixture of U(C2O4)2, U(C2O4)32– and U(C2O4)44–. It was shown [47, 48] how the concentration of these species varies with variation in oxalate ion concentration of the solution. The oxalate ion concentration in reaction mixtures of this study was ≤ 0.05 M. From literature [47, 48] it therefore follows that the main uranium(IV) species in the reaction mixtures is U(C2O4)32− and there may also be some U(C2O4)2. This means that two uranium(IV) species can be active in the reaction mixtures utilized in this study. However, since the concentration of U(C2O4)2 is very low and it is very insoluble (Ksp = 1 × 10–22 [48]), its influence can be regarded as insignificantly small, and the only reactive U(IV) species of note in the reaction mixture is U(C2O4)32–.
Variation of the hydrogen ion in the W(CN)83– reaction (Supplementary Information, Table S1, entries 13–27) and the Mo(CN)83– reaction (Table S2 entries 26–40) indicate a significant [H+] dependence (Fig. 5). It has been shown that U(C2O4)32– in aqueous medium exists as U(C2O4)3(H2O)22– [48] and that the complex ion can dissociate as shown in Eq. 9.
By abbreviating U(C2O4)3(H2O)22– as HA and U(C2O4)3(H2O)(OH)3– as A in Eq. 9, fitting of the observed experimental data for the W(CN)83– reaction at 34.9 °C to Eq. 10 [45, 49, 50]
This yields values for the equilibrium constant Ka = (1.6 ± 0.5) × 10–6 M−1. The rate constants for the oxidation of U(C2O4)3(H2O)22– (HA) and U(C2O4)3(H2O)(OH)3– (A) by W(CN)83– was obtained from the same fit to be kHA = (5 ± 1) × 10–5 s−1 and kA = (1.5 ± 0.3) × 10–2 s−1. When Eq. 10 is applied to the kinetic data of the MoV(CN)83– reaction data at 25.0 °C, the equillibrium constant was found to be Ka = (1.3 ± 0.8) × 10–6 M−1 while the rate constants for the MoV(CN)83– oxidation of U(C2O4)3(H2O)22– (HA) and U(C2O4)3(H2O)(OH)3– (A) were found as kHA = (9 ± 1) × 10–3 s−1 and kA = 1.1 ± 0.6 s−1.
In both cases kA is more than 100 times larger than kHA. This implies kHA is negligible compared to kA and U(C2O4)3(H2O)(OH)3– is regarded as the dominant reactive U(IV) species in these redox reactions.
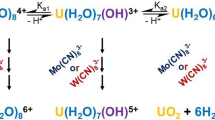
From the experimental results and discussion, a reaction mechanism, Scheme 1, is proposed.
The k1 and k-1 equilibrium steps are regarded as rate determining (slow) while steps k2 (rate constant of step 2, k2 not to be confused with k2 which is the second order rate constant of the k1 step) and k3 (rate constant of step 3) are considered fast.
Applying the steady state approximation to the intermediate binuclear complexes [(NC)7MV(CN)···(HO)UIV(C2O4)3(H2O)]6– and [(NC)7MIV(CN)···(HO)UV(C2O4)3(H2O)]6– and by applying Eq. 11,
The rate law shown in Eq. 12 can be derived. Equation 12 is also in agreement with the experimental results.
The activation parameters ∆H# and ∆S# have been obtained by application of the Eyring equation (Eq. 4). Least squares mathematical fitting of the observed temperature variation data to the Eyring equation (Eq. 4) is the modern (and more accurate) way to obtain activation parameters [51]. Utilizing second order rate constants, k2, a non-linear least squares fit [43] of the experimental data for varying the temperature of the reaction mixture (Supplementary Information Table S5, entries 28–34 for the W(CN)83– reaction and entries 48–55, for the Mo(CN)83– reaction) to Eq. 4 (Fig. 6), yield the activation enthalpy, ∆H#(WV(CN)83–) = 79.6 ± 0.5 kJ mol−1 and ∆H#(MoV(CN)83–) = 36.6 ± 0.2 kJ mol−1. The same fits yield the activation entropy, ∆S#(WV(CN)83–) = − 6.4 ± 0.2 JK−1 mol−1 and ∆S#(MoV(CN)83–) = − 99.7 ± 0.2 JK−1 mol−1. The obtained activation parameters have been validated by using Eq. 13 as explained by Lente [42] and others [22, 35].
A non-linear least squares fit of second order rate constant (M−1 s−1) and temperature (K) data to the exponential Eyring equation. [MV(CN)63−] = 5.0 × 10–4 M (M = W, red plot, or M = Mo, black plot). For both [UIV(C2O4)44–] = 5.0 × 10–3 M, µ(NaCl) = 1.055 M, [C2O42–] = 0.05 M. (Color figure online)
Conclusions
The oxidation of tetrakisoxalatouranate(IV) by the octacyanotungstate(V) and octacyanomolybdate(V) ions occur stoichiometrically in the ratio UIV(C2O4)44–:MV(CN)63– = 1:2 (M = W, Mo) and the reactions are first order in both reactants. The reactive form of UIV in these reactions in the pH range 1.6 – 4.99 is UIV(C2O4)3(H2O)(OH)3–. Variation of the pH of the reaction mixtures yield an acid dissociation constant of Ka ≈ 1.3 × 10–6 M−1 (pKa ≈ 5.80) at 25.0 °C and 1.6 × 10–6 M−1 (pKa = 5.89) at 34.9 °C for the equilibrium
This value is mutually consistent with the value obtained from kinetic studies of the hexacyanoferrate(III) ion oxidation of the tetrakisoxalatouranate(IV) ion [35] (Ka = 1.02 × 10–6 M−1, pKa = 5.99).
Our observations agree with those of Lente [44] that a sixfold excess of one reagent (here UIV(C2O4)44–) over the concentration of the limiting reagent, (here MV(CN)63–) is sufficient to study reactions kinetically under pseudo-order conditions. The traditional view of a ten-fold excess of one reactant in a reaction over another as the minimum requirement for pseudo-order conditions, is considered an artefact from an era when computational limitations required aggressive approximations.
The advent of strong computational abilities with least square fitting programmes allows calculation of rate constants quickly and more accurately than the traditional graphical approximation methods. The same applies to calculation of the activation parameters ∆H# and ∆S#. As Lente [44, 51], we conclude modern computational abilities with non-linear least squares fitting programmes applied directly to the Eyring equation (Eq. 4) allows calculation of ∆H# and ∆S# more easily and accurately directly from rate constant and temperature data.
References
Konig E (1962) Interpretation der absorptionsspektren der komplexionen [Mo(CN)8]4−, [Mo(CN)8]3−, [W(CN)8]4− und [W(CN)8]3−. Theoret Chim Acta 1:23–35
Perumareddi JR, Liehr AD, Adamson AW (1963) Ligand field theory of transition metal cyanide complexes. Part I. The zero, one and two electron or hole configurations. J Am Chem Soc 85:249–259
McGarvey BR (1966) The structure of the octacyanomolybdate(V) and -tungstate(V) ions from electron spin resonance. Inorg Chem 5:476–479
Hayes RG (1966) EPR studies of the Mo(CN)83− and W(CN)83− ions. The geometry of the ions. J Chem Phys 44:2210–2212
Adamson AW, Welker JP, Volpe M (1950) Exchange studies with complex ions. I. The exchange of radiocyanide with certain heavy metal complex cyanides. J Am Chem Soc 72:4030–4036
Dennis CR, Fourie E, Margerum DW, Swarts JC (2020) Kinetic advantage of inner sphere electron transfer reactions of copper(III, II) peptide complexes with cyano complexes of iron, molybdenum and tungsten. Trans Met Chem 45:147–157
Dennis CR, Margerum DW, Fourie E, Swarts JC (2020) A kinetic study of the electron-transfer reactions of nickel(III, II) tripeptide complexes with cyano complexes of molybdenum, tungsten, and iron. Inorg Chem 59:11695–11703
Leipoldt JG, Bok LDC, Dennis CR (1976) A kinetic study of the octacyanomolybdate(V) ion oxidation of arsenite in an alkaline medium. J Inorg Nucl Chem 38:1655–1657
Leipoldt JG, Bok LDC, Van Wyk AJ, Dennis CR (1977) A kinetic study of the octacyanotungstate(V) ion oxidation of arsenite in an alkalide medium. J Inorg Nucl Chem 39:2019–2020
Leipoldt JG, Bok LDC, Van Wyk AJ, Dennis CR (1977) A kinetic study of the reduction of the octacyanomolybdate(V) and octacyanotungstate(V) ions by hydrazine in an alkaline medium. React Kinet Catal Lett 6:467–474
Leipoldt JG, Dennis CR, Van Wyk AJ, Bok LDC (1978) A kinetic study of the osmium(Vlll) catalyzed reduction of octacyanomolybdate(V) and octacyanotungstate(V) ions by selenium(IV) in an alkaline medium. Inorg Chim Acta 31:187–190
Leipoldt JG, Bok LDC, Basson SS, Van Wyk AJ, Dennis CR, Cilliers PJ (1977) A kinetic study of the oxidation of thiourea by octacyanomolybdate(V) and octacyanotungstate(V) ions in an alkaline medium. React Kinet Catal Lett 8:93–99
Leipoldt JG, Dennis CR, Van Wyk AJ, Bok LDC (1979) A kinetic study of the Os(VIII) catalyzed oxidation of tellurium(IV) by octacyanomolybdate(V) and octacyanotungstate(V) ions in an alkaline medium. Inorg Chim Acta 34:237–240
Lamprecht GJ, Leipoldt JG, Dennis CR, Basson SS (1980) Kinetics of the octacyanomolybdate(V) ion oxidation of thiosulphate. React Kinet Catal Lett 13:269–275
Leipoldt JG, Dennis CR, Grobler EC (1983) Kinetics of the oxidation of the manganate ion by complex cyanides of Mo(V), W(V) and Fe(III) in alkaline solution. Inorg Chim Acta 77:L45–L46
Dennis CR, Basson SS, Leipoldt JG (1983) Kinetics and salt effects of the reduction of octacyanomolybdate(V) and octacyanotungstate(V) by sulphite ions. Polyhedron 2:1357–1362
Dennis CR, Leipoldt JG, Basson SS, Lamprecht GJ (1985) The oxidation of thiosulphate ions by octacyanotungstate(V) in weak acidic medium. Polyhedron 4:1621–1624
Dennis CR, Van Wyk AJ, Basson SS, Leipoldt JG (1987) Oxidation of hydrazine and methyl-substituted hydrazines by the cyano complexes of iron(III), molybdenum(V) and tungsten(V). A kinetic study. Inorg Chem 26:270–272
Van Wyk AJ, Dennis CR, Leipoldt JG, Basson SS (1987) A kinetic study of the oxidation of hydroxylamine by octacyanotungstate(V). Polyhedron 6:641–643
Dennis CR, Potgieter IM, Basson SS (2010) A kinetic study of the reduction of the octacyanomolybdate(V) ion by the hydroxide ion. Reac Kinet Mech Cat 99:63–68
Dennis CR, Potgieter IM, Basson SS (2011) A kinetic study of the oxidation of formaldehyde by the octacyanomolybdate(V) ion in aqueous alkaline medium. Reac Kinet Mech Cat 104:1–7
Dennis CR, Potgieter IM, Langner EHG, Fourie E, Swarts JC (2019) The oxidation of acetaldehyde by the octacyanomolybdate(V) ion in an aqueous alkaline medium. Transit Met Chem 44:161–165
Newton TW, Baker FB (1967) Aqueous oxidation-reduction reactions of uranium, neptunium, plutonium, and americium. Lanthanide/Actinide Chem 20:268–295
Bolotova GT, Golovnya (eds) (1964) Kompleksnye soedineniya urana, Akademiya nauk SSSR, Institut obschei i neorganicheskoi kimii (Complex uranium compounds, Academy of Sciences of the USSR, Institute of General and Inorganic Chemistry), Chapter 19
Kraus KA, Nelson F (1950) Hydrolytic behavior of metal ions. I. The acid constants of uranium(IV) and plutonium(IV). J Am Chem Soc 72:3901–3906
Kraus KA, Nelson F (1955) Hydrolytic behavior of metal ions. IV. The acid constant of uranium(IV) as a function of temperature. J Am Chem Soc 77:3721–3722
Betts RH (1955) Kinetics of the oxidation of uranium(IV) by iron(III) in aqueous solutions of perchloric acid. Can J Chem 33:1780–1791
Halpern J, Smith JG (1956) Kinetics of the oxidation of uranium(IV) by molecular oxygen in aqueous perchloric acid solution. Can J Chem 34:1419–1427
Newton TW (1959) The kinetics of the reaction between Pu(IV) and U(IV). J Phys Chem 63:1493–1497
Harkness AC, Halpern J (1959) Kinetics of the oxidation of uranium(IV) by thallium(III). J Am Chem Soc 81:3526–3529
Baker FB, Newton TW (1961) The reaction between uranium(IV) and hydrogen peroxide. J Phys Chem 65:1897–1899
Hassan RM (2011) A mechanistic approach of the kinetics of oxidation of uranium(IV) by hexachloroplatinate(IV) in aqueous perchlorate solutions. Evidence of the formation of a binuclear intermediate complex. J Phys Chem A 115:13338–13345
Hassan RM, Kojima T, Fukotumi H (1980) Kinetic study of the oxidation of uranium(IV) by ferricyanide ions in aqueous solutions. Bull Res Lab Nucl React Jpn 12:41–47
Hassan RM (1991) Kinetics of reaction of uranium(IV) and hexachloroiridate(IV) in acid perchlorate solutions. Evidence for a binuclear intermediate. J Chem Soc Dalton Trans. https://doi.org/10.1039/dt9910003003
Dennis CR, Van Zyl GJ, Fourie E, Basson SS, Swarts JC (2021) A kinetic study of the oxidation of the tetrakisoxalatouranate(IV) ion by the hexacyanoferrate(III) ion in an oxalate buffer medium. Reac Kinet Mech Cat 132:599–615
Leipoldt JG, Bok LDC, Cilliers PJ (1974) The preparation of potassium octacyanotungstate(IV) dihydrate. Z Anorg Allg Chem 407:350–352
Leipoldt JG, Bok LDC, Cilliers PJ (1974) The preparation of potassium octacyanomolybdate(IV) dihydrate. Z Anorg Allg Chem 409:343–344
Dennis CR, Van Wyk AJ, Basson SS, Leipoldt JG (1992) Synthesis of cesium octacyanomolybdate(V)- and cesium octacyanotungstate(V) dihydrate: a more successful method. Transit Met Chem 17:471–473
Basson SS, Bok LDC, Grobler SR (1974) Titrimetric and potentiometric determination of aqueous sulphide by octacyanomolybdate(V) and -tungstate(V) ions. Z Anorg Anal Chem 268:287–288
Marchi LE (1950) Potassium tetraoxalatouranate(IV). Inorg Synth 3:169–170
Gray GW, Spence JT (1971) Photochemical reduction of octacyanomolybdate(V) in aqueous solution. Inorg Chem 10:2751–2755
Lente G, Fabien I, Poe J (2005) A common misconception about the Eyring equation. New J Chem 29:759–760
"Scientist" software, version 2, MicroMath, Saint Louis, Missouri, USA
Lente G (2015) Chapter 3—deterministic kinetics in chemistry and systems biology. Springer, Cham, pp 61–65. https://doi.org/10.1007/978-3-319-15482-4
Wilkins RG (1991) The study of kinetics and mechanism of transition metal complexes, 2nd edn. Allyn and Bacon, Boston, p p8
Laidler KJ (1963) Reaction kinetics, vol 2. Pergamon Press Ltd, London
Zakharova FA, Moskvin AI (1960) The solubility product of uranium(IV) oxalate and the composition and dissociation constants of oxalato-uranium(IV) complexes in aqueous solution. Zhur Neorg Khim 5:1228–1233
Bolotova GT, Golovnya (eds) (1964) Kompleksnye soedineniya urana, Akademiya nauk SSSR, Institut Obschei i Neorganicheskoi Kimii (Complex uranium compounds, Academy of Sciences of the USSR, Institute of General and Inorganic Chemistry), Chapter 21
Swarts JC, Aquino MAS, Han J, Lam KY, Sykes AG (1995) Kinetic studies on the reduction of the tyrosyl radical of the R2 subunit of E. coli ribonucleotide reductase. Biochim Biophys Acta 1247:215–224
Han J, Swarts JC, Sykes AG (1996) Kinetic studies on the hydrazine and phenyl hydrazine reductions of Escherichia coli R2 subunit of ribonucleotide reductase. Inorg Chem 35:4629–4634
Lente G (2018) Facts and alternative facts in chemical kinetics: remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr Opin Chem Eng 21:76–83
Acknowledgements
The authors acknowledge the Central Research Fund of the University of the Free State, Bloemfontein, South Africa.
Author information
Authors and Affiliations
Contributions
GJvZ—Investigation; EF—Investigation, writing, editing; CRD—Investigation, writing, review, editing, project admin; SSB—Conceptualisation, methodology, review, editing, supervision; JCS—Conceptualization, methodology, review, editing, project admin supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors are not aware of any conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dennis, C.R., van Zyl, G.J., Fourie, E. et al. A kinetic study of the oxidation of the tetrakisoxalatouranate(IV) ion by the octacyanotungstate(V) and the octacyanomolybdate(V) ions in an acidic oxalate buffer medium. Reac Kinet Mech Cat 134, 615–627 (2021). https://doi.org/10.1007/s11144-021-02109-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02109-2