A method has been developed to process waste of tungsten–cobalt solid solutions by potentiostatic dissolution in phosphoric acid solutions. A dissolution mechanism depending on the electrode potential has been proposed. A technique for extracting tungsten from tungsten concentrates in chloride–metasilicate melts has been developed. Conditions for distributing tungsten and ore components between immiscible substances have been established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tungsten carbide–cobalt solid solutions have found wide industrial application. Their significance increases with use in modern technology. To return expensive components contained in solid-solution waste into the production cycle, methods for secondary processing of such waste need to be developed. Matrices of spent drilling and cutting tools and scrap can represent such waste. The development of new methods for processing solid-solution waste remains relevant [1,2,3].
The existing methods for extracting components from solid solutions differ not only in processing conditions but also in the nature of chemical reagents used. A substantial disadvantage of the methods involving dissolution of solid-solution components with various acids and their mixtures is that the reagents are toxic. This can be partially eliminated as diamond and hardmetal tools can be processed by anodic dissolution in hydroxide–chloride melts [4]. Tungsten and carbon move from the anodic material to the melt as tungstates and carbonates. The solid-solution materials accumulate near the cathode as nanosized metallic powders. The process results in tungsten oxide requiring further processing into tungsten carbide to be returned into the tool production cycle. The application of high-temperature selective extraction of tungsten from concentrates and secondary raw materials in halide–silicate melts is described in [5].
Cobalt and tungsten carbide were separated by anodic dissolution in phosphoric acid solutions. The use of phosphoric acid solutions as an electrolyte allows the components of spent hardmetal tools to be separated and tungsten carbide suitable for returning into the production cycle to be extracted. The ability of tungsten and tungsten carbide to be passivated in aqueous solutions determines the electrochemical behavior of these compounds. Electrode potentials, cathodic processes in electrodeposition of gases, and oxidation processes with their participation were mainly studied in hydrochloric and sulfuric acid solutions. The electrochemical deposition of hydrogen on a tungsten carbide electrode was found to be limited by the recombination of adsorbed hydrogen atoms. The shape of curves showing anodic polarization of tungsten in sulfuric acid solutions is indicative of metal transition from active dissolution to passive state [6, 7].
Tungsten carbide is one of the most important hardmetallic and catalytically active compounds. More than 60% of tungsten processed today is used to produce carbide. Tungsten carbide is commercially produced by two methods: carburization of metal and aluminothermic reduction of ores and concentrates. Both methods include a significant number (more than seven) of complex stages. The production of tungsten carbide powder by electrochemical synthesis from melts is in the pilot test stage [8, 9].
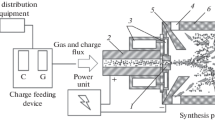
To extract tungsten compounds from ores and concentrates, environmentally safe dissolution in various salts is used. High-temperature selective extraction (HTSE) has been proposed as an alternative method. A method is under development to produce tungsten carbide by treating tungsten-containing melts with reducing gases, carbon, calcium carbide, etc. The first stage is to decompose the ore or concentrate by HTSE. The extracting components are sodium chloride and sodium metasilicate melts. When fused with tungsten concentrates (tungstates or scheelites), tungsten compounds move to the chloride phase and iron, manganese, and calcium oxides to the silicate one. The phases are separated by selective decantation. The degree of tungsten being removed to the chloride phase determines further feasibility of producing tungsten carbide by gas treatment of melts.
The objective of this paper is to conduct high-temperature selective extraction of tungsten from the concentrates and determine the process parameters (temperature, melt composition, extraction time).
Experimental Procedure
To study the anodic dissolution of electrodes from waste of WC–Co hardmetal, metallic cobalt, and tungsten carbide tools, a three-electrode electrochemical cell was used. The working electrolyte was a 1.25 molar solution (1.25 moles per 1 L of solution) of H3PO4 phosphoric acid. The electrodes were WC–6% Co (VK-6) rods. The tungsten carbide electrodes were produced from tungsten and carbon powders by hot pressing at 2200°C and 9.81 GPa. The cobalt electrodes were made of high-purity metallic foil. The electrodes’ surface area was 1–2 cm2. Before measurements, the working electrodes were carefully polished and finished. The polished surface was treated with alcohol and distilled water directly before measurements. A platinum plate 1.0–1.5 cm2 was used as an auxiliary electrode. The electrode potentials were measured relative to a saturated calomel electrode located beyond the electrochemical cell and connected with it through a salt bridge. All measurements were performed in a nitrogen atmosphere at 18°C. Stationary potentiostatic curves were plotted using a PI-50-1 potentiostat and PDP-4 recorder for measuring current intensity in time. Metallographic studies were conducted with a Neophot-21 electron microscope. The phase composition of solid samples was identified employing a DRON-4.0 diffractometer and the phase composition of gases employing a Selmichrom-1 chromatographer. Argon or helium was the carrier gas in the chromatographic analysis. The data were processed with an IBM-486 computer. The concentration curves showing the distribution of cobalt and tungsten in cross-sections were plotted with an MS-46 Cameca microprobe analyzer. The partial currents in the dissolution of metals were determined by comparing the recorded current and the solution analysis results.
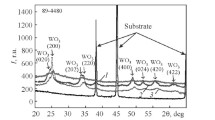
The tungsten concentrates used in the experiment had the following chemical composition, wt.%: 65.0–71.0 WO3, 8.5–9.2 FeO, 13.5–20.5 MnO, 0.5–1.5 Sb2O5, 0.5–1.2 SiO2, and 0.4–1.1 TiO2. Reagent-grade sodium chloride and metasilicate were used at the HTSE stage. The extraction process was carried out in silicon carbide crucibles. The crucibles were 50 mm in inner diameter and 150 mm in height. The HTSE charge contained (wt.%) 45 NaCl, 20 Na2SiO3, and 35 tungstate. The process temperature was 1000–1100°C and time up to 2 h. The liquid halide–tungstate phase was decanted into a separate crucible for further processing. The thicker silicate phase was filled with water. The insignificant amount of tungstate contained in the silicate phase was dissolved in water and removed from the leaching solution in the form of calcium tungstate.
Results and Discussion
Processing of Tungsten Carbide–Cobalt Solid-Solution Waste in Phosphoric Acid Solutions. Standard potentiostatic polarization curves for the anodic dissolution of VK-6 hardmetal, cobalt, and tungsten carbide and the reduction of hydrogen ions at the tungsten carbide electrode are shown in Fig. 1. The polarization curves are reproduced with cycling potential virtually without hysteresis. Mixing has hardly any effect on the curves either. The stationary potential of the VK-6 electrode is –(0.3–0.34) V and is intermediate between the stationary potentials of the components: –(0.55–0.57) V for cobalt and–(0.09–0.11) V for tungsten carbide. Three regions are clearly seen on the polarization curves for the VK-6 electrode (Fig. 1, curve 1). Logarithmic current density changes linearly with increasing potential in the first region at an electrode potential of –(0.30–0.05) V. At a potential of – 0.02 V, current sharply decreases and remains almost constant until the potential increases to 0.70 V (second region of the polarization curve). The current increases exponentially (third region) when the potential rises further. Anodic dissolution of the VK-6 rods proceeded in potentiostatic conditions at a potential of –(0.25–0.05) V. The current decreases slowly with time (for 10–12 h) and then remains practically unchanged (Fig. 2).
Two areas can be visually distinguished in the cross-section of a VK-6 rod after anodic dissolution (Fig. 3). The insoluble base and partially dissolved area are separated by a boundary. According to X-ray diffraction, the insoluble area consists of the WC phase. No cobalt or tungsten was found in it. These data were also confirmed by electron microprobe analysis.
Cross-sectional microphotograph of the VK-6 hardmetal sample after anodic dissolution in a 1.25 H3PO4 molar solution and concentration curves showing distribution of cobalt and tungsten between the soluble part and base: I—soluble electrode part; II—electrode base; electrode potential of –0.10 V; experimental time 10 h; ×600
The tungsten content of the dissolved area is lower than of the reference tungsten sample and is 93.1–93.8%, which corresponds to its weight content of the carbide.
To confirm this dissolution mechanism, the VK-6 rods were anodically dissolved at different potentials corresponding to the first and second regions of the polarization curve (Fig. 1). Table 1 summarizes data on the solutions analyzed. With higher potential, the cobalt and tungsten dissolution rates and W/Co weight ratio (ratio of the tungsten dissolution rate to the cobalt dissolution rate) increase in the total amount of dissolved metals. At potentials above 0.70 V, gas releases on the anodic surface. Chromatographic analysis revealed that the gas was carbon dioxide.
Analysis of the solutions after anodic dissolution (Table 1) and metallographic, X-ray diffraction, and electron microprobe analyses show that the Co–W phase dissolves selectively in the first region of the polarization curve, leaving WC grains in the rod. The dissolution process is presented as
According to Table 1, the tungsten content in the total amount of dissolved metals is 11.3–14.2%, which corresponds to its solubility in metallic cobalt [10, 11].
It should also be noted that the main portion of the total anodic current corresponds to the anodic current of cobalt dissolution. Hence, the polarization curves for the VK-6 and cobalt electrodes are similar visually and quantitatively (Fig. 1, curves 1 and 3).
The common dissolution of cobalt and tungsten significantly accelerates with higher potential. When it reaches –0.02 V, the current abruptly decreases and constitutes 15–20% of the maximum value. The passivation film consists of tungsten oxide and cobalt phosphate. When the potential rises further, the current first slowly increases and then jumps at 0.7 V.
This is attributed to the following reaction on the cathode:
at potentials that are positive in relation to the return potential. In this case, dissolved tungsten, except when it is present in insignificant amounts determined by reaction (2) and calculated using cobalt dissolution data, accumulates according to reaction (3). This is confirmed by data on the contribution of individual partial reactions to the total anodic process.
If the solution contains no oxygen, the cathodic current corresponds to the reduction of hydrogen ions (Fig. 1, curve 4). Extrapolation of the respective polarization curve to the entire potential axis (Ecor) corresponds to currents of the same order as those measured in cobalt dissolution without polarization. It can be assumed that the rate of dissolving the Co–W phase from hardmetals depends on the sample thickness, cobalt content of the hardmetal, and cobalt and tungsten carbide grain sizes. The substitution of WC–6% Co hardmetal particles 1.0–2.0 μm in size by WC–5% Co hardmetal particles 0.5–1.0 μm in size increases the dissolution rate by 1.1–1.3 times.
Selective dissolution of the Co–W phase can be used to process hardmetal scrap. It is desirable that cobalt was separated from the carbide at lower energy consumed. After dissolution of the Co–W phase, the remaining tungsten carbide is ground and can be used again in the production of hardmetal tools.
Tungsten Extraction from Tungsten Concentrates in Ion Melts. The optimum composition of the melt promoting the maximum effectiveness of tungsten extraction (as WO3) from the halide–tungstate phase and tungsten separation from iron and manganese oxides was established experimentally. The concentrations of NaCl, Na2SiO3, and (Fe, Mn)WO4 for separation of two immiscible liquids were chosen according to [12]. Figure 4 shows the effect of 35–60 wt.% sodium chloride and 10–40 wt.% sodium metasilicate.
The high HTSE effectiveness is due to the ability of sodium tungstate to mix with sodium chloride in any ratios and the immiscibility of the silicate phase, whose melting point is lower than 1000°C, with the halide–tungstate phase.
The most effective method for processing scheelite concentrates is to apply HTSE to mixtures of scheelite and tungstate in ratios from 1 : 4 to 2 : 1. The use of such mixtures allows the HTSE process to be conducted without addition of fluxes in the form of alkaline earth metal fluorides and aluminum oxide required in scheelite processing according to [19]. The mixtures in the above ratios allow more than 96% WO3 to be extracted into the halide–tungstate phase. The content of calcium, iron, and manganese oxides in this phase is lower than 2.5 wt.%. The halide–tungstate phase at combined concentrations contained 29–32 WO3, 0.03–0.12 CaO, 0.02–0.05 Fe2O3, and 0.01–0.04 wt.% MnO2.
Conclusions
The ranges of potentials for dissolving the tungsten carbide–cobalt solid solution that correspond to the selective dissolution of tungsten and cobalt and the separation of the solid tungsten carbide phase have been established.
The tungsten ores and concentrates have been found to decompose at 1050–1100°C in the sodium chloride–sodium metasilicate melts forming two immiscible phases: halide–tungstate and silicate. The former phase contains 96–99% tungsten and the latter phase contains more than 90% of different components.
References
T.M. Kurska, G.O. Chornobay, and S.B. Eremenko, Materials Science and Materials Technology [in Ukrainian], Univ. Tsyv. Zakh. Ukr., Kharkiv (2008), p. 136.
V.M. Garnets, Materials Science [in Ukrainian], Kondor, Kyiv (2009), p. 351.
V.V. Khilchevskii, S.E. Kondratyuk, and V.O. Stepanenko, Materials Science and Structural Material Technology [in Ukrainian], Tekhnika, Kyiv (2010), p. 465.
V.I. Shapoval, V.V. Malyshev, and N.M. Sushinskii, “Recovery of diamond and tungsten from spent cutting and drilling tools,” Ekotekhnol. Resursosber., No. 6, 46–50 (1999).
V.V. Malyshev, A.I. Gab, V.M. Shevchenko, and V.G. Glushakov, “Environmentally friendly and resource-saving extraction of tungsten from wolframite concentrates in molten salts,” Ekotekhnol. Resursosber., No. 3, 73–75 (2002).
P. Wassershoid and T. Welton, Ionic Liquids in Synthesis, Wiley–VCH Verlag GmbH (2008), p. 721.
K.R. Seddon, “Liquids for clean technology,” Chem. Technol. Biotechnol., 68, No. 4, 351–356 (1997).
V.V. Malyshev, High-Temperature Electrochemistry and Electrodeposition of Group IV–VIA Metals and Their Compounds in Ion Melts [in Ukrainian], Univ. Ukraina, Kyiv (2004), p. 323.
V.V. Malyshev, I.A. Novoselova, A.I. Gab, D.B. Shakhnin, I.M. Astrelin, and M. Gaune-Escard, “Chapter 3. Tungsten carbide: high temperature electrochemical synthesis from ionic melts, technologies of obtaining and regeneration,” Adv. Chem. Res., 33, 71–123 (2016).
V.V. Lyakishev (ed.), Phase Diagrams of Binary Metal Systems [in Russian], Mashinostroenie, Moscow (1996), p. 996.
R. Viswanadham (ed.), Science of Hard Materials, Springer, US (2012), p. 1012.
O.A. Ageev, A.E. Belyaev, N.S. Boltovets, et al., Interstitial Phases in Semiconductor Device and VLSI Technology [in Russian], Inst. Monokrist. Kharkiv (2008), p. 392.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 58, Nos. 3–4 (526), pp. 143–150, 2019.
Rights and permissions
About this article
Cite this article
Malyshev, V.V., Kushchevska, N.F. Production of Tungsten and Tungsten Carbide Powders. Powder Metall Met Ceram 58, 237–242 (2019). https://doi.org/10.1007/s11106-019-00069-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-019-00069-w