Abstract
The conjugates of soybean protein isolate (SPI) with xylose and fructose were prepared via Maillard reaction by wet heating at mass ratio 4:1 and 80 °C for several hours. Absorbance at 420 nm indicated the existence of an induction period for the Maillard reaction between SPI and the two monosaccharaides and xylose was more reactive than fructose. SDS–PAGE and thermogravity analysis confirmed the occurrence of Maillard reaction. Fluorescence analysis revealed that the Maillard reaction led to relaxed structure for SPI in the conjugates. The conjugation with the two monosaccharaides significantly improved the solubility, but decreased the emulsifying activity of SPI. ζ-Potential measurement showed that the reaction changed the charge density of SPI and the specific effect depended on the sugar type and reaction degree. It is concluded that the wet heating-induced Maillard reaction with monosaccharaides might be a promising way for SPI modification due to shortened reaction time, improved solubility and tailored charge density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean protein isolate (SPI) is produced from defatted soy meal by alkali extraction followed by acid precipitation at pH 4.5. SPI is an abundant and inexpensive raw material and is the most important plant-derived protein for the food industry. It has been widely used as emulsifying, thickening and gelling agents in various meat and dairy products [1].
To further promote the application of SPI, various physical [2], chemical [3] and enzymatic [4] treatments have been applied to enhance its functional properties. Among the methods, conjugation with polysaccharides through the Maillard reaction has been proved to be a promising way and has attracted extensive attentions due to its safety [5]. The Maillard reaction can be induced by both wet and dry heating and the latter is the most frequently used for SPI modification. For example, conjugation with soy soluble polysaccharide [6], maltodextrin [1, 5], gum acacia [5], dextran [7], fenugreek gum [8] and carboxymethyl cellulose [9] in dry heating conditions effectively increased the water solubility and many other properties of SPI.
However, the Maillard reaction induced by dry-heating often takes a long reaction time of up to several days or weeks, and the reaction extent is relatively hard to control due to the uneven contact between the reactants. From an industrial viewpoint, the dry-heating process is not attractive and no commercial conjugates have been manufactured yet [10].
Wet heating is another common way to allow the Maillard reaction between proteins and saccharides. This method has been widely used to modify multiple proteins [11], but its application in SPI modification is quite limited and the major purpose of these rare reports is to shorten the time required for SPI-saccharide conjugation. Fox example, Guan et al. [12] found that microwave heating sped up the graft reactions of SPI with maltose, dextran and soluble starch in wet heating conditions and the reaction rates were 7, 57 and 12.3 times of those of classical dry heating; Zhuo et al. [13] reported that the Maillard reaction with dextran in macromolecular crowding conditions greatly improved the emulsifying activity of SPI, but required a shorter time than dry heating.
The Maillard reaction of proteins with monosaccharaides is much faster than that with polysaccharides [14] and monosaccharaides might be alternatives to polysaccharides for shortened reaction time. However, in contrast to polysaccharides, conjugation with monosaccharaides might lead to either adverse or beneficial effects on the properties of proteins. For example, the reaction of caseinomacropeptide with lactose at mass ratio 1:1.5, 40 °C and 44% relative humidity improved the emulsifying activity of the protein [15], while that of casein remained unchanged upon glycation with glucose, lactose and ribose under wet conditions (60 °C, 1:1000 molar ratio protein:sugar, 6 days) [16]. To present, no researches have been made on the Maillard reaction between SPI and monosaccharaides induced by wet heating.
Hence, the purpose of this work is to investigate the Maillard reaction between SPI and two monosaccharaides (xylose and fructose) in wet heating conditions and the variation of partial properties of the resultant Maillard reaction products (MRPs). This work is expected to provide information for the practical application of the Maillard reaction in SPI modification.
Experimental
Chemicals and materials
SPI of food grade was purchased from Qingdao Tianxin Food Additives Co., Ltd. (Qingdao, China). Xylose and fructose of analytical grade as well as bovine serum albumin (BSA) were obtained from Tianjin BASF Chemical Co., Ltd. (Tianjin, China). All other reagents were of analytical grade.
Preparation of MRPs
The mixture of SPI and sugar (xylose or fructose) was dissolved in deionized water to yield a sugar to SPI mass ratio of 1:4 and final SPI concentration of 4% (w/v). The solution was adjusted to pH 9.0 with 1 mol/L NaOH and put in a tightly capped tube, which was then heated in 80 °C water bath for 10 h. At an interval of 2 h, aliquots of the reaction mixture were taken out, cooled on ice water and subjected to absorbance, protein content and ζ-potential determination or freeze-dried for other characterizations.
Absorbance at 420 nm
The Maillard reaction between SPI and the two monosaccharaides was monitored by measuring the absorbance at 420 nm [17]. The sampled aliquots were centrifuged at 4000 r/min for 10 min and the absorbance of the supernatants was read at 420 nm using a UV2000 spectrophotometer (Unico, Shanghai, China).
SDS–PAGE analysis
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) was performed according to a previous report [1] with minor modifications using 4% stacking gel and 7.5% separating gel. A total of 0.0150 g of each of the freeze-dried powders was dissolved in 1 mL deionized water and thoroughly vortexed. The solution was then centrifuged at 10,000 r/min for 10 min and the supernatant was boiled for 5 min. After cooling to room temperature, 10 µL of the solution was loaded onto the gel. Electrophoresis was run at 20 mA in the stacking gel and at 40 mA in the separating gel. After electrophoresis, the gel was stained with Coomassie brilliant blue G-250 solution. The electrophoresis gel was photographed by using the 6100 Chemiluminescent Imaging System (Tanon Science & Technology Co., Ltd, Shanghai, China).
Intrinsic fluorescence emission spectroscopy
The intrinsic emission fluorescence spectra were obtained by an F-2700 PC fluorophotometer (Tianmei Scientific Instrument Co., Ltd, China). The freeze-dried powders were dissolved in 10 mmol/L phosphate buffer (pH 7.0) to yield 1 mg/mL solutions. To minimize the contribution of tyrosine residues to the emission spectra, the protein solutions were excited at 334 nm, and emission spectra were recorded from 350 to 500 nm at a constant slit of 5 nm.
Thermogravimetric analysis
After the sampled aliquots were freeze-dried, 3 mg of each of the powders was loaded to a TGA/DSC 1-thermogravimetric analyzer (Mettler Toledo Instruments, OH, USA) for thermodynamics property analysis. The samples were sealed in aluminum pans and a sealed empty pan was used as reference. The scans were carried out at a heating rate of 10 °C/min across the 30–500 °C range. A nitrogen purge was applied throughout the measurement.
Solubility determination
The solubility of the conjugates was determined using Lowry’s method with minor modifications [18]. The sampled aliquots were centrifuged at 4000 r/min for 10 min and the protein content in the supernatant was determined by comparing with the BSA standard curve. The solubility of the conjugate was expressed in micrograms of protein dissolved per milliliter of aqueous solution.
Emulsifying activity index
The emulsion activity indexes (EAI) of the conjugates were measured according to the method of Zheng et al. [19] with minor modifications. That is, each 3 mL sample solution was homogenized at 12,000 rpm at 20 °C for 1 min with 1 mL soybean oil to form emulsion. From the bottom of each test tube, an aliquot of 0.5 mL was dispersed in 4.5 mL of sodium dodecyl sulphate solution (1% w/v) and the absorbance at 500 nm was read using a UV 2000 spectrophotometer. The EAI was then calculated according to the following equation:
where Φ is the oil phase volume (0.25), C is the weight of protein per unit volume of aqueous phase before the emulsion is formed, N is the dilution factor, and A is the absorbance of the diluted emulsions.
ζ-Potential measurement
The sampled aliquots were diluted with deionized to an approximate concentration and the ζ-potential was then measured using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK).
Statistical analysis
All the experiments were performed on triplicate samples and values were expressed as mean values SD. Differences between mean values were conducted using the one-way analysis of variance (ANOVA) by SPSS 16.0 software. Differences were statistically significant at p < 0.05.
Results and discussion
Changes in browning intensity
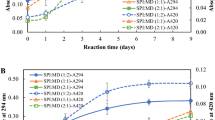
The absorbance at 420 nm indicated the browning degree of the Maillard reaction. The extent of browning as a function of heating time was shown in Fig. 1. It could be seen that the absorbance of the two MRPs remained nearly constant at the first 4 h, indicating the existence of an induction period for the Maillard reaction between SPI and the two monosaccharaides. This phenomenon has also been seen in the glucose/alanine system [20]. When the heating time increased to 6 h, the absorbance of the two MRPs increased significantly, implying the initiation of the reaction. As the reaction time further increased to 8 h or longer, the absorbance of the SPI-xylose MRP kept increasing, whereas that of the fructose-SPI MRP nearly remained unchanged. Besides, the absorbance of the SPI-xylose MRP was higher than of the SPI-fructose MRP in the entire reaction period selected, implying that xylose was more reactive than fructose.
SDS–PAGE
To confirm the covalent binding of sugars to SPI, SDS–PAGE was conducted by selecting reaction time 6 h as the representative and the results were given in Fig. 2. Compared with native SPI (lane a), heating of SPI at 60 °C for 6 h led to a smearing band on the top of the gel and the gradual disappearance of two 7S bands, indicating the formation of aggregates upon heating. The presence of xylose (lane d) and fructose (lane f) did not change the SDS–PAGE pattern of SPI. However, conjugation with xylose (lane c) or fructose (lane e) led to polydispersed bands at the top of the gel and reduced intensity of some 11S and 7S fractions, confirming the occurrence of Maillard reaction and the formation of conjugates with higher molecular weight. This result has also been seen in the SPI-NaCMC [9] and SPI-maltodextrin [1] conjugates. Besides, the intensities of some 11S and 7S fractions in the SPI-xylose MRP were much lower than that of the SPI-fructose MRP, illustrating that xylose was more susceptible to Maillard reaction with SPI than fructose. This result was consistent with the absorbance measurement results (Fig. 1).
Emission fluorescence spectroscopy
The fluorescence emission spectra of native SPI, wet-heated SPI, SPI–monosaccharide mixtures and conjugates were illustrated in Fig. 3 with heating time 6 h as a representative. The λmax of native SPI was 415 nm; after the conjugation with fructose and xylose, the λmax shifted to 404 and 411.5 nm respectively. This trend was consistent with the SPI-maltodextrin conjugate prepared by dry heating [5]. However, a reverse result was observed in fluorescence intensity compared with SPI-saccharide conjugates prepared by dry heating [5, 21], that is, the SPI-monosaccharide conjugates showed the highest intensity, followed by heated SPI, native SPI and SPI-monosaccharide mixture in sequence. This was because that the structure of SPI was more relaxed in aqueous solutions upon heating and the Maillard reaction made more fluorescigenic groups exposed. The presence of unreacted sugars could shield the fluorescence emission of SPI, thus leading to the least fluorescence intensity for the SPI-xylose/fructose mixtures. Such difference implied that wet heating and dry heating could led to different tertiary conformation for SPI and SPI in the wet-heated conjugates was more relaxed than the dry-heated counterparts.
Thermal properties
The effects of Maillard reaction with fructose and xylose on the thermogravimetric pattern of SPI were illustrated in Fig. 4 by selecting reaction time 6 h as a representative. In the temperature range 60–160 °C which could led to water evaporation, heated SPI suffered greater weight loss than the two MRPs, indicating that the Maillard reaction increased the water retention capacity of SPI. This could possibly be ascribed to the increased hydrophilicity of the MRPs due to the attachment of the monosaccharaides. As the temperature further increased, SPI started to decompose at around 230 °C. This starting temperature was consistent with the report of Schmidt and Soldi [22]. The SPI-xylose and SPI-fructose MRPs started to decompose at a lower temperature of 157.5 and 180 °C respectively and suffered greater weight loss than heated SPI in higher temperatures, implying that small volatile compounds were generated due to the Maillard reaction.
Solubility
As shown in Fig. 5, the presence of xylose or fructose increased the solubility of SPI, but no significant difference was observed between the two sugars. The same result has also been found in the presence of low-viscosity carboxymethyl cellulose and xanthan [9, 23]. Heat treatment increased the solubility of SPI. After heated at 80 °C for 2 and 10 h, the solubility of SPI increased by around 43 and 59% respectively compared with native SPI, whereas the increase at heating time 6 h was only 20%. This was because that the insoluble aggregates in SPI were dissociated and became soluble upon heating for 2 h; as the heating further proceeded to 6 h, the relaxed polypeptide chains aggregated again and the solubility decreased slightly consequently; as the heating time further elongated, the aggregates dissociated again and the solubility increased markedly.
The Maillard reaction further increased SPI solubility. After heated with xylose and fructose for 2 h, the solubility of SPI increased to 6400 and 7931 µg/mL respectively compared with 3983 and 5690 µg/mL of native and heated SPI. As the reaction time elongated to 6 h, the values further increased to 8598 and 8664 µg/mL respectively. However, as the reaction time increased to 10 h, the solubility of SPI declined to 6067 and 5679 µg/mL respectively, but was still significantly higher than native SPI, indicating that the Maillard reaction proceeded to the final stage and insoluble compounds were formed. These results indicated that moderate Maillard reaction with xylose and fructose by wet heating could significantly improve the solubility of SPI. The same results was also found in the SPI-glucose, SPI-carboxymethyl cellulose and SPI-maltodextrin/gum acacia conjugates prepared by dry heating [5, 9, 24].
The solubility of proteins is the most important factor for their physicochemical properties and correlates with functional properties such as emulsifiability, foaming and gelation [25]. The increased solubility of SPI implied that the SPI-xylose and SPI-fructose conjugates possibly possessed improved functional properties.
Emulsifying properties
Conjugation with polysaccharides has been demonstrated to be a promising way to improve the emulsifying properties of proteins [14] and this improvement has been attributed to the polysaccharide molecules that conjugated to protein molecules, thus providing additional steric hindrances between the emulsion droplets when the conjugated protein was adsorbed to the surface of the droplet [26]. Dry-heating is the preferred way to produce Maillard-type protein–polysaccharide conjugates and this method has been reported to increase the emulsifying properties of SPI [1, 5, 9].
As shown in Fig. 6, wet heating decreased the EAI of SPI, which was consistent with the finding of Zhuo et al. [13]. Conjugation with the two monosaccharaides further decreased this parameter. After reaction with xylose for 2 and 6 h, the EAI decreased by up to 54 and 65% respectively and that of the SPI-fructose conjugate was 44 and 70% respectively. As the incubation time further increased to 10 h, no further decrease in EAI was observed. It is very interesting that conjugation with glucose in dry heating condition improved the emulsifying properties of SPI [24]. Such difference implied that wet heating and dry heating could contribute different emulsifying properties to SPI.
ζ-Potential measurements
SPI has been used as a polyelectrolyte to encapsulate food ingredients through complex coacervation [27,28,29,30]. Hence, it is necessary to measure the ζ-potential of the MRPs. As shown in Fig. 7, all the three samples were still negatively charged. The Maillard reaction changed the charge density of SPI and the specific effect was dependent on the sugar species involved in the reaction and the reaction time. At reaction time 2 and 4 h, the absolute ζ-potential of the resultant SPI-xylose MRPs were higher than that of heated SPI, implying that the –NH2 groups in SPI participated in the reaction. As the reaction further proceeded, the absolute charge decreased greatly, indicating the generation of positively charged products. Concerning the SPI-fructose MRP, its positive charge decreased sharply at reaction duration 2 h and was lower than that of heated SPI. As the reaction time increased to 6 h, its negative charge increased. When the reaction time further elongated, the positive charge decreased slightly. Since SPI is quite promising as a polyelectrolyte wall material [31], Maillard reaction might be an effective way to modify its charge properties and a desired charge density could be tailored by varying the saccharide type or reaction degree.
Conclusions
There exists an induction period for the Maillard reaction between SPI and xylose or fructose in wet heating conditions and the reaction starts at 6 h or later. Xylose is more reactive than fructose and their conjugation with SPI leads to the formation of large molecules and more relaxed structure of SPI in the conjugates. The Maillard reaction with the two monosaccharaides improves the solubility, but decreases the emulsifying activity of SPI. Besides, the ζ-potential of the conjugates can be tailored by varying the sugar type and reaction time. Hence, the wet-heating Maillard reaction with monosaccharaides might be a promising way for SPI modification due to the shortened reaction time, improved solubility and tailored charge density of SPI.
References
J. Zhang, N. Wu, T. Lan, X. Yang, Improvement in emulsifying properties of soy protein isolate by conjugation with maltodextrin using high-temperature, short-time dry-heating Maillard reaction. Int. J. Food Sci. Tech. 49, 460–467 (2014)
H. Hu, J. Wu, E.C.Y. Li-Chan, L. Zhu, F. Zhang, X. Xu, G. Fan, L. Wang, X. Huang, S. Pan, Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocolloid. 30, 647–655 (2013)
W. Huang, X. Sun, Adhesive properties of soy proteins modified by sodium dodecyl sulfate and sodium dodecylbenzene sulfonate. J. Am. Oil Chem. Soc. 77, 705–708 (2000)
M. Qi, N.S. Hettiarachchy, U. Kalapathy, Solubility and emulsifying properties of soy protein isolates modified by pancreatin. J. Food Sci. 62, 1110–1115 (1997)
F. Xue, C. Li, X. Zhu, L. Wang, S. Pan, Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 51, 490–495 (2013)
Y. Yang, S.W. Cui, J. Gong, Q. Guo, Q. Wang, Y. Hua, A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocolloid. 48, 155–164 (2015)
N. Diftis, V. Kiosseoglou, Physicochemical properties of dry-heated soy protein isolate–dextran mixtures. Food Chem. 96, 228–233 (2006)
M. Kasran, S.W. Cui, H.D. Goff, Covalent attachment of fenugreek gum to soy whey protein isolate through natural Maillard reaction for improved emulsion stability. Food Hydrocolloid. 30, 552–558 (2013)
N. Diftis, V. Kiosseoglou, Improvement of emulsifying properties of soybean protein isolate by conjugation with carboxymethyl cellulose. Food Chem. 81, 1–6 (2003)
D. Zhu, S. Damodaran, J.A. Lucey, Formation of whey protein isolate (WPI)–dextran conjugates in aqueous solutions. J. Agric. Food Chem. 56, 7113–7118 (2008)
C.M. Oliver, L.D. Melton, R.A. Stanley, Creating proteins with novel functionality via the Maillard reaction: a review. Crit. Rev. Food Sci. 46, 337–350 (2006)
J.J. Guan, A.Y. Qiu, X.Y. Liu, Y.F. Hua, Y.H. Ma, Microwave improvement of soy protein isolate–saccharide graft reactions. Food Chem. 97, 577–585 (2006)
X.Y. Zhuo, J.R. Qi, S.W. Yin, X.Q. Yang, J.H. Zhu, L.X. Huang, Formation of soy protein isolate–dextran conjugates by moderate Maillard reaction in macromolecular crowding conditions. J. Sci. Food Agric. 93, 316–323 (2013)
F.C. de Oliveira, J.S. Coimbra, E.B. de Oliveira, A.D. Zuñiga, E.E. Rojas, Food protein-polysaccharide conjugates obtained via the Maillard reaction: a review. Crit. Rev. Food Sci. 56, 1108–1125 (2016)
F.J. Moreno, R. López-Fandiño, A. Olano, Characterization and functional properties of lactosyl caseinomacropeptide conjugates. J. Agric. Food Chem. 50, 5179–5184 (2002)
R.C. Groubet, J.M. Chobert, T. Haertle, Functional properties of milk proteins glycated in mild conditions. Sci. Aliment. 3, 423–438 (1999)
C.M.J. Brands, B.L. Wedzicha, M.A.J.S. van Boekel, Quantification of melanoidin concentration in sugar–casein systems. J. Agric. Food Chem. 50, 1178–1183 (2002)
J. Wang, X. Dong, S. Chen, J. Lou, Microencapsulation of capsaicin by solvent evaporation method and thermal stability study of microcapsules. Colloid J. 75, 26–33 (2013)
Z. Zheng, Y. Luo, L. Yao, J. Lu, G. Bu, Effects of Maillard reaction conditions on the functional properties of WPI chitosan oligosaccharide conjugates. J. Food Sci. Tech. 51, 3794–3802 (2014)
F.J. Morales, S. Jiménez-Pérez, Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 72, 119–125 (2001)
H. Jing, M. Yap, P.Y.Y. Wong, D.D. Kitts, Comparison of physicochemical and antioxidant properties of egg-white proteins and fructose and inulin Maillard reaction products. Food Bioprocess Tech. 4, 1489–1496 (2011)
V. Schmidt, C. Giacomelli, V. Soldi, Thermal stability of films formed by soy protein isolate–sodium dodecyl sulfate. Polym. Degrad. Stabil. 87, 25–31 (2005)
Y.R. Xie, N.S. Hettiarachchy, Xanthan gum effects on solubility and emulsification properties of soy protein isolate. J. Food Sci. 62, 1101–1104 (1997)
S. Tian, J. Chen, D.M. Small, Enhancement of solubility and emulsifying properties of soy protein isolates by glucose conjugation. J. Food Process. Pres. 35, 80–95 (2011)
Y. Li, Z. Chen, H. Mo, Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT-Food Sci. Technol. 40, 1167–1175 (2007)
E. Dickinson, V.B. Galazka, Emulsion stabilization by ionic and covalent complexes of β-lactoglobulin with polysaccharides. Food Hydrocolloid. 5, 281–296 (1991)
J.X. Xiao, G.Q. Huang, S.Q. Wang, Y.T. Sun, Microencapsulation of capsanthin by soybean protein isolate-chitosan coacervation and microcapsule stability evaluation. J. Appl. Polym. Sci. 131, 257–265 (2013)
D.V. Mendanha, S.E. Molina Ortiz, C.S. Favaro-Trindade, A. Mauri, E.S. Monterrey-Quintero, M. Thomazini, Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res. Int. 42, 1099–1104 (2009)
M.P. Nori, C.S. Favaro-Trindade, S.M. de Alencar, M. Thomazini, J.C. de Balieiro, C.J. Contreras Castillo, Microencapsulation of propolis extract by complex coacervation. LWT-Food Sci. Technol. 44, 429–435 (2011)
L.C. de Conto, C.R.F. Grosso, L.A.G. Gonçalves, Chemometry as applied to the production of omega-3 microcapsules by complex coacervation with soy protein isolate and gum Arabic. LWT-Food Sci. Technol. 53, 218–224 (2013)
A. Nesterenko, I. Alric, F. Silvestre, V. Durrieu, Vegetable proteins in microencapsulation: a review of recent interventions and their effectiveness. Ind. Crop Prod. 42, 469–479 (2013)
Acknowledgements
We thank the financial support from the National Science Foundation of China (Grant Number 31571890).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, LH., Sun, X., Huang, GQ. et al. Conjugation of soybean protein isolate with xylose/fructose through wet-heating Maillard reaction. Food Measure 12, 2718–2724 (2018). https://doi.org/10.1007/s11694-018-9889-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9889-y