Abstract
Maillard reaction (MR) with oat β-glucan changed the structure of soybean protein isolate (SPI), further leading to the enhancement of its functional properties. SPI was unfolded by MR, and the SPI conjugates with high molecular weight were identified. The water solubility of SPI was improved by cross-linking with hydrophilic β-glucan, while the hydrophobicity also increased along with the unfolding of the SPI. Cross-linking with β-glucan elevated the viscosity of SPI, thus enhancing viscosity-related physiological activities, including bile acid binding ability, fat binding capacity, and hypoglycemic activity, and the functional properties increased as the βG content involved in MR increased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As an ideal edible protein resource, soybean protein isolate exhibited distinct functional characteristics. Such as solubility, emulsifying property, gel property, and blistering property [1]. Due to its nutritional and functional advantages, SPI exhibits a huge application potential in the food industry. However, SPI may lose stability during different process conditions, such as a pH near its isoelectric point, an extreme ionic intensity, or a high temperature, hence resulting in reduced functional properties and further limiting its application in food industry. Therefore, some modification methods were used to improve the functional properties of SPI.
Possessing the character of green, mild, and efficiency, Maillard reaction (MR) is one of the most promising ways to improve the structure and function of protein [2]. Compared with monosaccharides and oligosaccharides, polysaccharides exhibit stronger molecular steric hindrance. The MR with polysaccharides endowed protein with more superior functional properties [2]. Xu’s work showed that chitosan enhanced the emulsifying property of SPI [3]. The solubility of pea protein isolates increased after MR with maltodextrin [4]. Pleurotus ostrectus β-glucan endowed oat protein with superior thermal stability and enhanced solubility [5]. Rice protein-glucan conjugates exhibited enhanced solubility, foaming and emulsifying properties [6].
As a linear non-starch polysaccharide, oat β-glucan (βG) exhibited many physiological activities, among which the hypoglycemic activity is the most impressive [7]. βG possesses high viscosity, water solubility, and because of many hydroxyl groups on its molecule, it can be expected to bring the improved water solubility to SPI through MR, and thus enhancing the functional property of SPI.
Research showed that the structure and functional properties of proteins conjugates may depend on the type and content of polysaccharides during MR [6, 8, 9]. In this work, MR was used to synthesize three SPI-βG conjugates. Then the structural property and functional characteristics of the conjugates were investigated. Our results will provide some foundation for the utilization of soybean protein isolate in food industry.
Materials and Methods
The material and methods section was presented as supplementary material.
Results and Discussion
Structural Information
Infrared Spectral Characterization and Secondary Structure
As depicted in Fig. S1a, all the samples showed similar infrared characteristic peak. For example, the C = O stretching vibrations at 1631 cm− 1 indicated amide I band, the peak at 1520 cm− 1 was N-H of amide II bending vibration, and the absorption band around 1240 cm− 1 was due to C-N stretching vibration and N-H deformation of amide III. The broad absorption around 3016 cm− 1 and 3637 cm− 1 resulted from the SPI’s N-H stretching vibration [10]. Compared with SPI, the slightly broadened absorption at 3020–3682 cm− 1 of conjugates was related to hydroxyl groups of βG. The enhanced absorption near 1036 cm− 1 of SPI conjugates indicated the C-N bonds between SPI and βG [11]. The reduced absorption band at 1523 cm− 1 of SPI conjugates revealed that amino group of SPI was consumed during MR.
The secondary structure was represented in Table S1. The α-helix and β-turn content of SPI increased slightly with the increasing βG in MR. Meanwhile, the random coils and β-sheets content of SPI displayed the opposite trend. The result may be caused by the denaturation of SPI, or by the cross-linking between SPI and polysaccharides [12]. Chen’s work showed that the change in the secondary structure resulted in a significant difference in the functional properties of protein [13].
Degree of Glycation
As shown in Table S2, the more βG content in Maillard reaction, the higher degree of glycation (DG) for SPI conjugates can be obtained, which is caused by the more cross-linking between βG and SPI. As expected, SPI: βG 1:1 showed the highest DG (21.01%). The phenomenon revealed that higher content of carbonyl groups can react with more amino groups of SPI [14], which further affect its functional property.
SDS-PAGE
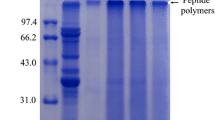
The SDS-PAGE of samples was represented in Fig. S1b. The characteristic bands of protein can be seen on the visualized gel of SPI (lane 1), and the banding pattern of SPI changed after MR. The conjugates (lanes 2 and 3) showed some darker and deeper stripes around 250 KDa, demonstrating components with high molecular weight were formed during MR of SPI and βG [15]. The color of the SPI conjugates bands at 7 and 11 S gradually became lighter as the βG content involved in MR increased. In summary, the increase in protein molecular weight is considered to be a key indicator of conjugates formation after Maillard reaction [5].
Fluorescence Spectrum
Fluorescence spectrum is affected by the tryptophan (Trp) group of protein and could assess SPI’s conformational variation and tertiary structure. MR with βG altered the structure of SPI, which influenced the exposure of Trp group. SPI’s fluorescence intensity exhibited a decrease and decreased as the βG content involved in MR increased (Fig. S1c). The phenomenon may be caused by the fact that shielding of Trp group by cross-linking between polysaccharides and proteins can lead to a reduction in fluorescence intensity [16]. The λmax (maximum absorption wavelength) indicates the microenvironment where Trp group is located, when λmax > 330 nm the Trp group is exposed to a polar microenvironment. The λmax was 337 (SPI), 341 (SPI: βG 4:1), 342 (SPI: βG 2:1), and 344 (SPI: βG 1:1) nm, respectively. The red-shift of λmax in SPI after MR suggested that SPI has been unfolded by MR and Trp group is more exposed to the solvent [17]. The polarity of the microenvironment where the fluorescence emitting group Trp is located increased, making the peptide chain more extended, and thus the spatial conformation of the protein changed. Meanwhile, the hydrophobic groups were also exposed by unfolded SPI, leading to SPI’s increased surface hydrophobicity. The covalent bonding with βG altered the structure of SPI, which resulted in a series of changes in its functional properties.
Physical and Chemical Properties
Solubility
As shown in Fig. S2, the lowest solubility of samples was observed around isoelectric point, which may be attributed to their structural similarity. Meanwhile, compared with SPI, the conjugates exhibited better solubility in the whole pH range, particularly at pH values (6–9). Among them, SPI: βG 1:1 exhibited the best solubility. The hydrophilic groups, mainly hydroxyl group of βG were introduced and adsorbed on the surface of SPI, improving the affinity of SPI to water, thus increasing the interaction between water molecules and SPI [18]. Research showed that the solubility of protein-polysaccharide conjugates can be controlled by the degree of MR [19]. In our work, the more βG was introduced, the higher the DG and the better solubility. Acacia gum introduced hydrophilic groups into soy protein isolates, enhancing the interaction between protein and water molecules, increasing the solubility of proteins [20].
Surface hydrophobicity (H 0)
H0 is one of the important factors that affect the solubility and emulsifying property of protein. As shown in Table S2, SPI exhibited the lowest H0, which was caused by the fact that majority of its hydrophobic residues was buried within the dense spherical zone [21]. MR endowed SPI with enhanced H0, which increased with the increase of βG content in reaction. The result may be caused by the increased solubility of SPI bonded with polar groups, exposing more hydrophobic groups. Meanwhile, the red-shift of λmax in SPI after MR suggested that SPI has been unfolded by MR [17]. The hydrophobic groups were exposed by unfolded SPI, leading to SPI’s increased surface hydrophobicity. Research showed that H0 of peanut isolate was also significantly enhanced after MR with dextran or gum arabic [22].
Rheological Property
Proteins’ rheological properties in food are tightly associated with its functional characteristics, which could directly affect the food quality and shelf-life [23]. The viscosity of SPI hardly changed within the range of shear rates of 0.1–100 s− 1, approaching an ideal Newtonian fluid (Fig. S3). However, the SPI conjugates showed shear thinning behavior of a pseudoplastic fluid, which was a result of the disrupting of irregularly curled polymer and its parallel alignment during shear [24]. Compared to SPI, SPI conjugates exhibited higher viscosity, which further affected the relevant characteristics.
Physiological Activities
The excellent physiological activity of βG is related to its viscosity, and since MR enhanced the viscosity of SPI, can it be expected that oat β-glucan will enhance or even confer SPI-related activity? Bile acid binding ability (BAB), fat binding capacity (FB), and glucose availability (GA) of SPI conjugates were shown in Table S2. The BAB of proteins has been associated with its cholesterol lowering. The BAB of SPI was 10.76%, and increased as the βG content involved in MR increased, and SPI: βG 1:1 showed a significant increase in BAB (47.70%). Whey protein isolate bounded to the bile salts through hydrogen bond interactions, and the increase in surface hydrophobicity indicated higher bile acid binding through hydrophobic interactions [25]. βG introduced hydrophilic groups to SPI during MR, enhancing the solubility and hydrophobicity (H0), which will result in the increased BAB of SPI. The MR product of glucose and L-lysine could adsorb bile acids and reduce plasma cholesterol [26].
The fat binding capacity of chitosan is closely related to its lipid-lowering effect [27]. SPI conjugates’ FB was improved by MR and increased as the βG content increased. Research revealed that hydroxyl group showed a positive effect on the fat binding capacity of macromolecules [28]. Meanwhile, the SPI helix structure was unfolded, exposing more hydrophobic groups and leading to the increased H0 [29]. The exposed hydrophobic regions on the protein surface can effectively interact with the hydrophobic portions of the fats, leading to improved FB [30].
In vitro digestion tests, the glucose availability was 20.34 (SPI), 12.58 (SPI: βG 4:1), 11.76 (SPI: βG 2:1) and 11.20 (SPI: βG 1:1) mmol/L, respectively. The result indicated that MR endowed SPI with enhanced hypoglycemic ability, and the ability increased as the βG content involved in MR increased. Generally, the enhancement of intestinal viscosity and reduction of pancreatic amylase activity can lower the digestibility and absorption of starch [31]. In the present study, the increase in hypoglycemic ability might be associated with the enhanced viscosity of SPI conjugates.
Carbohydrate digestion and uptake could be significantly reduced by the inhibition of α-glucosidase activity (IαG), which result in a lower blood glucose levels and an inhibition of postprandial hyperglycemia [32]. The IαG of SPI conjugates was depicted in Table S2. SPI barely demonstrated α-glucosidase activity, but the activity of SPI conjugates increased remarkably, among them SPI: βG 1:1 showed the best IαG (35.45%). Research showed that the IαG of chitosan was enhanced by MR with glucose [33], indicating MR was a resultful approach to increase SPI’s hypoglycemic effect.
Crossing with β-glucan increased the length of SPI’s molecular chain and thus increased the viscosity of SPI conjugates. This may be one of the reasons why viscosity-related properties such as bile acid binding ability, fat binding capacity, glucose availability and the inhibition of α-glucosidase activity were enhanced.
Conclusion
The content of β-glucan showed a great effect on the structure and function of SPI during the Maillard reaction. As β-glucan content increased, the degree of glycation of the Maillard reaction was elevated, and the water solubility and H0 of the SPI conjugates increased. The increase in DG meant that more β-glucan was bonded to SPI, which led to a higher viscosity of SPI conjugates and consequently a significant increase in viscosity-related physiological activities, especially, hypoglycemic activity. All the functional properties of SPI were positively correlated with the content of βG. Our result provided some theoretical information for the effective utilization of SPI.
Data Availability
Data will be made available on request.
References
Aidee SR, Sayra RB, José RR, Bertha BD, Sergio OSS, Cristina CH (2018) Phosphoesterification of soybean and peanut proteins with sodium trimetaphosphate (STMP): changes in structure to improve functionality for food applications. Food Chem 260:299–305. https://doi.org/10.1016/j.foodchem.2018.04.009
de Oliveira FC, Coimbra JSdR, de Oliveira EB, Zuñiga ADG, Rojas EEG (2016) Food protein-polysaccharide Conjugates obtained via the Maillard reaction: a review. Crit Rev Food Sci Nutr 56:1108–1125. https://doi.org/10.1080/10408398.2012.755669
Xu ZZ, Huang GQ, Xu TC, Liu LN, Xiao JX (2019) Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int J Biol Macromol 131:601–607. https://doi.org/10.1016/j.ijbiomac.2019.03.101
Kutzli I, Griener D, Gibis M, Schmid C, Dawid C, Baier SK et al (2020) Influence of Maillard reaction conditions on the formation and solubility of pea protein isolate-maltodextrin conjugates in electrospun fibers. Food Hydrocoll 101:105535. https://doi.org/10.1016/j.foodhyd.2019.105535
Zhong L, Ma N, Wu Y, Zhao L, Ma G, Pei F et al (2019) Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food Hydrocoll 87:459–469. https://doi.org/10.1016/j.foodhyd.2018.08.034
Cheng YH, Mu DC, Feng YY, Xu Z, Wen L, Chen ML et al (2022) Glycosylation of rice protein with dextran via the Maillard reaction in a macromolecular crowding condition to improve solubility. J Cereal Sci 103:103374. https://doi.org/10.1016/j.jcs.2021.103374
Bai J, Ren Y, Li Y, Fan M, Qian H, Wang L et al (2019) Physiological functionalities and mechanisms of β-glucans. Trends Food Sci Technol 88:57–66. https://doi.org/10.1016/j.tifs.2019.03.023
Laura JC, Villamiel M, Rosina LF (2007) Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll 21:433–443. https://doi.org/10.1016/j.foodhyd.2006.05.006
Spotti MJ, Loyeau PA, Marangón A, Noir H, Rubiolo AC, Carrara CR (2019) Influence of Maillard reaction extent on acid induced gels of whey proteins and dextrans. Food Hydrocoll 91:224–231. https://doi.org/10.1016/j.foodhyd.2019.01.020
Fu GM, Xu ZW, Luo C, Xu LY, Chen YR, Guo SL et al (2021) Modification of soy protein isolate by Maillard reaction and its application in microencapsulation of Limosilactobacillus reuteri. J Biosci Bioeng 132:343–350. https://doi.org/10.1016/j.jbiosc.2021.06.007
Gu F, Kim JM, Abbas S, Zhang X, Xia S, Chen Z (2009) Structure and antioxidant activity of high molecular weight Maillard reaction products from casein-glucose. Food Chem 120:505–511. https://doi.org/10.1016/j.foodchem.2009.10.044
Zhang T, Zhang H, Yang Z, Wang Y, Li H (2019) Black rice addition prompted the beer quality by the extrusion as pretreatment. Food Sci Nutr 7:3664–3674. https://doi.org/10.1002/fsn3.1223
Chen W, Ma X, Wang W, Lv R, Guo M, Ding T et al (2019) Preparation of modified whey protein isolate with gum acacia by ultrasound maillard reaction. Food Hydrocoll 95:298–307. https://doi.org/10.1016/j.foodhyd.2018.10.030
Meng Y, Zhao X, Jiang Y, Ban Q, Wang X (2023) Effect of Maillard reaction conditions on the gelation and thermal stability of whey protein isolate/d-tagatose conjugates. Food Chem 405:134928. https://doi.org/10.1016/j.foodchem.2022.134928
I LOA, Gabriela RC, Luz MGPA VM (2010) Conjugates of bovine serum albumin with chitin oligosaccharides prepared through the Maillard reaction. J Agric Food Chem 58:12000–12005. https://doi.org/10.1021/jf102869f
Zhang H, Yang J, Zhao Y (2015) High intensity ultrasound assisted heating to improve solubility, antioxidant and antibacterial properties of chitosan-fructose Maillard reaction products. LWT–Food Sci Technol 60:253–262. https://doi.org/10.1016/j.lwt.2014.07.050
Shen L, Tang C (2012) Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res Int 48:108–118. https://doi.org/10.1016/j.foodres.2012.03.006
Laura JC, Rosina LF, Olano A, Villamiel M (2005) Study on β-lactoglobulin glycosylation with dextran: effect on solubility and heat stability. Food Chem 93:689–695. https://doi.org/10.1016/j.foodchem.2004.09.050
Álvarez C, García V, Rendueles M, Díaz M (2012) Functional properties of isolated porcine blood proteins modified by Maillard’s reaction. Food Hydrocoll 28:267–274. https://doi.org/10.1016/j.foodhyd.2012.01.001
Mu L, Zhao H, Zhao M, Cui C, Liu L (2011) Physicochemical properties of soy protein isolates-acacia gum conjugates. Czech J Food Sci 29:129–136. https://doi.org/10.17221/339/2009-CJFS
Shigeru H, Shuryo N (1985) Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. J Food Sci 50:486–491. https://doi.org/10.1111/j.1365-2621.1985.tb13433.x
Li C, Xue H, Chen Z, Ding Q, Wang X (2014) Comparative studies on the physicochemical properties of peanut protein isolate-polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res Int 57:1–7. https://doi.org/10.1016/j.foodres.2013.12.038
Zhu Y, Gao H, Liu W, Zou L, McClements DJ (2020) A review of the rheological properties of dilute and concentrated food emulsions. J Texture Stud 51:45–55. https://doi.org/10.1111/jtxs.12444
Inglett GE, Chen D, Liu SX (2015) Functional properties of teff and oat composites. Food Nutr Sci 6:1591–1602. https://doi.org/10.4236/fns.2015.617164
Mei L, Fu Q, Guo T, Ji Q, Zhou Y (2022) Structural changes and cholesterol-lowering in denatured whey protein isolate: malic acid combined enzymolysis. Food Hydrocoll 127:107502. https://doi.org/10.1016/j.foodhyd.2022.107502
Ragot FI, Russell G, Schneeman BO (1992) Effect of Maillard reaction products on bile acid binding, plasma and hepatic lipids, and weight of gastrointestinal organs. J Agric Food Chem 40:1634–1640. https://doi.org/10.1021/jf00021a032
Biskup RC, Rokita B, Ulanski P, Rosiak JM (2005) Radiation-induced and sonochemical degradation of chitosan as a way to increase its fat-binding capacity. Nucl Instrum Methods Phys Res Sect B 236:383–390. https://doi.org/10.1016/j.nimb.2005.04.002
Jakobek L (2015) Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem 175:556–567. https://doi.org/10.1016/j.foodchem.2014.12.013
Marín ML, Selgas D, Pin C, Casas C (1992) Relationships of hydrophobicity and antigenic activity to fat-binding capacity of heat denatured meat proteins. J Sci Food Agric 60:505–508. https://doi.org/10.1002/jsfa.2740600416
Voutsinas LP, Nakai S (1983) A simple turbidimetric method for determining the fat binding capacity of proteins. J Agric Food Chem 31:58–63. https://doi.org/10.1021/jf00115a015
Regand A, Chowdhury Z, Tosh SM, Wolever TM, Wood P (2011) The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem 129:297–304. https://doi.org/10.1016/j.foodchem.2011.04.053
Hwang IG, Kim HY, Woo KS, Hong JT, Hwang BY, Jung JK et al (2011) Isolation and characterisation of an α-glucosidase inhibitory substance from fructose–tyrosine Maillard reaction products. Food Chem 127:122–126. https://doi.org/10.1016/j.foodchem.2010.12.099
Tran TN, Doan CT, Nguyen VB, Nguyen AD, Wang SL (2019) Anti-oxidant and anti-diabetes potential of water-soluble chitosan-glucose derivatives produced by Maillard reaction. Polymers 11:1714. https://doi.org/10.3390/polym11101714
Funding
The Natural Science Foundation of China supported this study (fund number: 31972142 and 31571914).
Author information
Authors and Affiliations
Contributions
Zhaonan Chu, Qiyun Zhang: Conceptualization, Data curation, Writing-Original draft preparation. Tao Sun: Writing-Reviewing and Editing. Xiaohui Li: Resources. Bin Xue: Validation. Jing Xie: Funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This study does not involve any human or animal testing.
Consent to Participate
All authors have read and approved the MS; and, that all are aware of its submission.
Consent for Publication
All authors agree to publish.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chu, Z., Zhang, Q., Li, X. et al. Effect of Oat β-Glucan on the Structure and Properties of Soybean Protein Isolate During Maillard Reaction. Plant Foods Hum Nutr 78, 552–556 (2023). https://doi.org/10.1007/s11130-023-01092-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01092-4